Electrolytic Gated Organic Field-Effect Transistors for Application in Biosensors—A Review
Abstract
:1. Introduction
2. General Concepts of Transistors
3. EGOFETs
4. Semiconducting Materials
4.1. Deposition Techniques
4.1.1. Vacuum Thermal Evaporation
4.1.2. Spin-Coating
4.1.3. Inkjet Printing and Spray Deposition
4.2. Semiconducting Materials
4.2.1. Generalities
4.2.2. Poly(3-hexylthiophene) (P3HT)
4.2.3. Poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene) (pBTTT)
5. Biosensors with EGOFETs
5.1. α-Sexithiophene (α6T)-Based EGOFET Sensor for Detection of Enzyme Activity
5.2. P3HT-Based EGOFET Sensor for DNA Detection
5.3. P3HT-Based EGOFET for Protein (Streptavidin) Detection
5.4. Non-Covalent Functionalization of the Semiconductor
5.5. pBTTT-Based EGOFET for Protein (Streptavidin) Detection
5.6. Gate Functionalization
5.7. Other Type of Electrolyte
5.8. Use of Nanoparticles
6. Conclusions and Outlooks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilkinson, A.; McNaught, A.D. IUPAC Compendium of Chemical Terminology, (the “Gold Book”); Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Nic, M.; Jirat, J.; Kosata, B. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford, UK, 1997; Available online: http://goldbook.iupac.org (accessed on 23 Feburary 2016).
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.W.; James, M. Principles of Transistor Circuits; Butterworths: London, UK, 1984. [Google Scholar]
- Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sarkar, D.; Banerjee, K. Proposal for tunnel-field-effect-transistor as ultra-sensitive and label-free biosensors. Appl. Phys. Lett. 2012, 100, 143108. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Pham, M.-C. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, A.; Koezuka, H.; Ando, T. Macromolecular electronic device: Field-effect transistor with a polythiophene thin film. Appl. Phys. Lett. 1986, 49, 1210–1212. [Google Scholar] [CrossRef]
- Kergoat, L.; Herlogsson, L.; Braga, D.; Piro, B.; Pham, M.C.; Crispin, X.; Berggren, M.; Horowitz, G. A water-gate organic field-effect transistor. Adv. Mater. 2010, 22, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional electrolyte-gated organic field-effect transistors. Adv. Mater. 2012, 24, 4511–4517. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, A.S.; Yuen, J.D.; Heeney, M.; McCulloch, I.; Moses, D.; Heeger, A.J. Beyond the metal-insulator transition in polymer electrolyte gated polymer field-effect transistors. Proc. Natl. Acad. Sci. USA 2006, 103, 11834–11837. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; Mohammadi, F.M.; Coppedè, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New opportunities for organic electronics and bioelectronics: Ions in action. Chem. Sci. 2013, 4, 1395–1409. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.D.; Dhoot, A.S.; Namdas, E.B.; Coates, N.E.; Heeney, M.; McCulloch, I.; Moses, D.; Heeger, A.J. Electrochemical doping in electrolyte-gated polymer transistors. J. Am. Chem. Soc. 2007, 129, 14367–14371. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kaake, L.G.; Cho, J.H.; Zhu, X.