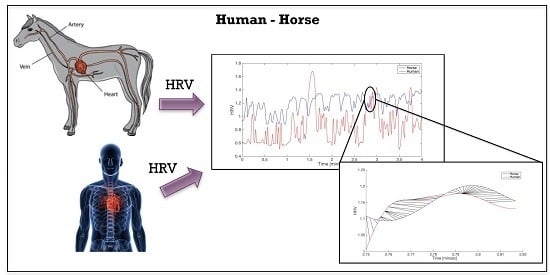
A Wearable System for the Evaluation of the Human-Horse Interaction: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
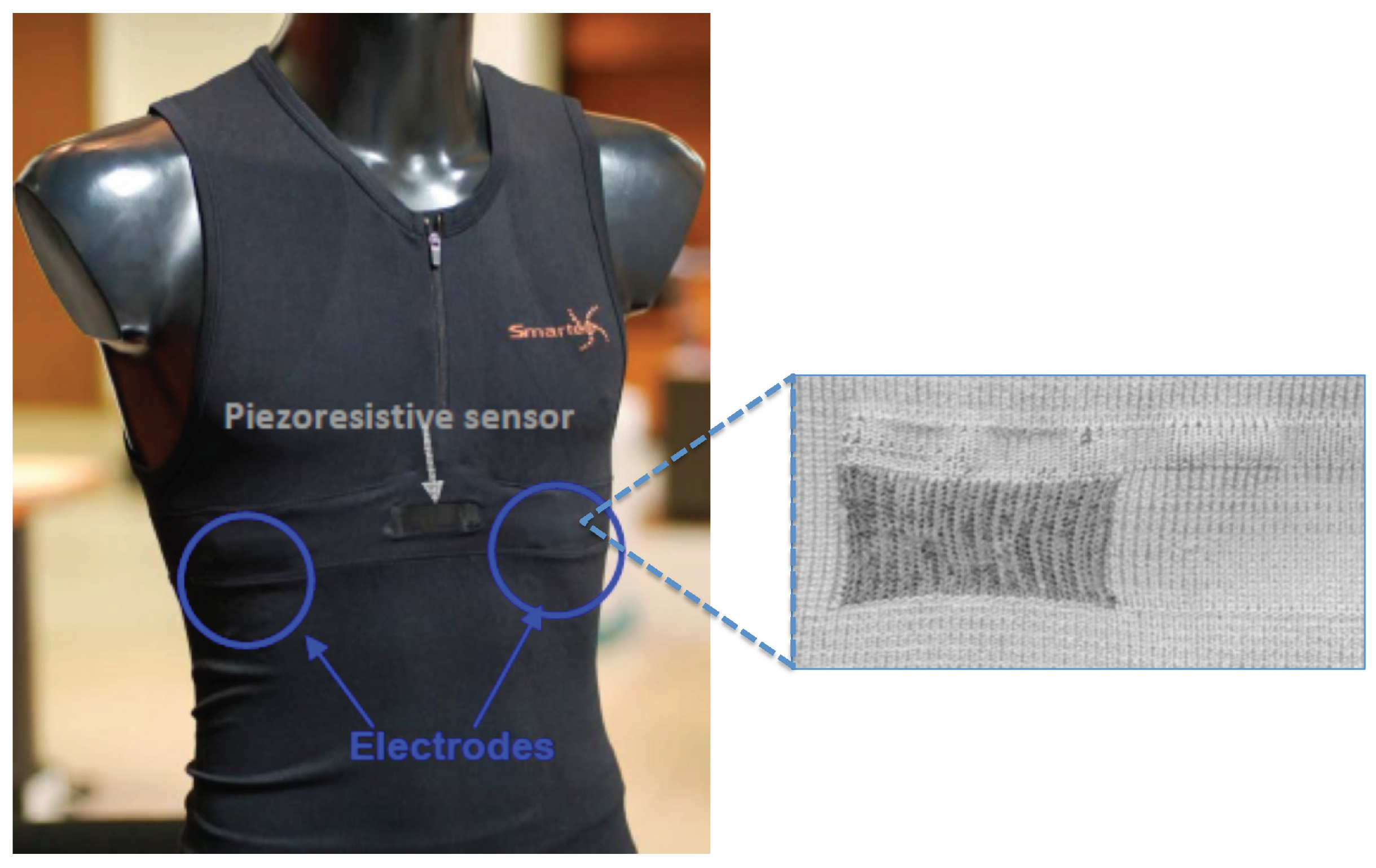
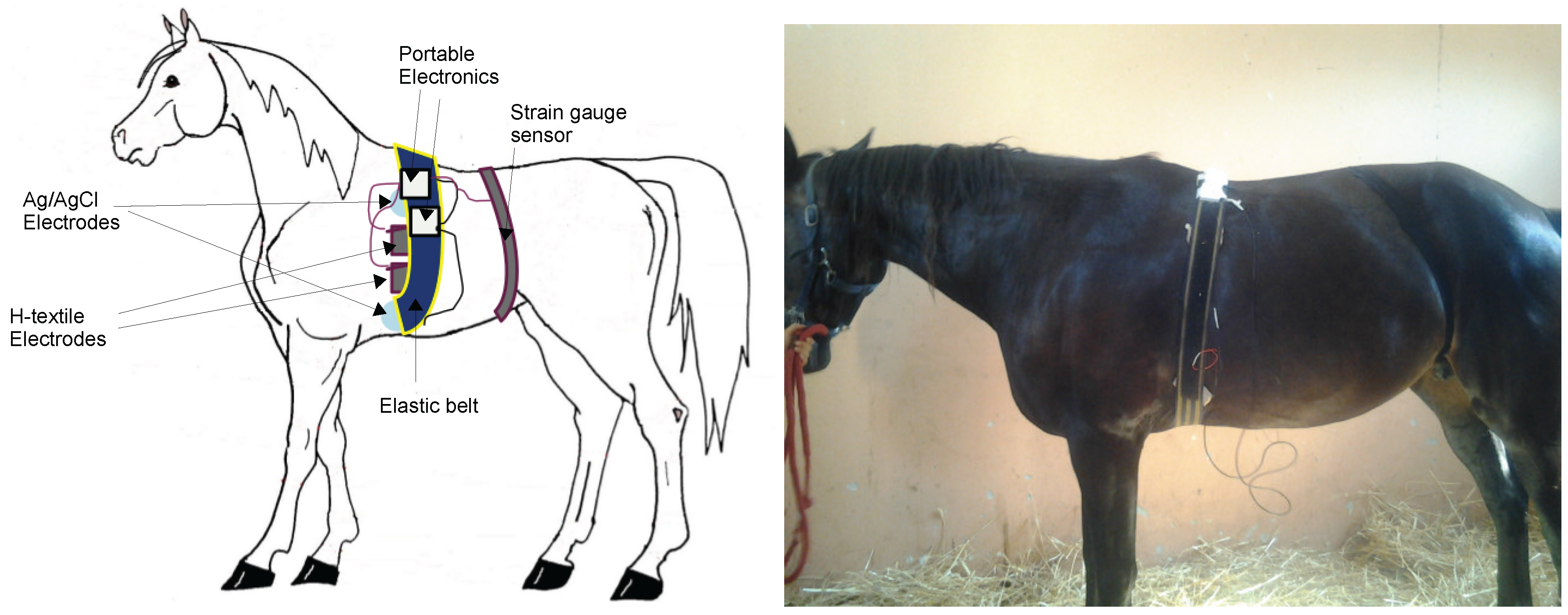
2.1. Equine Textile-Based ECG: Test and Validation
2.2. Protocol of Interaction
2.3. Signal Processing Chain for Human-Horse Interaction
2.3.1. Heart Rate Variability Estimation
2.3.2. Feature Estimation
2.3.3. Statistical Analysis and Pattern Recognition
3. Results
3.1. Results of the E-Textiles’ Validation and Testing
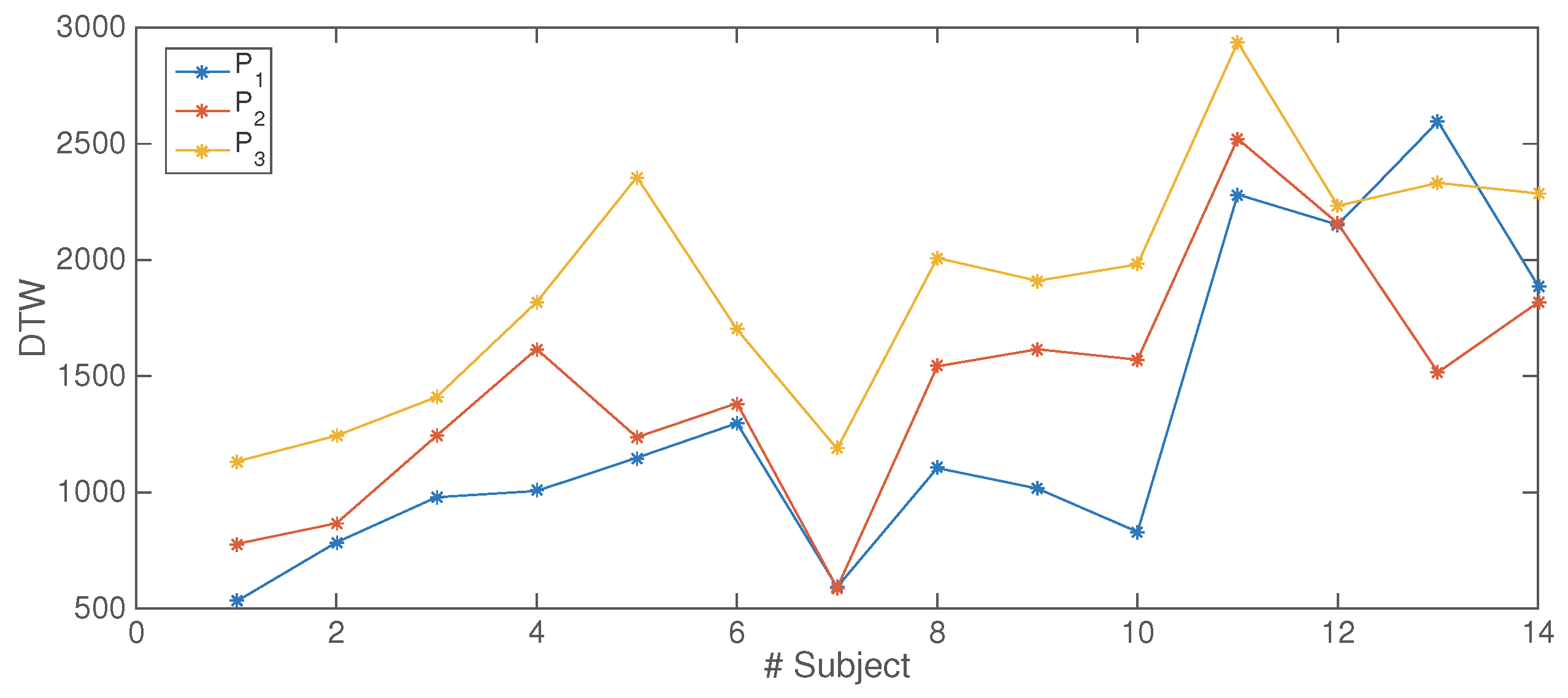
3.2. Results of the Human-Horse Interaction
4. Discussion and Conclusions
5. Ethical Statement
Author Contributions
Conflicts of Interest
References
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral assessment of horses in therapeutic riding programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]
