Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of local materials for formulation
- Extraction of sesame and soybeans oil: Sesame and soybean seeds were purchased from a local market of Bini-dang, Ngaoundere. The seeds were sorted to eliminate damaged grains and dirt. Seeds in good condition were washed thoroughly with clean water and sun dried in the open. All apparatus were washed and oven dried, and the soxhlet apparatus was set up in readiness for the experiment. The seeds were grounded mechanically, while soybean seeds were de-husked before grinding. Five hundred grams of each powdered sample was placed in a thimble made from thick filter paper and inserted into the center of the extractor. The soxhlet (1 L capacity) equipped with a condenser was placed onto a flask containing the hexane. The soxhlet was heated to 65 °C and allowed to reflux for about 8 h. It was then removed from the tube, dried in the oven, cooled in the desiccators, and weighed again to determine the amount of oil extracted [17].
- For lecithin: Lecithin was obtained by ethanolic extraction from soy residue after the defatting of soy powder with hexane. The soxhlet was heated to 80 °C and allowed to reflux for about 24 h.
- Obtention of Aloe vera gel protocol described by [18]: Leaves of Aloe vera were cut, washed, and then sliced an inch on both the upper and lower sides. The leaves were further cut and the pulp was removed thereafter. The pulp obtained was further crushed in a mechanical crusher. After the crushing of the pulp it was filtered in order to remove the attached fibres. The obtained sap was stored for future use.
- Additional information: The viscosity of the sesame oil was 54.2 cP. The viscosity of the soybean oil was 39.5 cP. The moisture content of the Aloe vera gel was 92.23%.
