Potential Use of Spin Traps to Control ROS in Antipollution Cosmetics—A Review
Abstract
:1. Introduction
2. Pollution and Its Effect on the Skin
3. Use of Antioxidants and Some Limitations Compared to Spin Traps
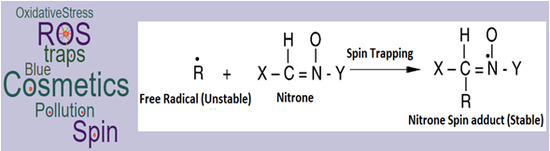
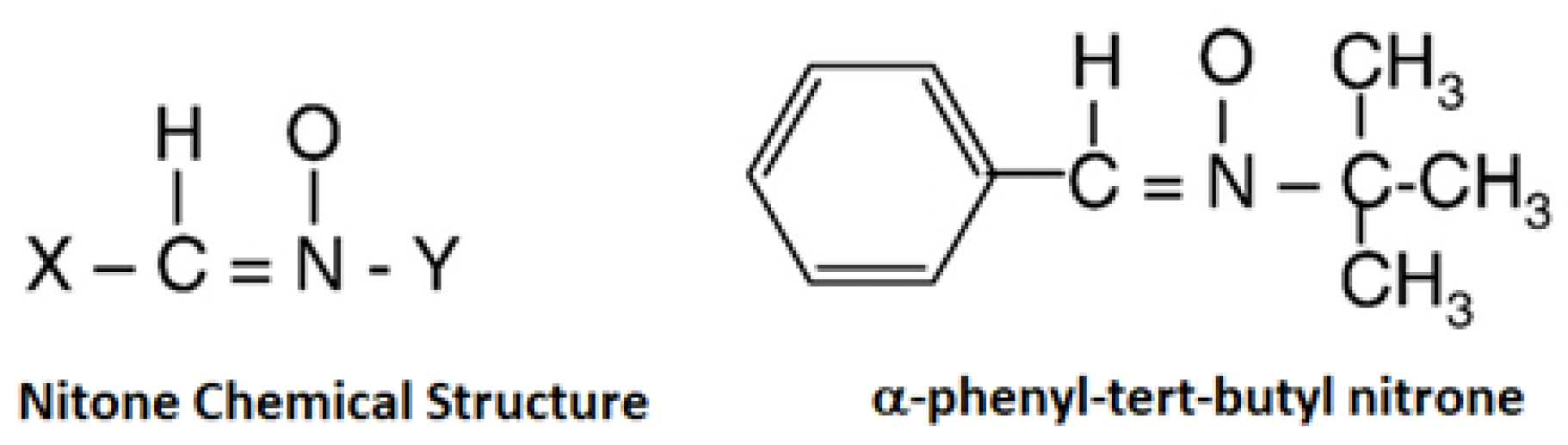
4. Spin Traps (Applications and Limitations)
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- WHO Website. Available online: http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/ (accessed on 15 December 2017).
- Beelen, R.; Hoek, G.; van den Brandt, P.A.; Goldbohm, R.A.; Fischer, P.; Schouten, L.J.; Armstrong, B.; Brunekreef, B.R. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 2008, 19, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Castano-Vinyals, G.; Cantor, K.P.; Malats, N.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Rothman, N.; Vermeulen, R.; Silverman, D.; et al. Air pollution and risk of urinary bladder cancer in a case-control study in Spain. Occup. Environ. Med. 2008, 65, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Lopaczynski, W.; Zeisel, S.H. Antioxidants, programmed cell death, and cancer. Nutr. Res. 2001, 21, 295–307. [Google Scholar] [CrossRef]
- Glade, M.J. The role of reactive oxygen species in Health and Disease. Nutrition 2003, 19, 401–403. [Google Scholar]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Menichini, E. Urban air pollution by polycyclic aromatic hydrocarbons: Levels and sources of variability. Sci. Total Environ. 1992, 116, 109–135. [Google Scholar] [CrossRef]
- Penning, T.M.; Burcynski, M.E.; Hung, C.F.; McCoull, K.D.; Palackal, N.T.; Tsuruda, L.S. Dihydrodiol dehydrogenase and polycyclic aromatic hydrocarbon activation: Generation of reactive and redox active o-quinones. Chem. Res. Toxicol. 1999, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Janzen, E.G.; Blackburn, B.J. Detection and identification of short-lived free radicals by electron spin resonance trapping techniques (spin trapping). Photolysis of organolead, -tin, and -mercury compounds. J. Am. Chem. Soc. 1969, 91, 4481–4490. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schaefer, H.; Otberg, N.; Teichmann, A.; Blume-Peytavi, U.; Sterry, W. Penetration of microparticles into human skin. Hautarzt 2004, 55, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Schäfer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hübenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lighting up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Jux, B.; Kadow, S.; Esser, C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 2009, 182, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W.; Aljuffali, I.A.; Huang, C.T.; Lee, C.W.; Fang, J.Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Tay, S.H. Environmental Factors, Toxicants and Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2014, 15, 16043–16056. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Loser, K.; Stander, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Angioni, C.; Ferreiros, N.; Schuh, C.D.; Suo, J.; Schreiber, Y.; Dawes, J.M.; Antunes-Martins, A.; Bennett, D.L.H.; McMahon, S.B.; et al. Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS ONE 2013, 8, e81228. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Ueta, M.; Katoh, N. TLR3 and Inflammatory Skin Diseases: From Environmental Factors to Molecular Opportunities. In Skin Stress Response Pathways; Springer: Berlin, Germany, 2016; pp. 235–249. [Google Scholar]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letašiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Rev. Environ. Health 2012, 11 (Suppl. 1), S12. [Google Scholar]