-Y.; Lodge, T.P.; Frisbie, C.D. Ion gel-gated polymer thin-film transistors: Operating mechanism and characterization of gate dielectric capacitance, switching speed, and stability. J. Phys. Chem. C 2009, 113, 8972–8981. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with cmos-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Yan, F. Organic semiconductors in organic thin-film transistor-based chemical and biological sensors. Polym. Rev. 2013, 53, 352–406. [Google Scholar] [CrossRef]
- Said, E.; Crispin, X.; Herlogsson, L.; Elhag, S.; Robinson, N.D.; Berggren, M. Polymer field-effect transistor gated via a poly (styrenesulfonic acid) thin film. Appl. Phys. Lett. 2006, 89, 143507. [Google Scholar] [CrossRef]
- Herlogsson, L.; Crispin, X.; Robinson, N.D.; Sandberg, M.; Hagel, O.J.; Gustafsson, G.; Berggren, M. Low-voltage polymer field-effect transistors gated via a proton conductor. Adv. Mater. 2007, 19, 97–101. [Google Scholar] [CrossRef]
- Said, E.; Larsson, O.; Berggren, M.; Crispin, X. Effects of the ionic currents in electrolyte-gated organic field-effect transistors. Adv. Funct. Mater. 2008, 18, 3529–3536. [Google Scholar] [CrossRef]
- Ono, S.; Miwa, K.; Seki, S.; Takeya, J. A comparative study of organic single-crystal transistors gated with various ionic-liquid electrolytes. Appl. Phys. Lett. 2009, 94, 063301. [Google Scholar] [CrossRef]
- Xia, Y.; Cho, J.H.; Lee, J.; Ruden, P.P.; Frisbie, C.D. Comparison of the mobility-carrier density relation in polymer and single-crystal organic transistors employing vacuum and liquid gate dielectrics. Adv. Mater. 2009, 21, 2174–2179. [Google Scholar] [CrossRef]
- Hamedi, M.; Herlogsson, L.; Crispin, X.; Marcilla, R.; Berggren, M.; Inganäs, O. Fiber-embedded electrolyte-gated field-effect transistors for e-textiles. Adv. Mater. 2009, 21, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Panzer, M.J.; He, Y.; Lodge, T.P.; Frisbie, C.D. Ion gel gated polymer thin-film transistors. J. Am. Chem. Soc. 2007, 129, 4532–4533. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, J.; Xia, Y.; Kim, B.; He, Y.; Renn, M.J.; Lodge, T.P.; Frisbie, C.D. Printable ion-gel gate dielectrics for low-voltage polymer thin-film transistors on plastic. Nat. Mater. 2008, 7, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, J.; He, Y.; Kim, B.; Lodge, T.P.; Frisbie, C.D. High-capacitance ion gel gate dielectrics with faster polarization response times for organic thin film transistors. Adv. Mater. 2008, 20, 686–690. [Google Scholar] [CrossRef]
- Facchetti, A. Dielectric materials: Gels excel. Nat. Mater. 2008, 7, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, G.; De Tullio, D.; Magliulo, M.; Mallardi, A.; Intranuovo, F.; Mulla, M.Y.; Favia, P.; Vikholm-Lundin, I.; Torsi, L. Detection beyond debye’s length with an electrolyte-gated organic field-effect transistor. Adv. Mater. 2014, 27, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, A. Contribution à la Modélisation des Transistors Organiques. Ph.D. Thesis, The University of Reims Champagne-Ardenne, Reims, France, 2009. [Google Scholar]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156–163. [Google Scholar] [CrossRef]