2.2.2. Screening of Factors of Each Phase
2.2.3. Experiment for the Optimization of the Base Cream Formulation
2.2.4. Preparation of Emulsion Base
2.2.5. Response Parameters
Creaming Index
Viscosity
Spreadability
Particle Size Determination
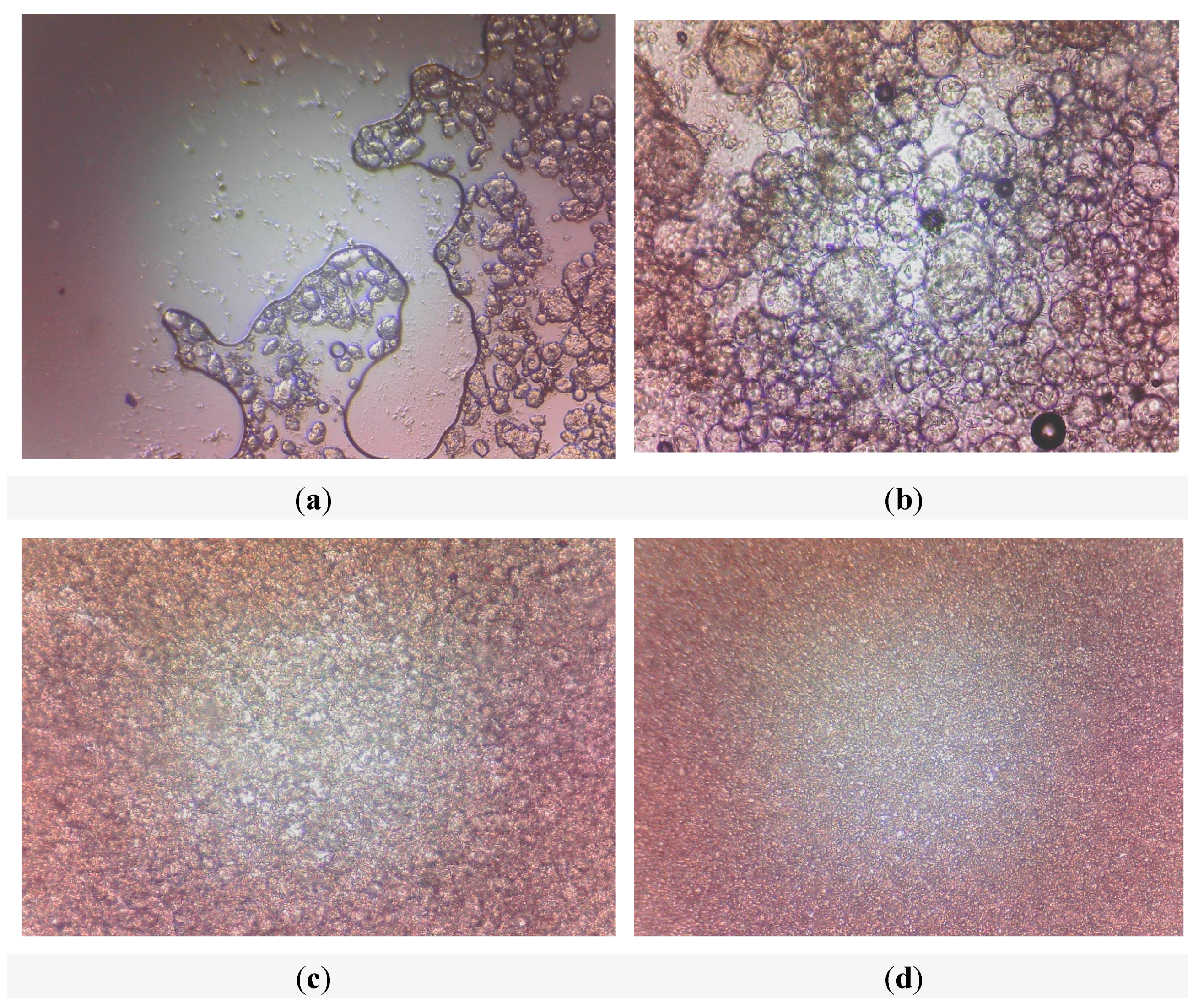
2.2.6. Microstructure Observation
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Screening of Base Cream Materials
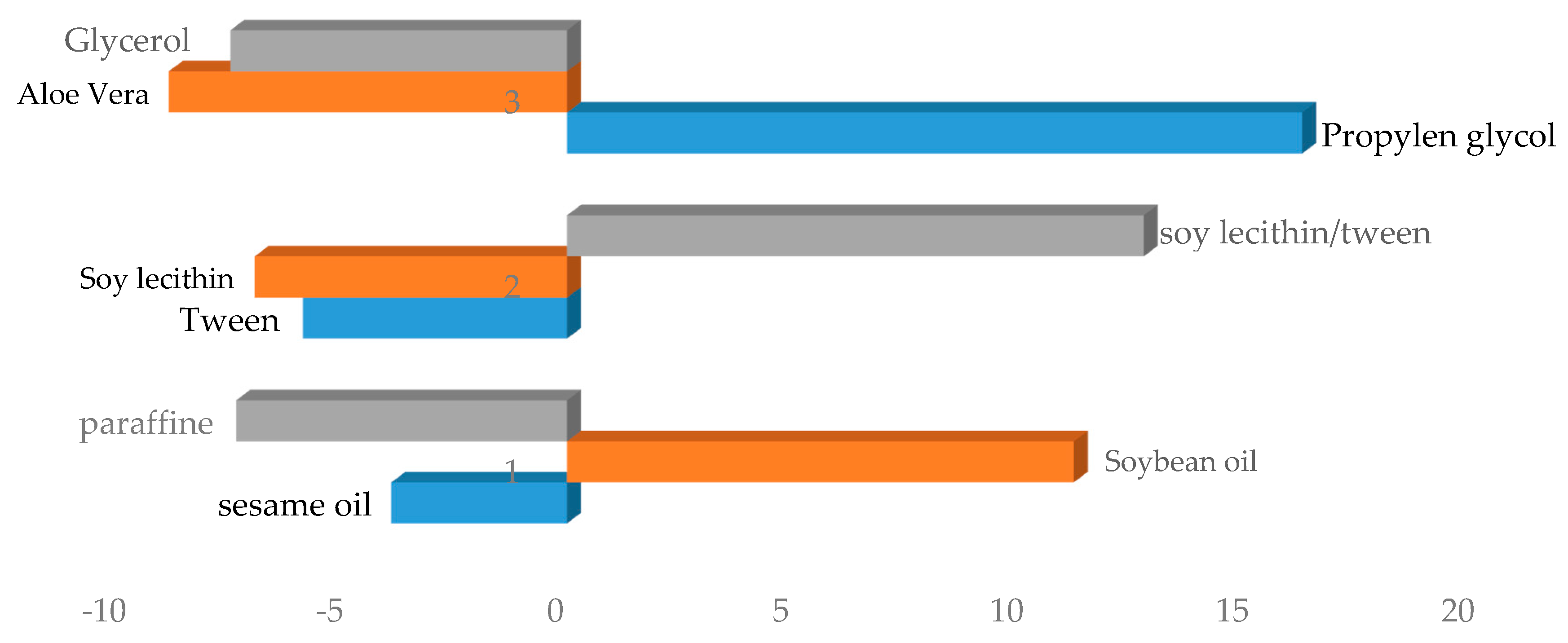
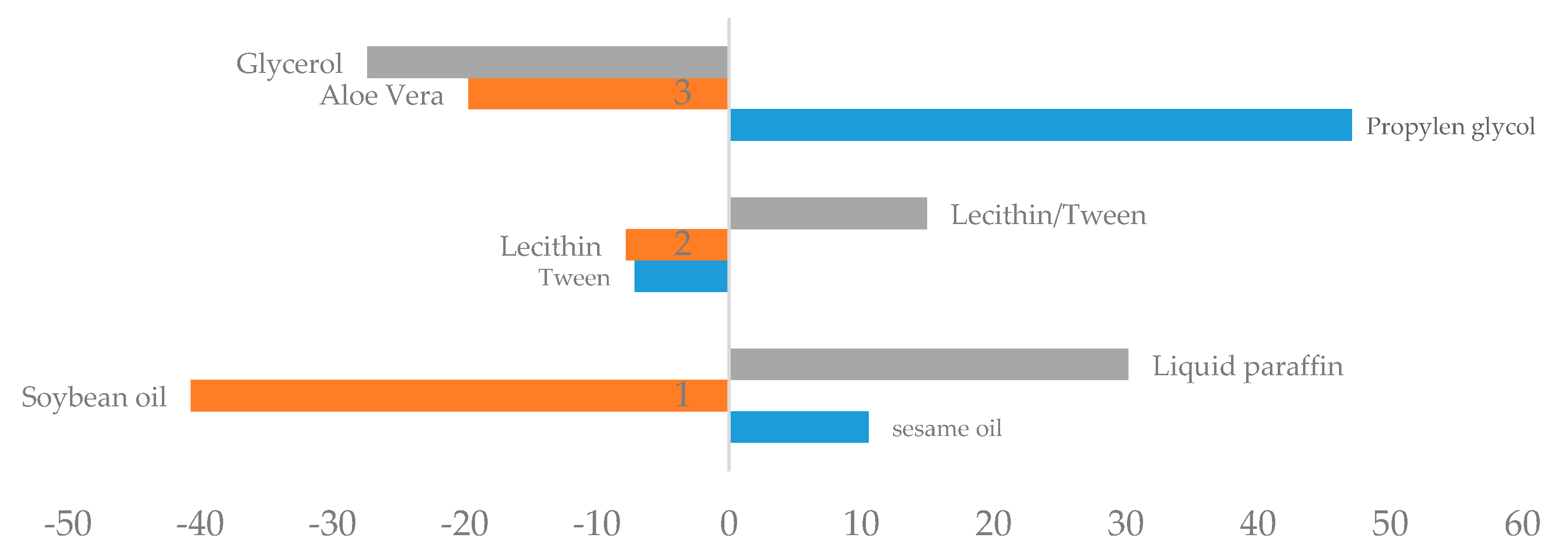
3.1.1. Effect of Different Phases on Creaming Index
3.1.2. Effect of Different Phases on Spreadability
3.1.3. Effect of Different Phases on Viscosity
3.2. Correlation between Responses
3.3. Optimization of Base Cream Formulation
3.3.1. Influence of Formulation on Viscosity
3.3.2. Influence of Formulation on Spreadability
3.3.3. Influence of Formulation on Particle Size
3.3.4. Optimization of Base Cream Formulation
3.3.5. Microstructure Observation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vinod, P.; Shaha, A.Y.; Flavian, S.R.A.; Dalia, S.M.M.E. A science based approach to topical drug classification system (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar]
- Lucinda, B.; Richard, K.; Benjamin, W.; Anna, W.; John, S.; Chi, W.C.; Saleh, T.; Mamta, G.B.; Gil, J.K.; Arthur, K.; et al. Topical drug classification. Int. J. Pharm. 2005, 295, 101–112. [Google Scholar]
- McClements, D.J. Reduced-fat foods: The complex science of developing diet-based strategies for tackling overweight and obesity. Adv. Nutr. 2015, 6, 338S–352S. [Google Scholar] [PubMed]
- Cañizares, P.; Martínez, F.; Lobato, J.; Rodrigo, M.A. Break-up of oil-in-wateremulsions by electrochemical techniques. J. Hazard. Mater. 2007, 145, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kralova, I.; Sjoblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 63–83. [Google Scholar]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2005, 70, 54–66. [Google Scholar]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field. Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [PubMed]