- Matsumu, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Hölzle, E.; Hönigsmann, H. UV-radiation–sources, wavelength, environment. J. Dtsch. Dermatol. Ges. 2005, 3 (Suppl. 2), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Madronich, S.; McKenzie, R.L.; Björn, L.O.; Caldwell, M.M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B 1998, 46, 5–19. [Google Scholar] [CrossRef]
- Situm, M.; Buljan, M.; Bulić, S.O.; Simić, D. The mechanisms of UV radiation in the development of malignant melanoma. Coll. Antropol. 2007, 1, 13–16. [Google Scholar]
- Garibyan, L.; Fisher, D.E. How sunlight causes melanoma. Curr. Oncol. Rep. 2010, 12, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I.B. Signaling by reactive oxygen and nitrogen species in skin diseases. Curr. Drug Metab. 2010, 11, 409–414. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Huang, J.L.; Sik, R.H.; Liu, J.; Waalkes, M.P.; Chignell, C.D. Expression profiling of human keratinocyte response to ultraviolet A: Implications in apoptosis. J. Investig. Dermatol. 2004, 122, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Kaspar, K.; Altmeyer, P.; Gambichler, T. UV transmission measurements of small skin specimens with special quartz cuvettes. Dermatology 2000, 201, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.A.; Kurt, F.; Jaenicke, R.; Rox, A. Erythema and melanogenesis action spectra of normal human skin. Photochem. Photobiol. 1982, 36, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Eskandarpour, M.; Hashemi, J.; Kanter, L. High gene mutation rate may contribute to hereditary skin cancers. J. Natl. Cancer Inst. 2003, 95, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ho, V.C.; Trotter, M.J.; Horsman, D.E.; Tron, V.A. Mutation in metastatic melanomas and primary melanomas from sun-exposed and sun-protected sites. J. Eur. Acad. Dermatol. Venereol. 1995, 4, 48–53. [Google Scholar] [CrossRef]
- Goodsell, D.S. The molecular perspective: Ultraviolet light and pyrimidine dimers. Oncologist 2001, 6, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed.; ASM Press: Washington, DC, USA, 2006; pp. 1118–1120. [Google Scholar]
- Whitmore, S.E.; Potten, C.S.; Chadwick, C.A.; Strickland, P.T.; Morison, W.L. Effect of photoreactivating light on UV radiation-induced alterations in human skin. Photodermatol. Photoimmunol. Photomed. 2001, 17, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Hatakeyama, Y.; Ohta, M.; Mori, T.; Nikaido, O. Establishment and characterization of a monoclonal-antibody recognising the Dewarisomers of (6-4) photoproducts. Photochem. Photobiol. 1993, 57, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Rosenstein, B.S. The use of specific radioimmunoassays to determine action spectra for the photolysis of (6-4) photoproducts. Photochem. Photobiol. 1987, 45, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.; Maisch, T.; Maier, M.; Engel, E.; Landthaler, M.; Bäumler, W. Singlet oxygen generation by UVA light exposure of endogenous photosensitizes. Biophys. J. 2006, 91, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Simon, J.D. Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin. Proc. Natl. Acad. Sci. USA 1998, 95, 10576–10578. [Google Scholar] [CrossRef] [PubMed]
- Albro, P.W.; Bilski, P.; Corbett, J.T.; Schroeder, J.L.; Chignell, C.F. Photochemical reactions and phototoxicity of sterols: Novel self-perpetuating mechanisms for lipid photooxidation. Photochem. Photobiol. 1997, 66, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T.; Roberts, M.J.; Jacobson, M.K.; Jacobson, E.L. 3-Hydroxypyridine Chromophores Are Endogenous Sensitizers of Photooxidative Stress in Human Skin Cells. J. Biol. Chem. 2004, 279, 30009–30020. [Google Scholar] [CrossRef] [PubMed]