- Schultz, P.N.; Remick-Barlow, G.; Robbins, L. Equine-assisted psychotherapy: A mental health promotion/intervention modality for children who have experienced intra-family violence. Health Soc. Care Community 2007, 15, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Roche, H.; Henry, S.; Visser, E.K. A review of the human–horse relationship. Appl. Anim. Behav. Sci. 2008, 109, 1–24. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human–animal relationship in farmed species: A critical review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How horses (Equus caballus) see the world: Humans as significant objects? Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Chamove, A.S.; Crawley-Hartrick, O.J.; Stafford, K.J. Horse reactions to human attitudes and behavior. Anthrozoös 2002, 15, 323–331. [Google Scholar] [CrossRef]
- Lesimple, C.; Fureix, C.; Menguy, H.; Hausberger, M. Human direct actions may alter animal welfare, a study on horses (Equus caballus). PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Hama, H.; Yogo, M.; Matsuyama, Y. Effects of stroking horses on both humans’ and horses’ heart rate responses1. Jpn. Psychol. Res. 1996, 38, 66–73. [Google Scholar] [CrossRef]
- Keeling, L.J.; Jonare, L.; Lanneborn, L. Investigating horse–human interactions: The effect of a nervous human. Vet. J. 2009, 181, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Koolagudi, S.G.; Rao, K.S. Emotion recognition from speech: A review. Int. J. Speech Technol. 2012, 15, 99–117. [Google Scholar] [CrossRef]
- Guidi, A.; Vanello, N.; Bertschy, G.; Gentili, C.; Landini, L.; Scilingo, E.P. Automatic analysis of speech F0 contour for the characterization of mood changes in bipolar patients. Biomed. Signal Process. Control 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Guidi, A.; Scilingo, E.; Gentili, C.; Bertschy, G.; Landini, L.; Vanello, N. Analysis of running speech for the characterization of mood state in bipolar patients. In Proceedings of the 2015 AEIT International Annual Conference (AEIT), Naples, Italy, 14–16 October 2015.
- Bianchi-Berthouze, N.; Cairns, P.; Cox, A.; Jennett, C.; Kim, W.W. On posture as a modality for expressing and recognizing emotions. In Proceedings of the Emotion and HCI Workshop at BCS HCI London, London, UK, 11–15 September 2006.