- Baines, D.; Seal, R. Natural Food Additives Ingredients and Flavourings; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Can, K.A.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the micro-encapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar]
- Cheung, L.; Wanasundara, J.; Nickerson, M.T. Effect of pH and NaCl on the emulsifying properties of a napin protein isolate. Food Biophys. 2015, 10, 30–38. [Google Scholar]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar]
- Silva, E.K.; Rosa, M.T.M.; Meireles, M.A.A. Ultrasound-assisted formation of emulsions stabilized by biopolymers. Curr. Opin. Food Sci. 2015, 5, 50–59. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Yang, M.; Liu, F.; Chen, Z. A novel process to efficiently form transglutaminase-set soy protein isolate-stabilized emulsion gels. LWT-Food Sci. Technol. 2013, 53, 15–21. [Google Scholar] [CrossRef]
- Rosa, M.; Kwiecien, M.; Tal-Figiel, B. Rheological investigations of pharmaceutical emulsions prepared with modified lecithin. Czas. Tech. 2013. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, O.P. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: A review. AAPS Pharm. Sci. Tech. 2005, 6, E298–E310. [Google Scholar] [CrossRef] [PubMed]
- Akpan, U.G.; Jimoh, A.; Mohammed, A.D. Extraction, Characterization and modification of castor seed oil. Leonardo J. Sci. 2006, 8, 43–52. [Google Scholar]
- Narayan, P.; Shruti, S.; Priyam, S.; Debabrata, C.; Suaib, L.; Sudeep, T. Development and evaluation of Aloe vera (L.) Burm based topical cream formulation. Ann. Phytomed. 2014, 3, 60–65. [Google Scholar]