- Cai, B.; Wang, S.; Huang, L.; Ning, Y.; Zhang, Z.; Zhang, G.-J. Ultrasensitive label-free detection of PNA-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 2014, 8, 2632–2638. [Google Scholar] [CrossRef] [PubMed]
- Vasu, K.; Chakraborty, B.; Sampath, S.; Sood, A. Probing top-gated field effect transistor of reduced graphene oxide monolayer made by dielectrophoresis. Solid State Commun. 2010, 150, 1295–1298. [Google Scholar] [CrossRef]
- Liao, R.; Tang, Z.; Lei, Y.; Guo, B. Polyphenol-reduced graphene oxide: Mechanism and derivatization. J. Phys. Chem. C 2011, 115, 20740–20746. [Google Scholar] [CrossRef]
- Liu, F.; Kim, Y.H.; Cheon, D.S.; Seo, T.S. Micropatterned reduced graphene oxide based field-effect transistor for real-time virus detection. Sens. Actuators B Chem. 2013, 186, 252–257. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Q.; Xu, C.; Li, H.; Wu, D.; Cai, Y.; Mao, K.; Cui, Z.; Du, B. Label-free electrochemical immunosensor for sensitive detection of kanamycin. Sens. Actuators B Chem. 2011, 155, 618–625. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Loan, P.T.K.; Hsu, C.-L.; Lee, Y.-H.; Wang, J.T.-W.; Wei, K.-H.; Lin, C.-T.; Li, L.-J. Label-free detection of DNA hybridization using transistors based on cvd grown graphene. Biosens. Bioelectron. 2013, 41, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, C.; Jang, J. High-performance field-effect transistor-type glucose biosensor based on nanohybrids of carboxylated polypyrrole nanotube wrapped graphene sheet transducer. Sens. Actuators B Chem. 2015, 208, 532–537. [Google Scholar] [CrossRef]
- Williams, G.; Kamat, P.V. Graphene-semiconductor nanocomposites: Excited-state interactions between zno nanoparticles and graphene oxide†. Langmuir 2009, 25, 13869–13873. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte-gated graphene field-effect transistors for detecting ph and protein adsorption. Nano Lett. 2009, 9, 3318–3322. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Piro, B.; Reisberg, S.; Nguyen, L.H.; Nguyen, T.D.; Duc, H.; Pham, M. An electrochemical elisa-like immunosensor for mirnas detection based on screen-printed gold electrodes modified with reduced graphene oxide and carbon nanotubes. Biosens. Bioelectron. 2014, 62, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.J. Electrical detection of DNA hybridization with single-base specificity using transistors based on cvd-grown graphene sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lan, F.; Yu, F.; Yu, J. Applications of graphene and related nanomaterials in analytical chemistry. New J. Chem. 2015, 39, 2380–2395. [Google Scholar] [CrossRef]