- Baragli, P.; Gazzano, A.; Martelli, F.; Sighieri, C. How Do Horses Appraise Humans’ Actions? A Brief Note over a Practical Way to Assess Stimulus Perception. J. Equine Vet. Sci. 2009, 29, 739–742. [Google Scholar] [CrossRef]
- Ekman, P. Facial expression and emotion. Am. Psychol. 1993, 48, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Keltner, D.; Ekman, P.; Gonzaga, G.C.; Beer, J. Facial expression of emotion. Am. Psychol. 1993, 48, 173–183. [Google Scholar]
- Ekman, P.; Levenson, R.W.; Friesen, W.V. Autonomic nervous system activity distinguishes among emotions. Science 1983, 221, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Pluta, M.; Osiński, Z. Variability of heart rate in primitive horses and their relatives as an indicator of stress level, behavioral conduct towards humans and adaptation to living in wild. Bull. Vet. Inst. Pulawy 2014, 58, 495–501. [Google Scholar] [CrossRef]
- Nardelli, M.; Valenza, G.; Greco, A.; Lanata, A.; Scilingo, E.P. Recognizing emotions induced by affective sounds through heart rate variability. IEEE Trans. Affect. Comput. 2015, 6, 385–394. [Google Scholar] [CrossRef]
- von Lewinski, M.; Biau, S.; Erber, R.; Ille, N.; Aurich, J.; Faure, J.M.; Möstl, E.; Aurich, C. Cortisol release, heart rate and heart rate variability in the horse and its rider: Different responses to training and performance. Vet. J. 2013, 197, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Strzelec, K.; Kędzierski, W.; Bereznowski, A.; Janczarek, I.; Bocian, K.; Radosz, M. Salivary cortisol Levels in horses and their riders during three-day-events. Bull. Vet. Inst. Pulawy 2013, 57, 237–241. [Google Scholar]
- Kang, O.D.; Lee, W.S. Changes in Salivary Cortisol Concentration in Horses during Different Types of Exercise. Asian-Australas. J. Anim. Sci. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Medica, P.; Aveni, F.; Ferlazzo, A. The potential role of training sessions on the temporal and spatial physiological patterns in young Friesian horses. J. Equine Vet. Sci. 2016, 47, 84–91. [Google Scholar] [CrossRef]
- Van Dyke Parunak, H.; Bisson, R.; Brueckner, S.; Matthews, R.; Sauter, J. A model of emotions for situated agents. In Proceedings of the Fifth International Joint Conference on Autonomous Agents and Multiagent Systems, Hakodate, Japan, 8–12 May 2006; ACM: New York, NY, USA, 2006; pp. 993–995. [Google Scholar]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; McComb, K. Functionally relevant responses to human facial expressions of emotion in the domestic horse (Equus caballus). Biol. Lett. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K. A language of their own: An interactionist approach to human-horse communication. Soc. Anim. 2004, 12, 299–316. [Google Scholar] [CrossRef]
- Rochais, C.; Henry, S.; Sankey, C.; Nassur, F.; Gorecka-Bruzda, A.; Hausberger, M. Visual attention, an indicator of human-animal relationships? A study of domestic horses (Equus caballus). Front. Psychol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, E.K.; Baldwin, A.; Schiltz, P.M. Heart rate variability in horses engaged in equine-assisted activities. J. Equine Vet. Sci. 2011, 31, 78–84. [Google Scholar] [CrossRef]
- Visser, E.; Van Reenen, C.; Van der Werf, J.; Schilder, M.; Knaap, J.; Barneveld, A.; Blokhuis, H. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol. Behav. 2002, 76, 289–296. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A. The relationship between behavioral and other welfare indicators of working horses. J. Equine Vet. Sci. 2013, 33, 1–12. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Birke, L.; Brandt, K. Mutual corporeality: Gender and human/horse relationships. In Women’s Studies International Forum; Elsevier: Amsterdam, The Netherlands, 2009; Volume 32, pp. 189–197. [Google Scholar]
- Proops, L.; McComb, K.; Reby, D. Cross-modal individual recognition in domestic horses (Equus caballus). Proc. Natil. Acad. Sci. USA 2009, 106, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Proops, L.; McComb, K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 3131–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLean, B. Equine-assisted therapy. J. Rehabil. Res. Dev. 2011, 48, ix–xii. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.E. All creatures great and small utilizing equine-assisted therapy to treat eating disorders. J. Psychol. Christianity 2005, 24, 65–67. [Google Scholar]
- Piette, D.; Norton, T.; Exadaktylos, V.; Berckmans, D. Real-time monitoring of the horse-rider dyad using body sensor network technology. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; IEEE: New York, NY, USA, 2016; pp. 287–291. [Google Scholar]
- Jansen, F.; Van der Krogt, J.; Van Loon, K.; Avezzu, V.; Guarino, M.; Quanten, S.; Berckmans, D. Online detection of an emotional response of a horse during physical activity. Vet. J. 2009, 181, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Crews, D. The Bond Between a Horse and a Human. Arizona State University: Phoenix, Arizona, July 2009. [Google Scholar]