- Wenczl, E.; van der Schans, G.P.; Roza, L.; Kolb, R.M.; Timmerman, A.J.; Smit, N.P.M.; Pavel, S.; Schothorst, A.A. (Pheo)Melanin Photosensitizes UVA-Induced DNA Damage in Cultured Human Melanocytes. J. Investig. Dermatol. 1998, 111, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.; Joshi, P.C. Tryptophan as an endogenous photosensitizer to elicit harmful effects of ultraviolet B. Indian J. Biochem. Biophys. 1992, 29, 296–298. [Google Scholar] [PubMed]
- Defedericis, H.C.; Patrzyc, H.B.; Rajecki, M.J.; Budzinski, E.E.; Lijima, H.; Dawidzik, J.B.; Evans, M.S.; Greene, K.E.; Box, H.C. Singlet oxygen-induced DNA damage. Radiat. Res. 2006, 165, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.T.; Madzak, C.; Sarasinm, A.; DI Mascio, P.; Sies, H.; Menckm, C.F.M. Singlet oxygen induced DNA damage and mutagenicity in a single-stranded SV40-based shuttle vector. Photochem. Photobiol. 1992, 55, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shafirovich, V.; Shapiro, R.; Geacintov, N.E.; Broyde, S. Spiroimino dihydantoin lesions derived from guanine oxidation structures, energetics, and functional implications. Biochemistry 2005, 44 (Suppl. 16), 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.G. Biochemical basis of ozone toxicity. Free Radic. Biol. Med. 1990, 9, 245–265. [Google Scholar] [CrossRef]
- Gruber, J.V.; Tay, A.; Holtz, R. Protecting the skin against ozone. J. Cosmet. Sci. 2005, 56, 348–349. [Google Scholar]
- Sitch, S.; Cox, P.M.; Collins, W.J.; Huntingford, C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.C. Free radical reactions in biology: Initiation of lipid autoxidation by ozone and nitrogen dioxide. Environ. Health Perspect. 1979, 16, 180–181. [Google Scholar]
- Han, S.J.; Kwak, M.K.; Han, D.H.; Kim, S.H.; Jang, A.S. Ozone Exposure Suppresses Proliferative Response in Mice Skin. Korean J. Intern. Med. 2012, 27, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Traber, M.G.; Podda, M.; Tsang, K.; Cross, C.E.; Packer, L. Ozone depletes tocopherols and tocotrienols topically applied to murine skin. FEBS Lett. 1997, 401, 167–170. [Google Scholar] [CrossRef]
- Fortino, V.; Maioli, E.; Torricelli, C.; Davis, P.; Valacchi, G. Cutaneous MMPs are differently modulated by environmental stressors in old and young mice. Toxicol. Lett. 2007, 173, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Traber, M.G.; Tsang, K.; Cross, C.E.; Packer, L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic. Biol. Med. 1997, 23, 385–391. [Google Scholar] [CrossRef]
- Thiele, J.J.; Traber, M.G.; Polefka, T.G.; Cross, C.E.; Packer, L. Ozone-exposure depletes vitamin E and induces lipid peroxidation in murine stratum corneum. J. Investig. Dermatol. 1997, 108, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Weber, S.U.; Luu, C.; Cross, C.E.; Packer, L. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000, 466, 165–168. [Google Scholar] [CrossRef]
- Pryor, W.C.; Church, D.F. The reaction of ozone with unsaturated fatty acids: Aldehydes and hydrogen peroxide as mediators of ozone toxicity. In Oxidative Damage and Repair: Chemical, Biological and Medical Aspects; Davies, K.J.A., Ed.; Pergamon Press: New York, NY, USA, 1991; pp. 496–504. [Google Scholar]
- Valacchi, G.; van der Vliet, A.; Schock, B.C.; Okamoto, T.; Obermuller-Jevic, U.; Cross, C.E.; Packer, L. Ozone exposure activates oxidative stress responses in murine skin. Toxicology 2002, 179, 163–170. [Google Scholar] [CrossRef]
- He, Q.C.; Tavakkol, A.; Wietecha, K.; Begum-Gafur, R.; Ansari, S.A.; Polefka, T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int. J. Cosmet. Sci. 2006, 28, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pagnin, E.; Okamoto, T.; Corbacho, A.M.; Olano, E.; Davis, P.A.; van der Vliet, A.; Packer, L.; Cross, C.E. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem. Biophys. Res. Commun. 2003, 305, 741–746. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Zaid, M.A.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Maes, D.; Mukhta, H. Aryl Hydrocarbon Receptor Is an Ozone Sensor in Human Skin. J. Investig. Dermatol. 2009, 129, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Podda, M.; Packer, L. Tropospheric ozone: An emerging environmental stress to skin. Biol. Chem. 1997, 378, 1299–1305. [Google Scholar] [PubMed]