- Tran, H.; Piro, B.; Reisberg, S.; Duc, H.; Pham, M. Antibodies directed to rna/DNA hybrids: An electrochemical immunosensor for micrornas detection using graphene-composite electrodes. Anal. Chem. 2013, 85, 8469–8474. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, M.; Li, J. Solution-gated graphene transistors for chemical and biological sensors. Adv. Healthc. Mater. 2014, 3, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Herlogsson, L.; Piro, B.; Pham, M.C.; Horowitz, G.; Crispin, X.; Berggren, M. Tuning the threshold voltage in electrolyte-gated organic field-effect transistors. Proc. Natl. Acad. Sci. USA 2012, 109, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, S.; Braun, S.; Fahlman, M.; Crispin, X.; Berggren, M. Effect of gate electrode work-function on source charge injection in electrolyte-gated organic field-effect transistors. Adv. Funct. Mater. 2014, 24, 695–700. [Google Scholar] [CrossRef]
- Demelas, M.; Lai, S.; Spanu, A.; Martinoia, S.; Cosseddu, P.; Barbaro, M.; Bonfiglio, A. Charge sensing by organic charge-modulated field effect transistors: Application to the detection of bio-related effects. J. Mater. Chem. B 2013, 1, 3811–3819. [Google Scholar] [CrossRef]
- Myers, J.D.; Xue, J. Organic semiconductors and their applications in photovoltaic devices. Polym. Rev. 2012, 52, 1–37. [Google Scholar] [CrossRef]
- Alberga, D.; Mangiatordi, G.F.; Torsi, L.; Lattanzi, G. Effects of annealing and residual solvents on amorphous p3ht and pbttt films. J. Phys. Chem. C 2014, 118, 8641–8655. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.; Friend, R.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.; Spiering, A.; Janssen, R.A.; Meijer, E.; Herwig, P. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Liu, Z.; Tsao, H.N.; Müllen, K.; Minari, T.; Noh, Y.-Y.; Sirringhaus, H. Improving solution-processed n-type organic field-effect transistors by transfer-printed metal/semiconductor and semiconductor/semiconductor heterojunctions. Org. Electron. 2014, 15, 1884–1889. [Google Scholar] [CrossRef]
- Noh, Y.-Y.; Zhao, N.; Caironi, M.; Sirringhaus, H. Downscaling of self-aligned, all-printed polymer thin-film transistors. Nat. Nanotechnol. 2007, 2, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Sinno, H.; Nguyen, H.T.; Hägerström, A.; Fahlman, M.; Lindell, L.; Coulembier, O.; Dubois, P.; Crispin, X.; Engquist, I.; Berggren, M. Amphiphilic semiconducting copolymer as compatibility layer for printing polyelectrolyte-gated ofets. Org. Electron. 2013, 14, 790–796. [Google Scholar] [CrossRef]
- Wang, X.; Nilsson, D.; Norberg, P. Printable microfluidic systems using pressure sensitive adhesive material for biosensing devices. Biochim. Biophys. Acta 2013, 1830, 4398–4401. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Sachnov, S.; Gruber, M.; Gannott, F.; Spallek, S.; Schweiger, M.; Krückel, J.; Kaschta, J.; Spiecker, E.; Wasserscheid, P. Spray-coatable ionogels based on silane-ionic liquids for low voltage, flexible, electrolyte-gated organic transistors. J. Mater. Chem. C 2014, 2, 2423–2430. [Google Scholar] [CrossRef]
- Buth, F.; Kumar, D.; Stutzmann, M.; Garrido, J. Electrolyte-gated organic field-effect transistors for sensing applications. Appl. Phys. Lett. 2011, 98, 153302. [Google Scholar] [CrossRef]