- Sankey, C.; Henry, S.; André, N.; Richard-Yris, M.A.; Hausberger, M. Do horses have a concept of person? PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Sankey, C.; Richard-Yris, M.A.; Leroy, H.; Henry, S.; Hausberger, M. Positive interactions lead to lasting positive memories in horses, Equus caballus. Anim. Behav. 2010, 79, 869–875. [Google Scholar] [CrossRef]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Narayanan, K.; Govindan, R.; Gopinathan, M. Unstable periodic orbits in human cardiac rhythms. Phys. Rev. E 1998, 57, 4594–4602. [Google Scholar] [CrossRef]
- Poon, C.S.; Merrill, C.K. Decrease of cardiac chaos in congestive heart failure. Nature 1997, 389, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Keogh, E.J.; Pazzani, M.J. Scaling up dynamic time warping for datamining applications. In Proceedings of the Sixth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Boston, MA, USA, 20–23 August 2000; ACM: New York, NY, USA, 2000; pp. 285–289. [Google Scholar]
- Rabiner, L.; Juang, B.H. Fundamentals of Speech Recognition; Signal Processing Series; Prentice Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Caiani, E.; Porta, A.; Baselli, G.; Turie, M.; Muzzupappa, S.; Pieruzzi, F.; Crema, C.; Malliani, A.; Cerutti, S. Warped-average template technique to track on a cycle-by-cycle basis the cardiac filling phases on left ventricular volume. In Proceedings of the 1998 Computers in Cardiology, Cleveland, OH, USA, 13–16 September 1998; IEEE: Cleveland, OH, USA, 1998; pp. 73–76. [Google Scholar]
- Valenza, G.; Nardelli, M.; Lanata, A.; Gentili, C.; Bertschy, G.; Kosel, M.; Scilingo, E.P. Predicting Mood Changes in Bipolar Disorder through Heartbeat Nonlinear Dynamics. IEEE J. Biomed. Health Inform. 2016, 20, 1034–1043. [Google Scholar] [CrossRef]
- Guidi, A.; Salvi, S.; Ottaviano, M.; Gentili, C.; Bertschy, G.; de Rossi, D.; Scilingo, E.P.; Vanello, N. Smartphone Application for the Analysis of Prosodic Features in Running Speech with a Focus on Bipolar Disorders: System Performance Evaluation and Case Study. Sensors 2015, 15, 28070–28087. [Google Scholar] [CrossRef] [PubMed]
- Lorussi, F.; Rocchia, W.; Scilingo, E.P.; Tognetti, A.; De Rossi, D. Wearable, redundant fabric-based sensor arrays for reconstruction of body segment posture. IEEE Sens. J. 2004, 4, 807–818. [Google Scholar] [CrossRef]
- Lanata, A.; Valenza, G.; Scilingo, E.P. A novel EDA glove based on textile-integrated electrodes for affective computing. Med. Biol. Eng. Comput. 2012, 50, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Valenza, G.; Nardelli, M.; Bianchi, M.; Citi, L.; Scilingo, E.P. Force-Velocity Assessment of Caress-like Stimuli through the Electrodermal Activity Processing: Advantages of a Convex Optimization Approach. IEEE Trans. Hum.-Mach. Syst. 2016, 1–10. [Google Scholar] [CrossRef]
- Ödman, S.; Öberg, P.A. Movement-induced potentials in surface electrodes. Med. Biol. Eng. Comput. 1982, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Webster, J. Medical Instrumentation: Application and Design; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- De Talhouet, H.; Webster, J.G. The origin of skin-stretch-caused motion artifacts under electrodes. Physiol. Meas. 1996, 17, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Thakor, N.; Webster, J. The origin of skin potential and its variations. In Proceedings of the 31st Annual Conference on Engineering in Medicine and Biology, Atlanta, GA, USA, 1978; Volume 20, p. 212.
- Milanesi, M.; Martini, N.; Vanello, N.; Positano, V.; Santarelli, M.; Paradiso, R.; De Rossi, D.; Landini, L. Multichannel techniques for motion artifacts removal from electrocardiographic signals. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’06, New York, NY, USA, 31 August –3 September 2006; IEEE: New York, NY, USA, 2006; pp. 3391–3394. [Google Scholar]
- Martini, N.; Milanesi, M.; Vanello, N.; Positano, V.; Santarelli, M.; Landini, L. A real-time adaptive filtering approach to motion artefacts removal from ECG signals. Int. J. Biomed. Eng. Technol. 2010, 3, 233–245. [Google Scholar] [CrossRef]
- Scheffer, C.; van Oldruitenborgh-Oosterbaan, M.S. Computerized ECG recording in horses during a standardized exercise test. Vet. Q. 1996, 18, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Vitale, V.; Balocchi, R.; Varanini, M.; Sgorbini, M.; Macerata, A.; Sighieri, C.; Baragli, P. The effects of restriction of movement on the reliability of heart rate variability measurements in the horse (Equus caballus). J. Vet. Behav. Clin. Appl. Res. 2013, 8, 400–403. [Google Scholar] [CrossRef]
- Young, L.; Van Loon, G. Diseases of the heart and vessels. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of Equine Athlete; Elsevier: Amsterdam, The Netherlands, 2013; pp. 695–744. [Google Scholar]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Suh, M. E-Textiles for Wearability: Review of Integration Technologies. Available online: http://www.textileworld.com/textile-world/features/2010/04/e-textiles-for-wearability-review-of-integration-technologies/ (accessed on 20 April 2010).