- Fabbrocini, G.; Triassi, M.; Mauriello, M.C.; Torre, G.; Annunziata, M.C.; De Vita, V.; Pastore, F.; D’Arco, V.; Monfrecola, G. Epidemiology of Skin Cancer: Role of Some Environmental Factors. Cancers 2010, 2, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175 (Suppl. 2), 26–29. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Birch-Machin, M.A. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant defenses in eukaryotic cells. In Free Radicals: From Basic Science to Medicine; Poli, G., Albano, E., Dianzani, M.U., Eds.; Birkhauser Verlag: Basel, Switzerland, 1993; pp. 365–373. [Google Scholar]
- Makino, E.; Vega, V.; Kadoya, K.; Fleck, T.; Mehta, R. A novel blend of antioxidants minimizes UV-induced DNA damage markers in human skin. J. Am. Acad. Dermatol. 2016, 74 (Suppl. 1), AB11. [Google Scholar]
- Valacchi, G.; Sticozzi, C.; Chen, N.; Demaude, J.; Krol, Y.; Oresajo, C. Antioxidant mixtures protect against ozone induced damage in human reconstructed skin model. J. Am. Acad. Dermatol. 2016, 74 (Suppl. 1), AB31. [Google Scholar]
- Valacchi, G.; Sticozzi, C.; Chen, N.; Krol, Y.; Oresajo, C. Antioxidants prevent ozone-induced oxidative damage in human keratinocytes. J. Am. Acad. Dermatol. 2015, 72 (Suppl. 1), AB28. [Google Scholar]
- Rosen, G.M.; Finkelstein, E. Use of spin traps in biological systems. Adv. Free Radic. Biol. Med. 1985, 1, 345–375. [Google Scholar] [CrossRef]
- Marchand, V.; Charlier, N.; Verrax, J.; Buc-Calderon, P.; Levêque, P.; Gallez, B. Use of a cocktail of spin traps for fingerprinting large range of free radicals in biological systems. PLoS ONE 2017, 12, e0172998. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Hensley, K.; Forster, M.J.; Kelleher-Andersson, J.A.; Wood, P.L. Nitrones, their value as therapeutics and probes to understand aging. Mech. Ageing Dev. 2002, 123, 1021–1031. [Google Scholar] [CrossRef]
- Floyd, R.A.; Hensley, K.; Forster, M.J.; Kelleher-Andersson, J.A.; Wood, P.L. Nitrones as neuroprotectants and antiaging drugs. Ann. N. Y. Acad. Sci. 2002, 959, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Hensley, K. Nitrone inhibition of age-associated oxidative damage. Ann. N. Y. Acad. Sci. 2000, 899, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Konishi, T. Enhancement of hydroxyl radical generation in the Fenton reaction by alpha-hydroxy acid. Biochem. Mol. Biol. Int. 1998, 46, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Groth, N.; Herrling, T. Cutaneous tolerance to nitroxide free radicals and nitrone spin traps in the guinea pig. Toxicology 1998, 126, 33–40. [Google Scholar] [CrossRef]
- Fuchs, J.; Groth, N.; Herrling, T. Biological magnetic resonance. In In-Vivo EPR (ESR); Springer: New York, NY, USA, 2003; Volume 18, pp. 483–513. [Google Scholar]
- Haselof, R.F.; Mertsch, K.; Rohde, A.; Baeger, A.; Grigor’ev, I.A.; Blasig, I.E. Cytotoxicity of spin trapping compounds. FEBS Lett. 1997, 418, 73–75. [Google Scholar] [CrossRef]
- Janzen, E.G.; Poyer, J.L.; Schaefer, C.F.; Downs, P.E.; DuBose, C.M. Biological spin trapping. II. Toxicity of nitrone spin traps: Dose-ranging in the rat. Biochem. Biophys. Methods 1995, 30, 239–247. [Google Scholar] [CrossRef]
- Proctor, P.H. Topical Spin Trap Composition and Method. U.S. Patent 5,723,502, 3 March 1998. [Google Scholar]