- Peky, N.; Cristina, R.; Vladi, C.; Maria, T.; Elfriede, B.; Silvia, B. Optimization of Pothomorphe umbellata (L.) Miquel topical formulations using experimental design. Int. J. Pharm. 2008, 353, 149–159. [Google Scholar]
- Evi-Maria, V.; Eforia, T.; Nikoleta, X.; Anna-Maria, D. Stability Study of O/W Cosmetic Emulsions Using Rosmarinus officinalis and Calendula officinalis Extracts. Open J. Appl. Sci. 2012, 2, 139–145. [Google Scholar]
- Tsatsop, R.K.; Djiobie, G.; Regonne, K.; Bama, V.; Mbawala, A.; Ngassoum, M. Optimization of rheological properties in the formulation of an ointment base from natural ingredients. Int. J. Sci. Technol. Res. 2017, 6, 113–121. [Google Scholar]
- Panda, P. Formulation and evaluation of topical dosage form of Alangium salvifolium linn. and their wound healing activity. Asian J. Pharm. Sci. Res. 2011, 28, 10–22. [Google Scholar]
- Seamus, L.M.; Ruth, H.; Daniel, M. Effect of lecithin and monoglycerides on the heat stability of a model infant formula emulsion. Food Hydrocolloids 2008, 22, 888–898. [Google Scholar]
- Shchipunov, Y.A.; Schmiedel, P. Phase behavior of lecithin at the oil/water interface. Langmuir 1996, 12, 6443–6445. [Google Scholar] [CrossRef]
- Boyd, J.; Parkinson, C.; Sherman, P. Factors affecting emulsion stability, and the HLB concept. J. Colloid Interface Sci. 1972, 41, 359–370. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, D.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the Extraction Process of Sesame Seed’S Oil Using Response Surface Method on the Industrial Scale. Ind. Crops Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- Ilievska, B.; Loftsson, T.; Hjalmarsdottir, M.A.; Asgrimsdottir, G.M. Topical Formulation Comprising Fatty Acid Extract from Cod Liver Oil: Development, Evaluation and Stability Studies. Mar. Drugs 2016, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Reiss, H. Entropy-induced dispersion of bulk liquids. J. Colloid Interface Sci. 1975, 53, 61–70. [Google Scholar] [CrossRef]