- Scarpa, G.; Idzko, A.; Götz, S.; Neumaier, T.; Thalhammer, S. Biocompatibility studies of solution-processable organic thin-film transistors for sensing applications. In Proceedings of the IEEE International Conference on Nano/Molecular Medicine and Engineering (NANOMED), Tainan, Taiwan, 18–21 October 2009; pp. 265–268.
- Magliulo, M.; Mallardi, A.; Mulla, M.Y.; Cotrone, S.; Pistillo, B.R.; Favia, P.; Vikholm-Lundin, I.; Palazzo, G.; Torsi, L. Electrolyte-gated organic field-effect transistor sensors based on supported biotinylated phospholipid bilayer. Adv. Mater. 2013, 25, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12, 12–20. [Google Scholar] [CrossRef]
- Angione, M.D.; Cotrone, S.; Magliulo, M.; Mallardi, A.; Altamura, D.; Giannini, C.; Cioffi, N.; Sabbatini, L.; Fratini, E.; Baglioni, P. Interfacial electronic effects in functional biolayers integrated into organic field-effect transistors. Proc. Natl. Acad. Sci. USA 2012, 109, 6429–6434. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Mok, S.M.; Yu, J.; Chan, H.L.; Yang, M. Label-free DNA sensor based on organic thin film transistors. Biosens. Bioelectron. 2009, 24, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, F.; Kuiper, M.J.; Poolman, B.; Brouwer, F.; Hummelen, J.C.; de Leeuw, D.M.; De Boer, B.; Blom, P.W. Organic field-effect transistor-based biosensors functionalized with protein receptors. J. Appl. Phys. 2010, 108, 124501. [Google Scholar] [CrossRef]
- Roberts, M.E.; Mannsfeld, S.C.; Queraltó, N.; Reese, C.; Locklin, J.; Knoll, W.; Bao, Z. Water-stable organic transistors and their application in chemical and biological sensors. Proc. Natl. Acad. Sci. USA 2008, 105, 12134–12139. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Piro, B.; Berggren, M.; Pham, M.-C.; Yassar, A.; Horowitz, G. DNA detection with a water-gated organic field-effect transistor. Org. Electron. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Magliulo, M.; Pistillo, B.R.; Mulla, M.Y.; Cotrone, S.; Ditaranto, N.; Cioffi, N.; Favia, P.; Torsi, L. PE-CVD of hydrophilic-COOH functionalized coatings on electrolyte gated field-effect transistor electronic layers. Plasma Process. Polym. 2013, 10, 102–109. [Google Scholar] [CrossRef]
- Cotrone, S.; Ambrico, M.; Toss, H.; Angione, M.D.; Magliulo, M.; Mallardi, A.; Berggren, M.; Palazzo, G.; Horowitz, G.; Ligonzo, T. Phospholipid film in electrolyte-gated organic field-effect transistors. Org. Electron. 2012, 13, 638–644. [Google Scholar] [CrossRef]
- McCulloch, I.; Heeney, M.; Chabinyc, M.L.; DeLongchamp, D.; Kline, R.J.; Colle, M.; Duffy, W.; Fischer, D.; Gundlach, D.; Hamadani, B.; et al. Semiconducting Thienothiophene Copolymers: Design, Synthesis, Morphology, and Performance in Thin-Film Organic Transistors. Adv. Mater. 2009, 21, 1091–1109. [Google Scholar] [CrossRef]
- Minamiki, T.; Minami, T.; Kurita, R.; Niwa, O.; Wakida, S.-I.; Fukuda, K.; Kumaki, D.; Tokito, S. Accurate and reproducible detection of proteins in water using an extended-gate type organic transistor biosensor. Appl. Phys. Lett. 2014, 104, 243703. [Google Scholar] [CrossRef]
- Dumitru, L.M.; Manoli, K.; Magliulo, M.; Palazzo, G.; Torsi, L. Low-voltage solid electrolyte-gated ofets for gas sensing applications. Microelectron. J. 2014, 45, 1679–1683. [Google Scholar] [CrossRef]