- Barclay, L.R.C.; Dakin, K.A.; Khor, J.A.Y. The autoxidation of thiol aminoacids and ascorbate and their cooperative effects as antioxidants with trolox in micelles and lipid bilayers. Res. Chem. Intermed. 1995, 21, 467–488. [Google Scholar] [CrossRef]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Ferrero, L. Detection and identification of free radicals generated by UV and visible light in Ex Vivo human skin. IFSCC Mag. 2008, 11, 297–315. [Google Scholar]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Kim, Y.K.; Matsui, M.; Chung, J.H. Possible role of infrared or heat in sun-induced changes of dermis of human skin in vivo. J. Dermatol. Sci. 2012, 66, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Bonnet, M.; Marques, S.; Numa, M.; Doucet, O. Low to moderate doses of infrared A irradiation impair extracellular matrix homeostasis of the skin and contribute to skin photodamage. Skin Pharmacol. Physiol. 2015, 28, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, M.J.; Kim, M.S.; Lee, S.; Kim, Y.K.; Lee, D.H.; Lee, C.W.; Cho, K.H.; Chung, J.H. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J. Dermatol. Sci. 2008, 50, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-Violet Light Irradiation Dose Dependently Decreases Carotenoids in Human Skin, Which Indicates the Generation of Free Radicals. Oxid. Med. Cell. Longev. 2015, 2015, 579675. [Google Scholar] [CrossRef] [PubMed]
- Wheeland, R.G.; Dhawan, S. Evaluation of self-treatment of mild-to-moderate facial acne with a blue light treatment system. J. Drugs Dermatol. 2011, 10, 596–602. [Google Scholar] [PubMed]
- Weinstabl, A.; Hoff-Lesch, S.; Merk, H.F.; von Felbert, V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology 2011, 223, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.M.; Otero, M.E.; van Erp, P.E.J.; Gerritsen, M.J.P.; van de Kerkhof, P.C.M. Efficacy of blue light vs. red light in the treatment of psoriasis: A double-blind, randomized comparative study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Langer, E.; Seemann, M.; Seemann, G.; Fell, I.; Saloga, J.; Grabbe, S.; von Stebut, E. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS ONE 2011, 6, e20566. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.; van Erp, P.E.; van de Kerkhof, P.C.; Gerritsen, R.M. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ribier, A.L.; Nguyen, Q.L.; Simonnet, J.T.; Boussouira, B. Use of a Spin Trap in a Cosmetic or Dermatological Composition. U.S. Patent 5569663 A, 29 October 1996. [Google Scholar]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vávrová, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Son, S.U.; Lim, J.W.; Kang, T.; Jung, J.; Lim, E.K. Hyaluronan-Based Nanohydrogels as Effective Carriers for Transdermal Delivery of Lipophilic Agents: Towards Transdermal Drug Administration in Neurological Disorders. Nanomaterials 2017, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Forbes, B.; Martin, G.P. The use of hyaluronan in topical drug delivery. In Hyaluronan: Biomedical, Medical and Clinical Aspects; Kennedy, J., Phillips, G.O., Williams, P.A., Hascall, V., Eds.; Woodhead Publishers: Cambridge, UK, 2002; pp. 249–256. [Google Scholar]
- Brown, M.B.; Martin, G.P. Comparison of the effect of hyaluronan and other polysaccharides on drug skin partitioning. Int. J. Pharm. 2001, 225, 113–121. [Google Scholar] [CrossRef]
- Sawant, P.D.; Luu, D.; Ye, R.; Buchta, R. Drug release from hydroethanolic gels. Effect of drug’s lipophilicity (log P), polymer-drug interactions and solvent lipophilicity. Int. J. Pharm. 2010, 396, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Sawant, P.D.; Liu, X.Y.; Ho, P.C.L.; Chan, Y.W.; Chan, S.Y. SMGA Gels for the skin permeation of haloperidol. J. Control. Release 2005, 106, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Truth Aging Website. Available online: https://www.truthinaging.com/review/spin-trap-what-is-it (accessed on 15 December 2017).