- Baer, R.J.; Wolkow, M.D.; Kasperson, K.M. Effect of Emulsifiers on the Body and Texture of Low Fat Ice Cream. J. Dairy Sci. 1997, 80, 3123–3132. [Google Scholar] [CrossRef]
- Gowda, D.C.; Neelisiddaiah, B.; Anjaneyalu, Y.V. Structural Studies of polysaccharides from Aloe vera. Carbohydr. Res. 1979, 72, 201–205. [Google Scholar]
- Pattanayak, S.; Nayack, S.S.; Dinda, S.C.; Panda, K.; Naval, K.P. Evaluation of herbal ointments formulated with methanolic extract of Cajanus scarabaeides. J. Pharm. Allied Health Sci. 2011, 1, 47–59. [Google Scholar]
- Pasupathi, A.; Palanisamy, P.; Jaykar, B.; Margret, C.; Venkateswarlu, B.S. Formulation, development, evaluation of calcitriol and clobetasol propionate ointment. Indian J. Res. Pharm. Biotechnol. 2013, 1, 95. [Google Scholar]
- Amit, S.; Saraswati, B.; Kamalesh, U.; Kumud, U. Formulation and Evaluation of a Novel Herbal Gel of Equisetum arvense Extract. J. Pharmacogn. Phytochem. 2013, 1, 80–86. [Google Scholar]
- Hamilton, R. Structure and general properties of mineral and vegetables oils used a spray adjuvant. J. Pestic. Sci. 1993, 37, 141–146. [Google Scholar] [CrossRef]
- Quintana, S.E.; Franco, J.M.; Garcia-Zapateiro, L.A. Physicochemical and bromatological characteristics of arenca (Triportheus magdalenae), and rheological properties of oil-in-water emulsions containing isolated protein. Ciênc. Agrotecnol. 2015, 39, 634–641. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; Marcel Decker: New York, NY, USA, 2003. [Google Scholar]
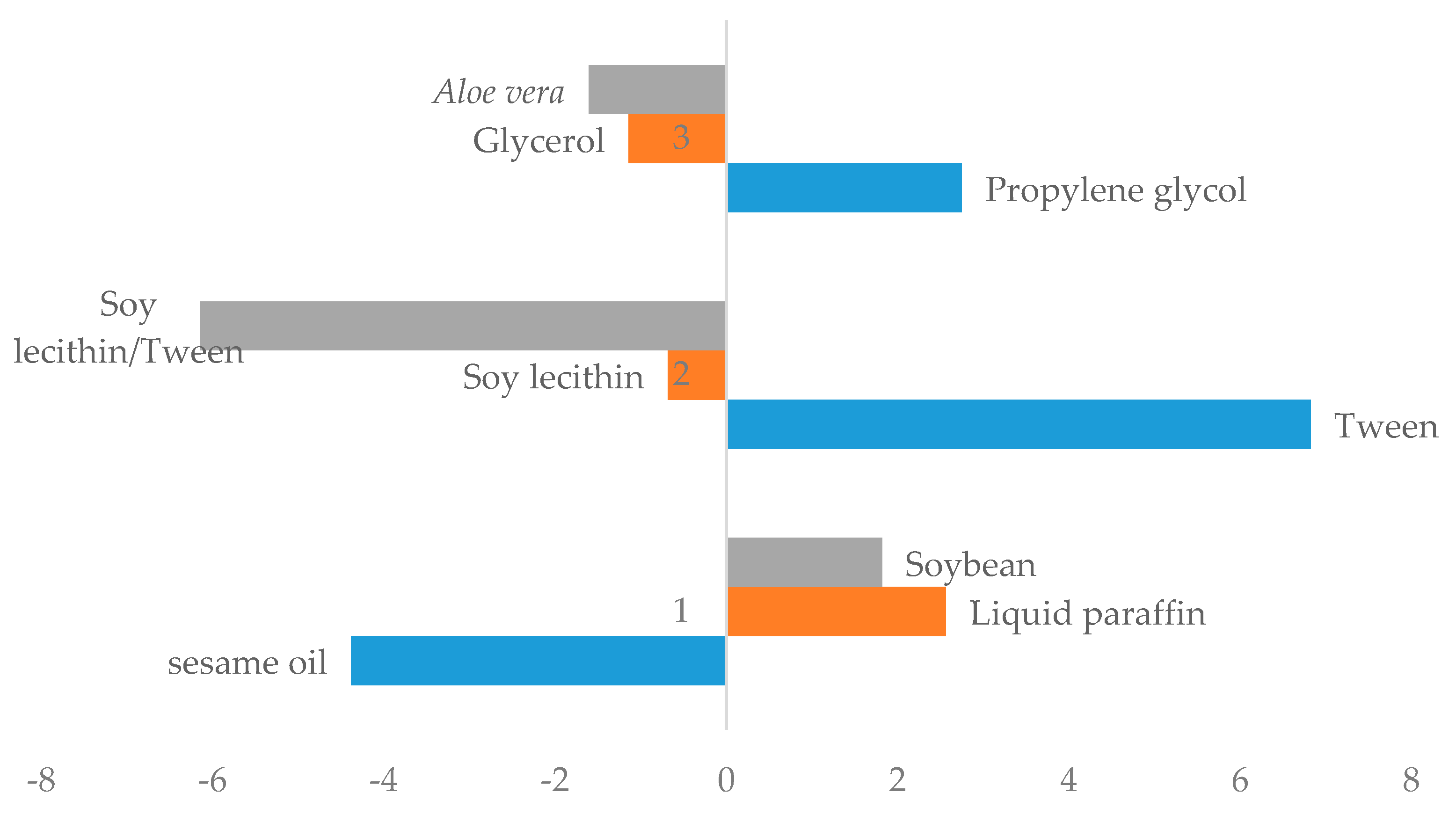
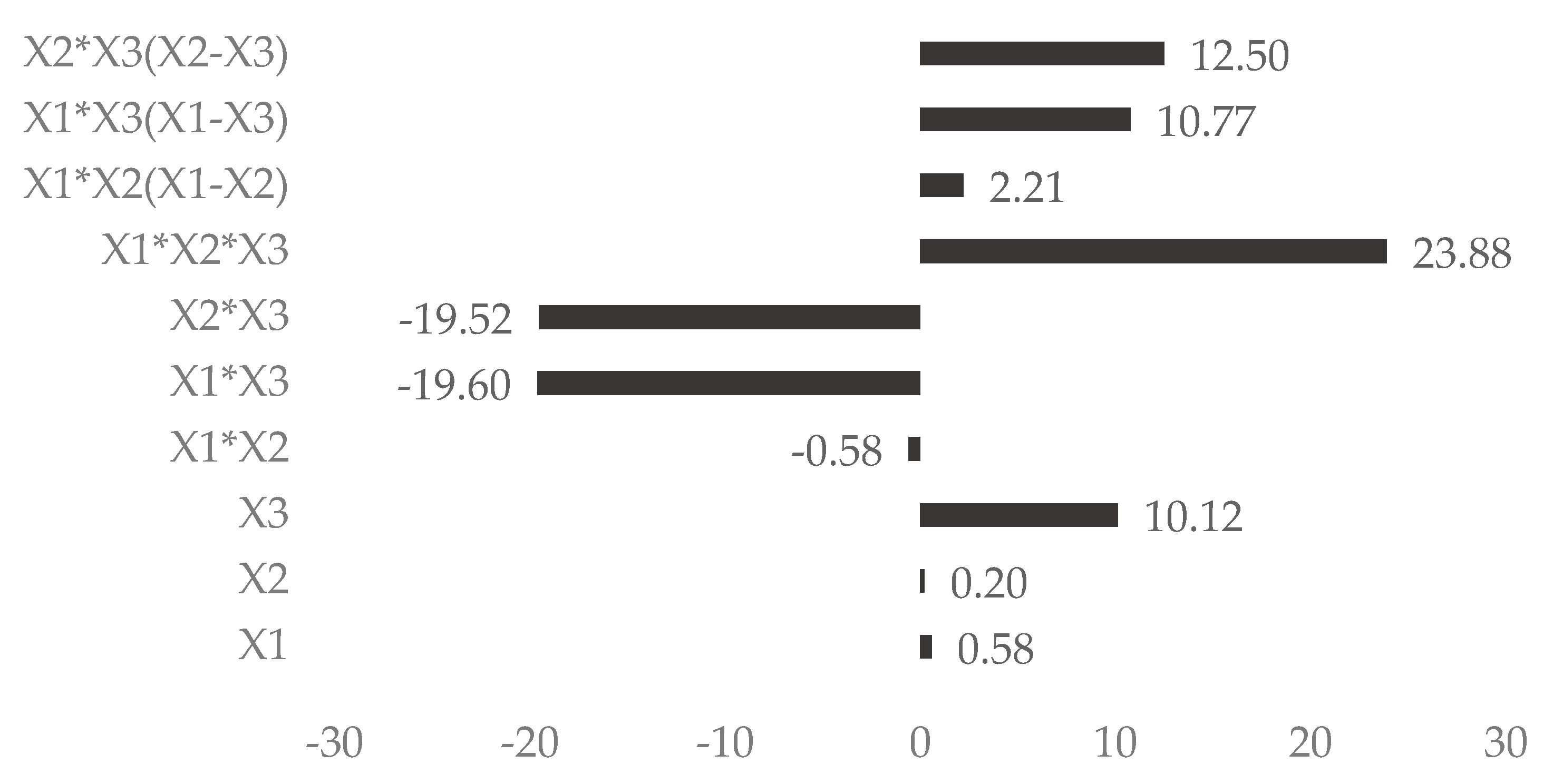