- Mulla, M.; Seshadri, P.; Torsi, L.; Manoli, K.; Mallardi, A.; Ditaranto, N.; Santacroce, M.; Di Franco, C.; Scamarcio, G.; Magliulo, M. Uv crosslinked poly (acrylic acid): A simple method to bio-functionalize electrolyte-gated ofet biosensors. J. Mater. Chem. B 2015, 3, 5049–5057. [Google Scholar] [CrossRef]
- Mulla, M.Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-modulated transistor detects odorant binding protein chiral interactions. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Frechet, J.M. Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight. Adv. Mater. 2003, 15, 1519–1522. [Google Scholar] [CrossRef]
- Chang, J.-F.; Sun, B.; Breiby, D.W.; Nielsen, M.M.; Sölling, T.I.; Giles, M.; McCulloch, I.; Sirringhaus, H. Enhanced mobility of poly (3-hexylthiophene) transistors by spin-coating from high-boiling-point solvents. Chem. Mater. 2004, 16, 4772–4776. [Google Scholar] [CrossRef] [Green Version]
- Heffner, G.W.; Pearson, D.S. Molecular characterization of poly (3-hexylthiophene). Macromolecules 1991, 24, 6295–6299. [Google Scholar] [CrossRef]
- Bao, Z.; Dodabalapur, A.; Lovinger, A.J. Soluble and processable regioregular poly (3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 1996, 69, 4108–4110. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Tessler, N.; Friend, R.H. Integrated optoelectronic devices based on conjugated polymers. Science 1998, 280, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Hugger, S.; Thomann, R.; Heinzel, T.; Thurn-Albrecht, T. Semicrystalline morphology in thin films of poly (3-hexylthiophene). Colloid Polym. Sci. 2004, 282, 932–938. [Google Scholar]
- Cho, S.; Lee, K.; Yuen, J.; Wang, G.; Moses, D.; Heeger, A.J.; Surin, M.; Lazzaroni, R. Thermal annealing-induced enhancement of the field-effect mobility of regioregular poly (3-hexylthiophene) films. J. Appl. Phys. 2006, 100, 114503. [Google Scholar] [CrossRef]
- Gurau, M.C.; Delongchamp, D.M.; Vogel, B.M.; Lin, E.K.; Fischer, D.A.; Sambasivan, S.; Richter, L.J. Measuring molecular order in poly (3-alkylthiophene) thin films with polarizing spectroscopies. Langmuir 2007, 23, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Alexiadis, O.; Mavrantzas, V.G. All-atom molecular dynamics simulation of temperature effects on the structural, thermodynamic, and packing properties of the pure amorphous and pure crystalline phases of regioregular p3ht. Macromolecules 2013, 46, 2450–2467. [Google Scholar] [CrossRef]
- Mårdalen, J.; Samuelsen, E.J.; Gautun, O.R.; Carlsen, P.H. Chain configuration of poly (3-hexylthiophene) as revealed by detailed x-ray diffraction studies. Solid State Commun. 1991, 77, 337–339. [Google Scholar] [CrossRef]
- Prosa, T.; Winokur, M.; Moulton, J.; Smith, P.; Heeger, A. X-ray structural studies of poly (3-alkylthiophenes): An example of an inverse comb. Macromolecules 1992, 25, 4364–4372. [Google Scholar] [CrossRef]
- Marchant, S.; Foot, P. Annealing behaviour of conductive poly (3-hexylthiophene) films. Polymer 1997, 38, 1749–1751. [Google Scholar] [CrossRef]