- Mintel Website. Available online: http://www.mintel.com/press-centre/beauty-and-personal-care/anti-pollution-claims-on-beauty-products-in-asia-pacific-grow-by-40-in-two-years (accessed on 15 December 2017).

| Molecule | Symbol | Free Radical Structure |
|---|---|---|
| Oxygen | O2 |  |
| Hydrogen Peroxide | H2O2 |  |
| Superoxide anion | ●O−2 |  |
| Peroxide | ●O−22 |  |
| Hydroxyl Radical | ●OH |  |
| Hydroxyl ion | OH− |  |
| No. | Pollution Type | Skin Indications |
|---|---|---|
| 1 | Ultraviolet radiation | Extrinsic skin aging and skin cancers |
| 2 | Cigarette smoke | Premature aging, psoriasis, acne and skin cancers, atopic dermatitis and eczema |
| 3 | Polyaromatic hydrocarbons | Extrinsic skin aging, pigmentation, cancers and acneiform eruptions |
| 4 | Volatile organic compounds | Atopic Dermatitis |
| Spin-Trap Group | Spin-Traps | Cytotoxiciy (Inhibitory concentration at half maximum, IC50, mM) [91] | Cutaneous Irritation Potency(IP) at 100 mM [91] | Benefits |
|---|---|---|---|---|
| Nitrones | α-Phenyl-tert-butyl nitrone | 9.37 ± 0.26 (Low toxicity) | Slightly irritant (IP = 1.4) | Slightly-irritant, non-sensitising, high trans-epidermis water loss (TEWL), and low cytotoxicity |
| 5,5-dimethyl-1-pyrroline N-oxide | 138.34 ± 2.22 (Least toxic) | Non-irritant (IP = 0) | Non-irritant, non-sensitising, low TEWL and least cytotoxic | |
| Nitroxides | 2,2,5,5-tetramethyl-3-oxazolidinoxyl | NA | Slightly irritant (IP = 0.9) | Slightly-irritant, non-sensitising and high TEWL |
| 2,2,5,5-tetramethyl-1-dihydro-pyrrolinoxyl | NA | Non-irritant (IP = 0) | Non-irritant, non-sensitising, and low TEWL | |
| 2,2,3,4,5,5-hexamethyl-imidazoline-1-yloxyl | NA | Non-irritant (IP = 0) | Non-irritant, non-sensitising and low TEWL | |
| 3,3,5,5-tetramethylpyrroline-N-oxide | 0.72 ± 0.05; Highly cytotoxic | Slightly irritant (IP = 1.7) | Slightly-irritant, non-sensitising and high TEWL | |
| Hydroxylamine-TEMPO | 2,2,6,6-tetramethyl-1-hydroxypiperidine | NA | Non-irritant (IP = 0) | Non-irritant, non-sensitising, low TEWL |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, P.D. Potential Use of Spin Traps to Control ROS in Antipollution Cosmetics—A Review. Cosmetics 2018, 5, 8. https://doi.org/10.3390/cosmetics5010008
Sawant PD. Potential Use of Spin Traps to Control ROS in Antipollution Cosmetics—A Review. Cosmetics. 2018; 5(1):8. https://doi.org/10.3390/cosmetics5010008
Chicago/Turabian StyleSawant, Prashant D. 2018. "Potential Use of Spin Traps to Control ROS in Antipollution Cosmetics—A Review" Cosmetics 5, no. 1: 8. https://doi.org/10.3390/cosmetics5010008