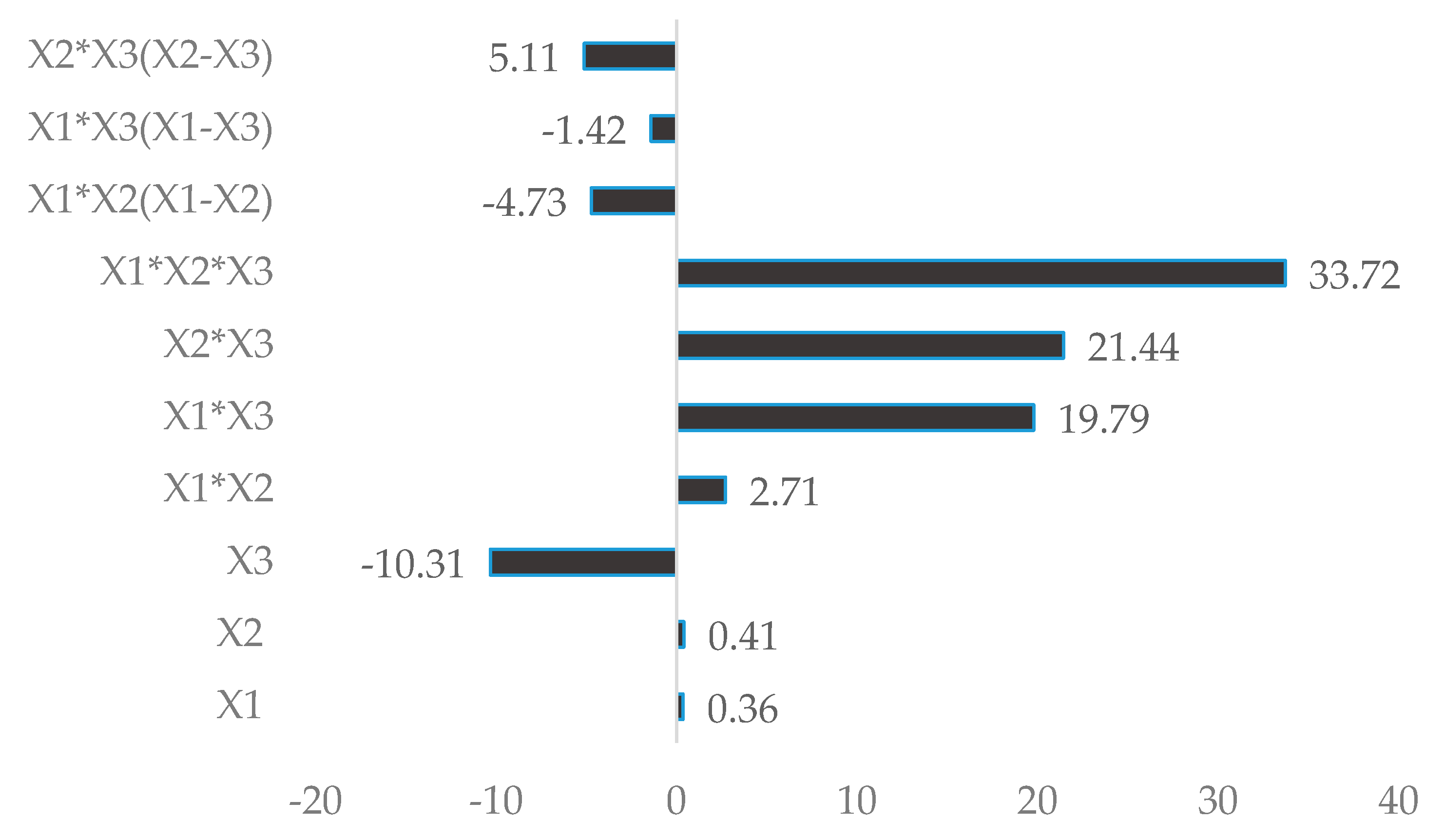
| Phase (Percentage) | Material | Coded Variable | Level of Variable |
|---|---|---|---|
| Oil phase (20%) | Soybean oil | X1 | 1 |
| Sesame oil | 2 | ||
| Liquid paraffin | 3 | ||
| Aqueous phase (30%) | Aloe vera gel | X2 | 1 |
| Glycerol | 2 | ||
| Propylene glycol | 3 | ||
| Emulsyfing phase (8%) | Lecithin | X3 | 1 |
| Tween | 2 | ||
| Lecithin/tween | 3 |
| Raw Material | Abbreviation | Lower Limit (%) | Upper Limit (%) |
|---|---|---|---|
| Selected oil phase | X1 | 20 | 25 |
| Selected aqueous phase | X2 | 28 | 32 |
| Selected emulsifying phase | X3 | 8 | 10 |
| Runs | Oil Phase | Emulsifying | Aqueous Phase | Creaming Index (%) | Spreadability (g·cm/s) | Viscosity (cP) |
|---|---|---|---|---|---|---|
| 1 | 1 | 3 | 1 | 0.0 ± 0.0 | 31.02 ± 1.12 | 360 ± 10 |
| 2 | 2 | 3 | 1 | 0.0 ± 0.0 | 20.99 ± 1.01 | 340 ± 7 |
| 3 | 1 | 1 | 1 | 8.33 ± 0.12 | 20.46 ± 1.09 | 320 ± 5 |
| 4 | 1 | 2 | 1 | 0.0 ± 0.0 | 22.39 ± 1.15 | 328 ± 8 |
| 5 | 2 | 1 | 1 | 16.66 ± 0.14 | 17.87 ± 1.15 | 415 ± 12 |
| 6 | 2 | 2 | 1 | 28.33 ± 0.10 | 15.07 ± 0.51 | 425 ± 11 |
| 7 | 3 | 1 | 1 | 25.00 ± 0.11 | 22.60 ± 1.14 | 229 ± 8 |
| 8 | 3 | 2 | 1 | 16.67 ± 0.14 | 21.57 ± 1.17 | 275 ± 6 |
| 9 | 3 | 3 | 1 | 10.00 ± 0.05 | 21.99 ± 0.25 | 290 ± 4 |
| 10 | 1 | 1 | 2 | 20.00 ± 0.13 | 9.043 ± 0.05 | 370 ± 6 |
| 11 | 1 | 2 | 2 | 1.66 ± 0.05 | 20.62 ± 1.01 | 367 ± 5 |
| 12 | 1 | 3 | 2 | 1.66 ± 0.06 | 22.18 ± 1.10 | 315 ± 11 |
| 13 | 2 | 1 | 2 | 15.67 ± 0.17 | 12.31 ± 0.053 | 480 ± 14 |
| 14 | 2 | 2 | 2 | 8.33 ± 0.02 | 14.90 ± 0.95 | 410 ± 11 |
| 15 | 2 | 3 | 2 | 1.66 ± 0.05 | 19.27 ± 1.10 | 419 ± 9 |
| 16 | 3 | 1 | 2 | 20.00 ± 0.17 | 20.44 ± 1.10 | 420 ± 7 |
| 17 | 3 | 2 | 2 | 1.66 ± 0.03 | 22.60 ± 1.11 | 412 ± 10 |
| 18 | 3 | 3 | 2 | 0.0 ± 0.0 | 19.64 ± 1.12 | 390 ± 9 |
| 19 | 1 | 1 | 3 | 8.33 ± 0.11 | 18.37 ± 1.10 | 415 ± 11 |