- Favia, P.; Sardella, E.; Gristina, R.; d’Agostino, R. Novel plasma processes for biomaterials: Micro-scale patterning of biomedical polymers. Surf. Coat. Technol. 2003, 169, 707–711. [Google Scholar] [CrossRef]
- Kergoat, L.; Battaglini, N.; Miozzo, L.; Piro, B.; Pham, M.-C.; Yassar, A.; Horowitz, G. Use of poly (3-hexylthiophene)/poly (methyl methacrylate)(p3ht/pmma) blends to improve the performance of water-gated organic field-effect transistors. Org. Electron. 2011, 12, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Toss, H.; Suspene, C.; Piro, B.; Yassar, A.; Crispin, X.; Kergoat, L.; Pham, M.-C.; Berggren, M. On the mode of operation in electrolyte-gated thin film transistors based on different substituted polythiophenes. Org. Electron. 2014, 15, 2420–2427. [Google Scholar] [CrossRef]
- Suspène, C.; Piro, B.; Reisberg, S.; Pham, M.-C.; Toss, H.; Berggren, M.; Yassar, A.; Horowitz, G. Copolythiophene-based water-gated organic field-effect transistors for biosensing. J. Mater. Chem. B 2013, 1, 2090–2097. [Google Scholar] [CrossRef]
- Chabinyc, M.L.; Endicott, F.; Vogt, B.D.; DeLongchamp, D.M.; Lin, E.K.; Wu, Y.; Liu, P.; Ong, B.S. Effects of humidity on unencapsulated poly (thiophene) thin-film transistors. Appl. Phys. Lett. 2006, 88, 113514. [Google Scholar] [CrossRef]
- Hoshino, S.; Yoshida, M.; Uemura, S.; Kodzasa, T.; Takada, N.; Kamata, T.; Yase, K. Influence of moisture on device characteristics of polythiophene-based field-effect transistors. J. Appl. Phys. 2004, 95, 5088–5093. [Google Scholar] [CrossRef]
- Caronna, T.; Forte, M.; Catellani, M.; Meille, S.V. Photodegradation and photostabilization studies of poly (3-butylthiophene) in the solid state. Chem. Mater. 1997, 9, 991–995. [Google Scholar] [CrossRef]
- Manoli, K.; Dumitru, L.M.; Mulla, M.Y.; Magliulo, M.; Franco, C.D.; Santacroce, M.V.; Scamarcio, G.; Torsi, L. A comparative study of the gas sensing behavior in p3ht-and pbttt-based otfts: The influence of film morphology and contact electrode position. Sensors 2014, 14, 16869–16880. [Google Scholar] [CrossRef] [PubMed]
- Do, K.; Huang, D.M.; Faller, R.; Moulé, A.J. A comparative md study of the local structure of polymer semiconductors p3ht and pbttt. Phys. Chem. Chem. Phys. 2010, 12, 14735–14739. [Google Scholar] [CrossRef] [PubMed]
- Porrazzo, R.; Bellani, S.; Luzio, A.; Lanzarini, E.; Caironi, M.; Antognazza, M.R. Improving mobility and electrochemical stability of a water-gated polymer field-effect transistor. Org. Electron. 2014, 15, 2126–2134. [Google Scholar] [CrossRef]
- Al Naim, A.F.; Grell, M. Organic solvents as gate media for thin-film transistors. J. Appl. Phys. 2012, 112, 114502. [Google Scholar] [CrossRef]