- Gargiulo, G.; Bifulco, P.; Calvo, R.A.; Cesarelli, M.; Jin, C.; Van Schaik, A. Mobile biomedical sensing with dry electrodes. In Proceedings of the 2008 International Conference on Intelligent Sensors, Sensor Networks and Information Processing, ISSNIP 2008, Sydney, Australia, 15–18 December 2008; IEEE: New York, NY, USA, 2008; pp. 261–266. [Google Scholar]
- Seoane, F.; Ferreira, J.; Alvarez, L.; Buendia, R.; Ayllón, D.; Llerena, C.; Gil-Pita, R. Sensorized garments and textrode-enabled measurement instrumentation for ambulatory assessment of the autonomic nervous system response in the atrec project. Sensors 2013, 13, 8997–9015. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.; Catarino, A.P.; Rocha, A.; Postolache, O. Health monitoring using textile sensors and electrodes: An overview and integration of technologies. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), ISCTE, University of Lisbon, Lisbon, Portugal, 11–12 June 2014; IEEE: New York, NY, USA, 2014; pp. 1–6. [Google Scholar]
- Lanatà, A.; Valenza, G.; Greco, A.; Gentili, C.; Bartolozzi, R.; Bucchi, F.; Frendo, F.; Scilingo, E.P. How the Autonomic Nervous System and Driving Style Change With Incremental Stressing Conditions during Simulated Driving. IEEE Trans. Intell. Transp. Syst. 2015, 16, 1505–1517. [Google Scholar] [CrossRef]
- Zito, D.; Pepe, D.; Neri, B.; De Rossi, D.; Lanata, A.; Tognetti, A.; Scilingo, E.P. Wearable system-on-a-chip UWB radar for health care and its application to the safety improvement of emergency operators. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; IEEE: New York, NY, USA, 2007; pp. 2651–2654. [Google Scholar]
- Ishijima, M. Cardiopulmonary monitoring by textile electrodes without subject-awareness of being monitored. Med. Biol. Eng. Comput. 1997, 35, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Gemignani, A.; Scilingo, E.; De Rossi, D. Knitted bioclothes for cardiopulmonary monitoring. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Cancun, Mexico, 17–21 September 2003; IEEE: New York, NY, USA, 2003; Volume 4, pp. 3720–3723. [Google Scholar]
- Milanesi, M.; Vanello, N.; Positano, V.; Santarelli, M.; Paradiso, R.; Rossi, D.D.; Landini, L. Frequency domain approach to blind source separation in ECG monitoring by wearable system. In Proceedings of the 2005 Computers in Cardiology, Lyon, France, 25–28 September 2005; IEEE: New York, NY, USA, 2005; pp. 767–770. [Google Scholar]
- Scilingo, E.P.; Gemignani, A.; Paradiso, R.; Taccini, N.; Ghelarducci, B.; De Rossi, D. Performance evaluation of sensing fabrics for monitoring physiological and biomechanical variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, T.; Watanabe, H.; Ando, H.; Ishikawa, T.; Kobayashi, K. Noninvasive measurement of heartbeat, respiration, snoring and body movements of a subject in bed via a pneumatic method. IEEE Trans. Biomed. Eng. 2005, 52, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Bianchi, A.; Lau, K.; Scilingo, E. Psyche: Personalised monitoring systems for care in mental health. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 1–4 September 2010; IEEE: New York, NY, USA, 2010; pp. 3602–3605. [Google Scholar]
- Peltokangas, M.; Verho, J.; Vehkaoja, A. Night-time EKG and HRV monitoring with bed sheet integrated textile electrodes. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Valenza, G.; Mancuso, C.; Scilingo, E.P. Robust multiple cardiac arrhythmia detection through bispectrum analysis. Expert Syst. Appl. 2011, 38, 6798–6804. [Google Scholar] [CrossRef]
- Zito, D.; Pepe, D.; Neri, B.; Zito, F.; De Ross, D.; Lanatà, A. Feasibility study and design of a wearable system-on-a-chip pulse radar for contactless cardiopulmonary monitoring. Int. J. Telemed. Appl. 2008, 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Zito, D.; Pepe, D.; Mincica, M.; Zito, F.; De Rossi, D.; Lanata, A.; Scilingo, E.; Tognetti, A. Wearable system-on-a-chip UWB radar for contact-less cardiopulmonary monitoring: Present status. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 21–24 August 2008; IEEE: New York, NY, USA, 2008; pp. 5274–5277. [Google Scholar]
- Lanata, A.; Scilingo, E.P.; De Rossi, D. A multimodal transducer for cardiopulmonary activity monitoring in emergency. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Betella, A.; Zucca, R.; Cetnarski, R.; Greco, A.; Lanatà, A.; Mazzei, D.; Tognetti, A.; Arsiwalla, X.D.; Omedas, P.; De Rossi, D.; et al. Inference of human affective states from psychophysiological measurements extracted under ecologically valid conditions. In Using Neurophysiological Signals that Reflect Cognitive or Affective State, Frontiers in Neuroscience; Frontiers Media SA: Lausanne, Switzerland, 2014; p. 66. [Google Scholar]
- Zhang, Y.T.; Poon, C.C.; Chan, C.H.; Tsang, M.W.; Wu, K.F. A health-shirt using e-textile materials for the continuous and cuffless monitoring of arterial blood pressure. In Proceedings of the 2006 3rd IEEE/EMBS International Summer School on Medical Devices and Biosensors, Cambridge, MA, USA, 4–6 September 2006; IEEE: New York, NY, USA, 2006; pp. 86–89. [Google Scholar]
- Chan, C.; Zhang, Y. Continuous and long-term arterial blood pressure monitoring by using h-Shirt. In Proceedings of the 2008 International Conference on Information Technology and Applications in Biomedicine (ITAB 2008), Shenzhen, China, 30–31 May 2008; IEEE: New York, NY, USA, 2008; pp. 267–269. [Google Scholar]
- Arshad, A. A Study on Health Monitoring System: Recent Advancements. IIUM Eng. J. 2014, 15, 87–99. [Google Scholar]
- Baha, S. T wave shape as fitness indicator in racehorse. A study by the Holter method. Rev. Méd. Vét. 1991, 142, 125–129. [Google Scholar]
- Raekallio, M. Long term ECG recording with Holter monitoring in clinically healthy horses. Acta Vet. Scand. 1991, 33, 71–75. [Google Scholar]
- Reef, V.; Marr, C.; Hammett, B. Holter monitoring in the management of atrial fibrillation following conversion. In Proceedings of the 11th American College of Veterinary Internal Medicine Forum, Washington, DC, USA, 20 May 1993; pp. 610–613.