| 20 | 1 | 2 | 3 | 0.83 ± 0.01 | 18.96 ± 1.09 | 419 ± 12 |
| 21 | 1 | 3 | 3 | 0.0 ± 0.10 | 15.70 ± 0.05 | 417 ± 10 |
| 22 | 2 | 1 | 3 | 15.00 ± 0.11 | 13.75 ± 0.05 | 412 ± 8 |
| 23 | 2 | 2 | 3 | 15.00 ± 0.10 | 21.00 ± 1.03 | 421 ± 10 |
| 24 | 2 | 3 | 3 | 3.33 ± 0.09 | 22.19 ± 1.07 | 450 ± 14 |
| 25 | 3 | 1 | 3 | 13.33 ± 0.14 | 22.39 ± 1.12 | 409 ± 6 |
| 26 | 3 | 2 | 3 | 1.60 ± 0.02 | 19.64 ± 1.08 | 419 ± 8 |
| 27 | 3 | 3 | 3 | 8.33 ± 0.09 | 17.70 ± 1.05 | 290 ± 2 |
| Responses | Coefficient Correlation | Goal | |
|---|---|---|---|
| Height | Viscosity | 0.71 | Moderately strong |
| Spreadability | Viscosity | 0.95 | Strong |
| Spreadability | Height | 0.66 | Average |
| Variables Level | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Viscosity | Spreadability | Particle Size | |||||||
| N° | X1 | X2 | X3 | Observed. | Adjusted. | Observed. | Adjusted. | Observed. | Adjusted. |
| 1 | 24 | 28 | 8 | 734 | 785.4 | 28.9 | 34.5 | 34.6 | 21.8 |
| 2 | 20 | 32 | 8 | 293 | 276.0 | 154.4 | 140.3 | 22.9 | 25.1 |
| 3 | 22 | 28 | 10 | 640 | 611.6 | 54.5 | 57.7 | 8.7 | 3.7 |
| 4 | 20 | 30 | 10 | 100 | 382.8 | 34.9 | 24.9 | 34.6 | 25.0 |
| 5 | 22.75 | 28.75 | 8.5 | 760 | 747.8 | 46.3 | 34.2 | 104.7 | 106.3 |
| 6 | 20.75 | 30.75 | 8.5 | 218 | 221.4 | 57 | 65.7 | 138.0 | 140.7 |
| 7 | 21.75 | 28.75 | 9.5 | 220 | 240.2 | 54.5 | 45.6 | 138.0 | 142.0 |
| 8 | 20.75 | 29.75 | 9.5 | 83 | 54.1 | 38.9 | 39.9 | 158.4 | 158.7 |
| 9 | 22 | 30 | 8 | 320 | 332.7 | 46.3 | 48.2 | 60.2 | 64.5 |
| 10 | 23 | 28 | 9 | 386.7 | 406.3 | 35.4 | 36.8 | 69.1 | 77.0 |
| 11 | 20 | 31 | 9 | 240.3 | 264.4 | 45 | 51.9 | 69.1 | 77.4 |
| 12 | 21 | 29 | 10 | 426 | 473.6 | 40.2 | 49.5 | 79.4 | 98.3 |
| 13 | 21.5 | 29.5 | 9 | 284 | 253.1 | 42.3 | 42.62 | 208.9 | 186.3 |
| 14 | 20 | 32 | 8 | 266.7 | 785.4 | 132.3 | 34.5 | 30.2 | 21.8 |