- Cho, E.; Risko, C.; Kim, D.; Gysel, R.; Cates Miller, N.; Breiby, D.W.; McGehee, M.D.; Toney, M.F.; Kline, R.J.; Bredas, J.-L. Three-dimensional packing structure and electronic properties of biaxially oriented poly (2, 5-bis (3-alkylthiophene-2-yl) thieno [3, 2-b] thiophene) films. J. Am. Chem. Soc. 2012, 134, 6177–6190. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; MacDonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Wagner, R.; Zhang, W. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 2006, 5, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.N.; Müllen, K. Improving polymer transistor performance via morphology control. Chem. Soc. Rev. 2010, 39, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Schmoltner, K.; Kofler, J.; Klug, A.; List-Kratochvil, E. Electrolyte-gated organic field-effect transistors for sensing in aqueous media. SPIE Proc. 2013. [Google Scholar] [CrossRef]
- Casalini, S.; Dumitru, A.C.; Leonardi, F.; Bortolotti, C.A.; Herruzo, E.T.; Campana, A.; de Oliveira, R.F.; Cramer, T.; Garcia, R.; Biscarini, F. Multiscale sensing of antibody-antigen interactions by organic transistors and single-molecule force spectroscopy. ACS Nano 2015, 9, 5051–5062. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Hwang, J.-C.; Chueh, Y.-L.; Chang, T.-H.; Cheng, Y.-Y.; Lyu, P.-C. Hydrated bovine serum albumin as the gate dielectric material for organic field-effect transistors. Org. Electron. 2013, 14, 2645–2651. [Google Scholar] [CrossRef]
- Dumitru, L.; Manoli, K.; Magliulo, M.; Ligonzo, T.; Palazzo, G.; Torsi, L. A hydrogel capsule as gate dielectric in flexible organic field-effect transistors. APL Mater. 2015, 3, 014904. [Google Scholar] [CrossRef]
- Poghossian, A.; Bäcker, M.; Mayer, D.; Schöning, M.J. Gating capacitive field-effect sensors by the charge of nanoparticle/molecule hybrids. Nanoscale 2015, 7, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Hammock, M.L.; Knopfmacher, O.; Naab, B.D.; Tok, J.B.H.; Bao, Z. Investigation of Protein Detection Parameters Using Nanofunctionalized Organic Field-Effect Transistors. ACS Nano 2013, 7, 3970–3980. [Google Scholar] [CrossRef] [PubMed]








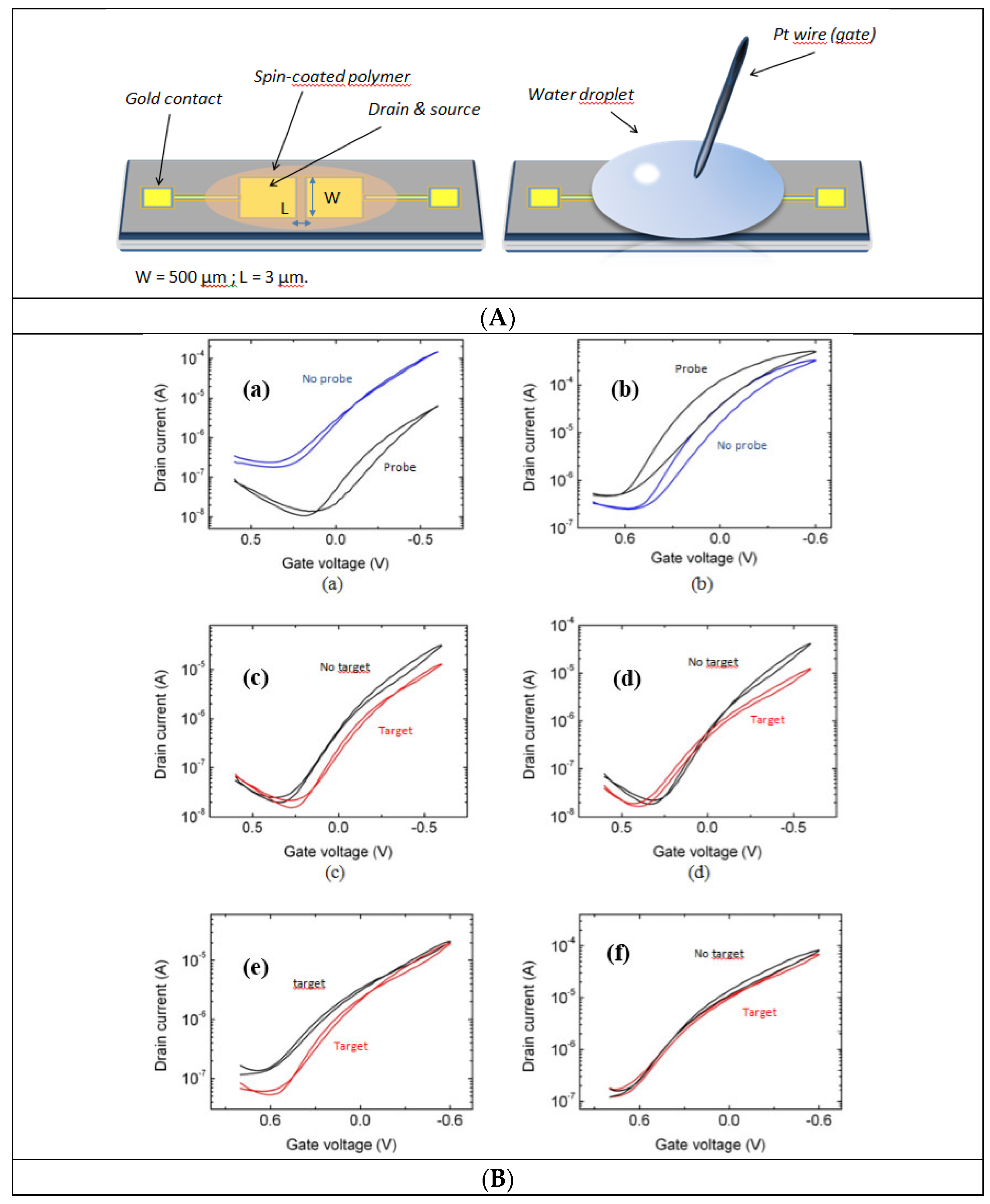












| ODN grafting (Figure 8a,b) | ||
| ODN probe | ∆VG min / V | Ioff (bare film) / Ioff (probe-modified film) |
| Yes | −0.31 ± 0.05 | 11.1 ± 3.7 |
| No | +0.16 ± 0.05 | 0.5 ± 0.12 |
| DNA hybrid. in PBS (Figure 8c,d) | ||
| ODN target | ∆VG min / V | Ioff (probe-modified film) / Ioff (hybridization) |
| HIV | −0.06 ± 0.02 | 1.7 ± 0.45 |
| RAND | −0.03 ± 0.03 | 1.3 ± 0.28 |
| DNA hybrid. in H2O (Figure 8e,f) | ||
| ODN probe | ∆VGmin / V | Ioff (probe-modified film) / Ioff (hybridization) |
| HIV | −0.03 ± 0.02 | 3.4 ± 1.5 |
| RAND | −0.04 ± 0.07 | 1.04 ± 0.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Noël, V.; Piro, B. Electrolytic Gated Organic Field-Effect Transistors for Application in Biosensors—A Review. Electronics 2016, 5, 9. https://doi.org/10.3390/electronics5010009
Wang D, Noël V, Piro B. Electrolytic Gated Organic Field-Effect Transistors for Application in Biosensors—A Review. Electronics. 2016; 5(1):9. https://doi.org/10.3390/electronics5010009
Chicago/Turabian StyleWang, Denjung, Vincent Noël, and Benoît Piro. 2016. "Electrolytic Gated Organic Field-Effect Transistors for Application in Biosensors—A Review" Electronics 5, no. 1: 9. https://doi.org/10.3390/electronics5010009