- Scheffer, C.; Robben, J.; Sloet, O.O.M. Continuous monitoring of ECG in horses at rest and during exercise. Vet. Rec. 1995, 137, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybern. C (Appl. Rev.) 2010, 40, 1–12. [Google Scholar] [CrossRef]
- Lanata, A.; Guidi, A.; Baragli, P.; Paradiso, R.; Valenza, G.; Scilingo, E.P. Removing movement artifacts from equine ECG recordings acquired with textile electrodes. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: New York, NY, USA, 2015; pp. 1955–1958. [Google Scholar]
- Pandian, P.; Mohanavelu, K.; Safeer, K.; Kotresh, T.; Shakunthala, D.; Gopal, P.; Padaki, V. Smart Vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Guidi, A.; Baragli, P.; Valenza, G.; Scilingo, E.P. A Novel Algorithm for Movement Artifact Removal in ECG Signals Acquired from Wearable Systems Applied to Horses. PLoS ONE 2015, 10, e0140783. [Google Scholar] [CrossRef] [PubMed]
- Lanatà, A.; Scilingo, E.P.; Nardini, E.; Loriga, G.; Paradiso, R.; De-Rossi, D. Comparative evaluation of susceptibility to motion artifact in different wearable systems for monitoring respiratory rate. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Citi, L.; Gentili, C.; Lanata, A.; Scilingo, E.P.; Barbieri, R. Characterization of depressive states in bipolar patients using wearable textile technology and instantaneous heart rate variability assessment. IEEE J. Biomed. Health Inform. 2015, 19, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Lanatà, A.; Scilingo, E.P.; Rossi, D.D. Towards a smart glove: Arousal recognition based on textile electrodermal response. In Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 1–4 September 2010; IEEE: New York, NY, USA, 2010; pp. 3598–3601. [Google Scholar]
- Valenza, G.; Gentili, C.; Lanatà, A.; Scilingo, E.P. Mood recognition in bipolar patients through the PSYCHE platform: Preliminary evaluations and perspectives. Artif. Intell. Med. 2013, 57, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, M.; Loriga, G.; Taccini, N.; Paradiso, R. Sensing fabrics for monitoring physiological and biomechanical variables: E-textile solutions. In Proceedings of the 2006 3rd IEEE/EMBS International Summer School on Medical Devices and Biosensors, Cambridge, MA, USA, 4–6 September 2006; pp. 1–4.
- Verheyen, T.; Decloedt, A.; De Clercq, D.; Deprez, P.; Sys, S.; van Loon, G. Electrocardiography in horses, part 1: How to make a good recording. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 331–336. [Google Scholar]
- Saul, J.P. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology 1990, 5, 32–37. [Google Scholar]
- Berntson, G.G. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Liñares, L.; Méndez, A.J.; Lado, M.J.; Olivieri, D.N.; Vila, X.A.; Gómez-Conde, I. An open source tool for heart rate variability spectral analysis. Comput. Methods Progr. Biomed. 2011, 103, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Signorini, M.G.; Cogliati, C.; Mezzetti, S.; Porta, A.; Cerutti, S.; Malliani, A. Non-linear dynamics and chaotic indices in heart rate variability of normal subjects and heart-transplanted patients. Cardiovasc. Res. 1996, 31, 441–446. [Google Scholar] [CrossRef]
- Valenza, G.; Nardelli, M.; Bertschy, G.; Lanata, A.; Scilingo, E. Mood states modulate complexity in heartbeat dynamics: A multiscale entropy analysis. Europhys. Lett. 2014, 107. [Google Scholar] [CrossRef]
- Valenza, G.; Greco, A.; Lanata, A.; Sebastiani, L.; Menicucci, D.; Gemignani, A.; Scilingo, E.P. Combining electroencephalographic activity and instantaneous heart rate for assessing brain–heart dynamics during visual emotional elicitation in healthy subjects. Phil. Trans. R. Soc. A. 2016, 374, 441–446. [Google Scholar]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 3, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vinod, K.; Saxena, S. Sampling frequency of the RR interval time series for spectral analysis of heart rate variability. J. Med. Eng. Technol. 2004, 28, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Anosov, O.; Patzak, A.; Kononovich, Y.; Persson, P.B. High-frequency oscillations of the heart rate during ramp load reflect the human anaerobic threshold. Eur. J. Appl. Physiol. 2000, 83, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.J. Calculating metrics of cardiac chronotropy: A pragmatic overview. Psychophysiology 2002, 39, S18. [Google Scholar]