| 15 | 24 | 28 | 8 | 840 | 276.0 | 35.6 | 140.3 | 11.4 | 25.1 |
| Parameters | Viscosity | Spreadability | Particle Size | |||
|---|---|---|---|---|---|---|
| Coefficient | Probability | Coefficient | Probability | Coefficient | Probability | |
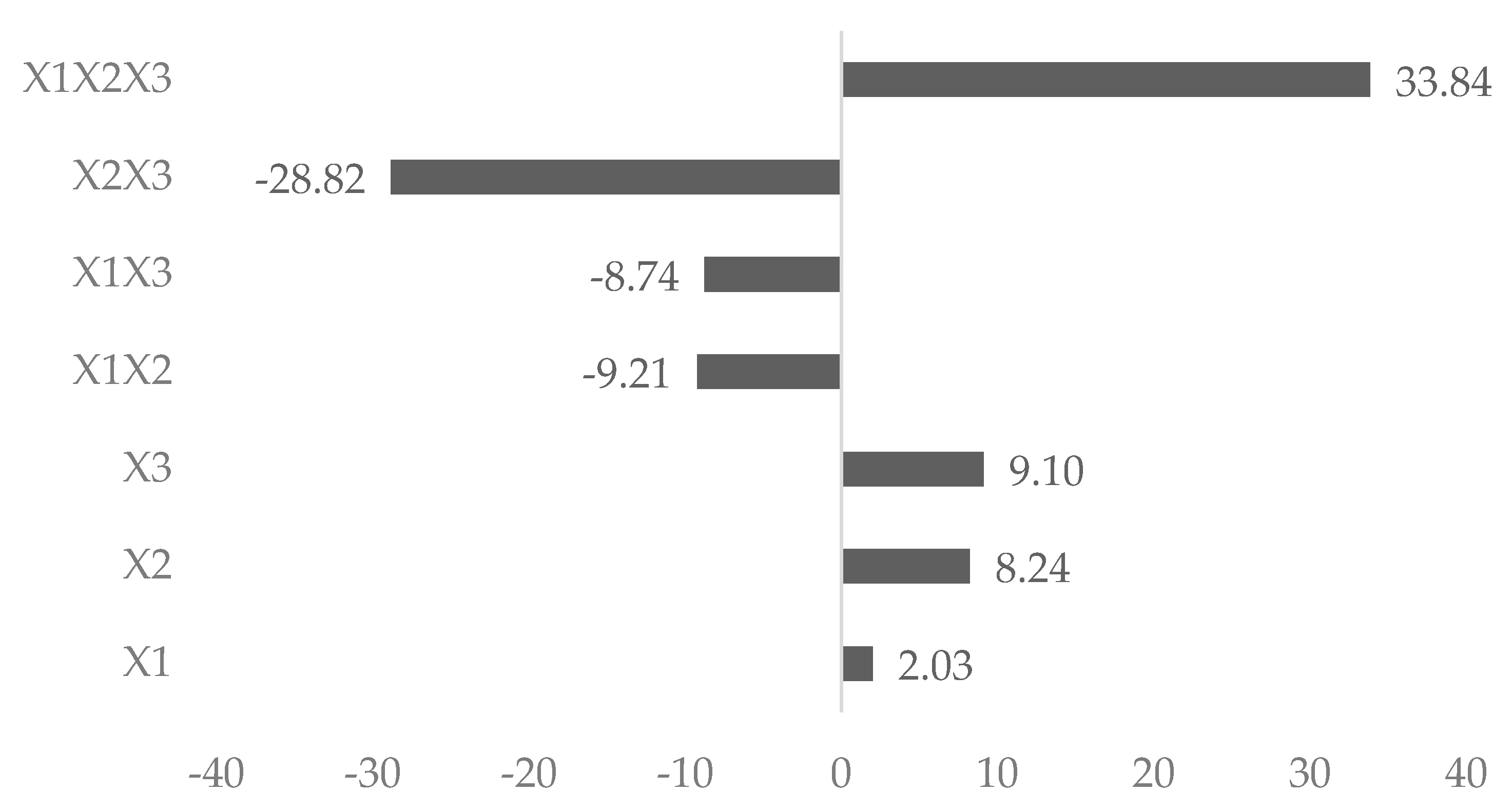
| X1 | 785.4 | 34.5 | 21.8 | |||
| X2 | 276.0 | 140.4 | 25.1 | |||
| X3 | 13,733.3 | 155.1 | −626.1 | |||
| X1 × X2 | −792.2 | 0.01 | −156.9 | 0.007 | 164.5 | 0.01 |
| X1 × X3 | −26,590.8 | 0.0009 | −148.2 | 0.2 | 1201.7 | 0.0009 |
| X2 × X3 | −26,487.5 | 0.0009 | −491.1 | 0.002 | 1302.3 | 0.0008 |
| X1 × X2 × X3 | 32,404.4 | 0.002 | 576.5 | 0.06 | 2048.3 | 0.002 |
| X1 × X2(X1 − X2) | 3013.8 | 0.004 | −287.2 | 0.003 | ||
| X1 × X3(X1 − X3) | 14,610.4 | 0.002 | −86.3 | 0.002 | ||
| X2 × X3(X2 − X3) | 16,964.6 | 0.001 | −310.2 | 0.0010 | ||
| R2 | 98.25 | 95.44 | 96.92 | |||
| R2 adj | 95.09 | 92.02 | 91.39 | |||
| Lack of fit | 0.24 | 0.68 | 0.23 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DJIOBIE TCHIENOU, G.E.; TSATSOP TSAGUE, R.K.; MBAM PEGA, T.F.; BAMA, V.; BAMSECK, A.; DONGMO SOKENG, S.; NGASSOUM, M.B. Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients. Cosmetics 2018, 5, 7. https://doi.org/10.3390/cosmetics5010007
DJIOBIE TCHIENOU GE, TSATSOP TSAGUE RK, MBAM PEGA TF, BAMA V, BAMSECK A, DONGMO SOKENG S, NGASSOUM MB. Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients. Cosmetics. 2018; 5(1):7. https://doi.org/10.3390/cosmetics5010007
Chicago/Turabian StyleDJIOBIE TCHIENOU, Gertrude Eleonore, Roli Karole TSATSOP TSAGUE, Therese Florence MBAM PEGA, Vera BAMA, Albert BAMSECK, Selestin DONGMO SOKENG, and Martin Benoît NGASSOUM. 2018. "Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients" Cosmetics 5, no. 1: 7. https://doi.org/10.3390/cosmetics5010007