- Müller, M. Dynamic time warping. In Information Retrieval for Music and Motion; Springer-Verlag: Berlin, Heidelberg, 2007; pp. 69–84. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar]
- Lanata, A.; Greco, A.; Valenza, G.; Scilingo, E.P. A pattern recognition approach based on electrodermal response for pathological mood identification in bipolar disorders. In Proceedings of the 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; IEEE: New York, NY, USA, 2014; pp. 3601–3605. [Google Scholar]
- Nardelli, M.; Valenza, G.; Cristea, I.A.; Gentili, C.; Cotet, C.; David, D.; Lanata, A.; Scilingo, E.P. Characterizing psychological dimensions in non-pathological subjects through autonomic nervous system dynamics. Front. Comput. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Valenza, G.; Nardelli, M.; Gentili, C.; Scilingo, E.P. Complexity index from a personalized wearable monitoring system for assessing remission in mental health. IEEE J. Biomed. Health Inform. 2015, 19, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Daftari, J.; Rao, R.R. Heart rate wavelet coherence analysis to investigate group entrainment. Pervasive Mob. Comput. 2016, 28, 21–34. [Google Scholar] [CrossRef]
- Lanata, A.; Valenza, G.; Scilingo, E.P. Eye gaze patterns in emotional pictures. J. Ambient Intell. Humaniz. Comput. 2013, 4, 705–715. [Google Scholar] [CrossRef]
- Lazzeri, N.; Mazzei, D.; Greco, A.; Rotesi, A.; Lanatà, A.; De Rossi, D.E. Can a humanoid face be expressive? A psychophysiological investigation. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.V.; Alfonso, L.A.; Llabre, M.M.; Fernandez, M.I. Project Stride: An Equine-Assisted Intervention to Reduce Symptoms of Social Anxiety in Young Women. Explor. J. Sci. Heal. 2015, 11, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bass, M.M.; Duchowny, C.A.; Llabre, M.M. The effect of therapeutic horseback riding on social functioning in children with autism. J. Autism Dev. Disord. 2009, 39, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, M.P.; Betella, A.; Lanatá, A.; Scilingo, E.P.; Bernardet, U.; Verschure, P.F. The social perceptual salience effect. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Lanatà, A.; Armato, A.; Valenza, G.; Scilingo, E.P. Eye tracking and pupil size variation as response to affective stimuli: A preliminary study. In Proceedings of the 2011 5th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth), Dublin, Ireland, 23–26 May 2011; IEEE: New York, NY, USA, 2011; pp. 78–84. [Google Scholar]


| Ag/AgCl | E-Textile | |
|---|---|---|
| h1 | 77, 48 | 32, 37 |
| h2 | 54, 50 | 35, 00 |
| h3 | 51, 49 | 39, 74 |
| h4 | 30, 44 | 27, 51 |
| h5 | 54, 44 | 32, 66 |
| h6 | 47, 02 | 41, 96 |
| h7 | 47, 89 | 35, 03 |
| Feature | vs. | vs. | vs. |
|---|---|---|---|
| DTW | 0.0353 (↑) | 0.0026 (↑) | 0.0018 (↑) |
| vs. | vs. | vs. | |
|---|---|---|---|
| human | 7.32E-04 (↑) | 4.63E-01 | 3.66E-03 (↓) |
| horse | 1.38E-01 | 4.4E-02 (↑) | 8.21E-01 |
| 85.7143 | 7.1429 | 7.1429 | |
| 21.4286 | 78.5714 | 0.0000 | |
| 0.0000 | 7.1429 | 92.8571 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidi, A.; Lanata, A.; Baragli, P.; Valenza, G.; Scilingo, E.P. A Wearable System for the Evaluation of the Human-Horse Interaction: A Preliminary Study. Electronics 2016, 5, 63. https://doi.org/10.3390/electronics5040063
Guidi A, Lanata A, Baragli P, Valenza G, Scilingo EP. A Wearable System for the Evaluation of the Human-Horse Interaction: A Preliminary Study. Electronics. 2016; 5(4):63. https://doi.org/10.3390/electronics5040063
Chicago/Turabian StyleGuidi, Andrea, Antonio Lanata, Paolo Baragli, Gaetano Valenza, and Enzo Pasquale Scilingo. 2016. "A Wearable System for the Evaluation of the Human-Horse Interaction: A Preliminary Study" Electronics 5, no. 4: 63. https://doi.org/10.3390/electronics5040063