Atomic Layer Growth of InSe and Sb2Se3 Layered Semiconductors and Their Heterostructure
Abstract
:1. Introduction
2. Materials and Methods
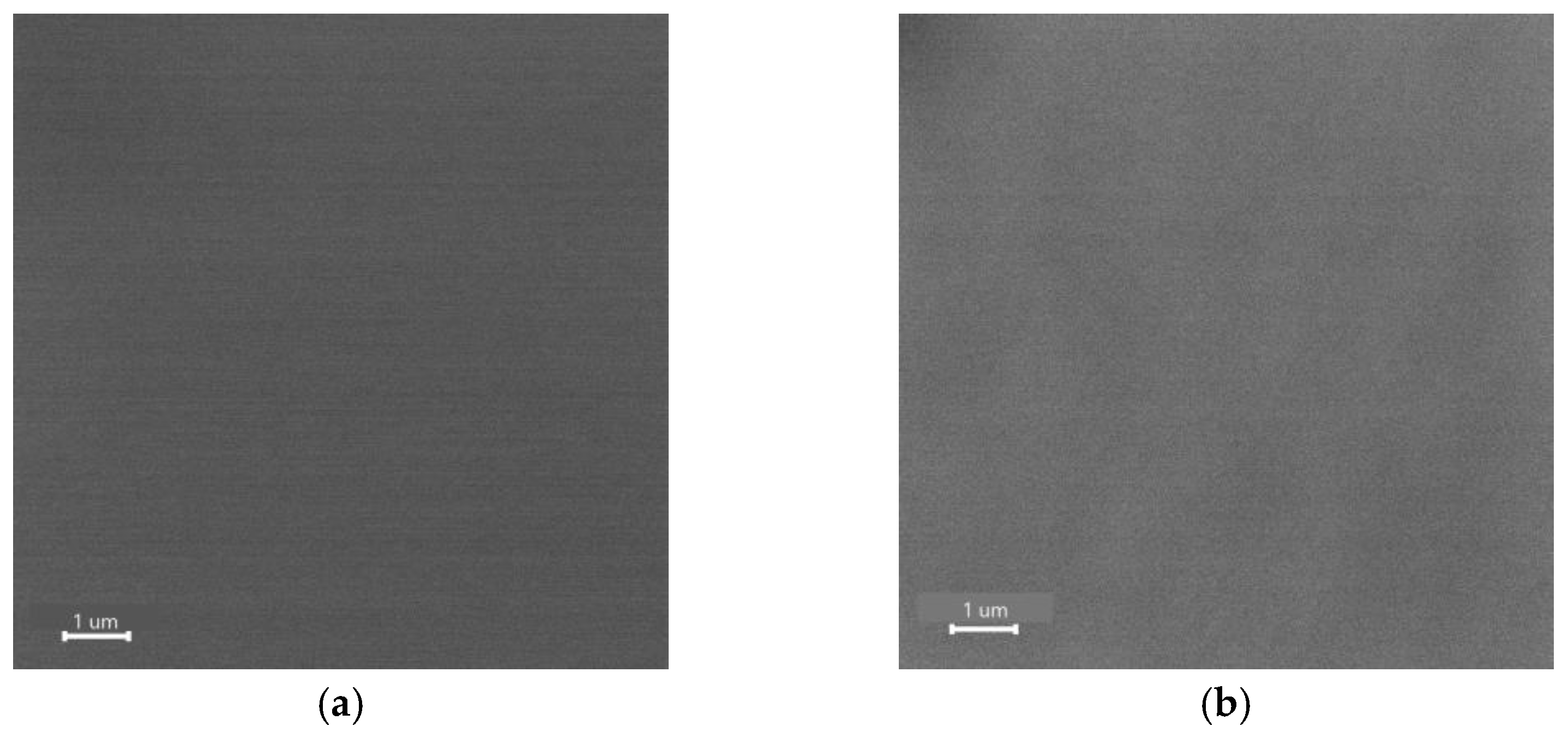
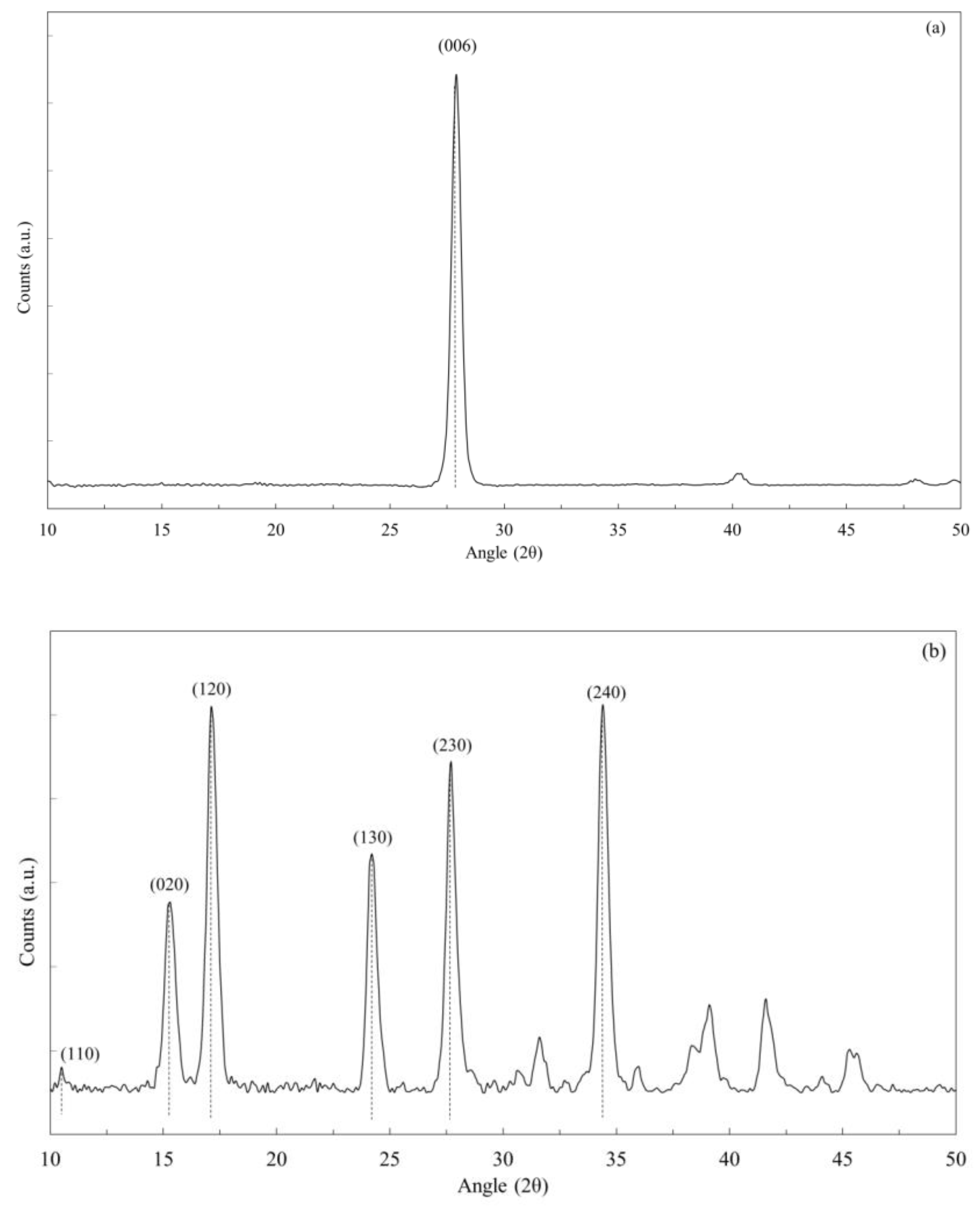
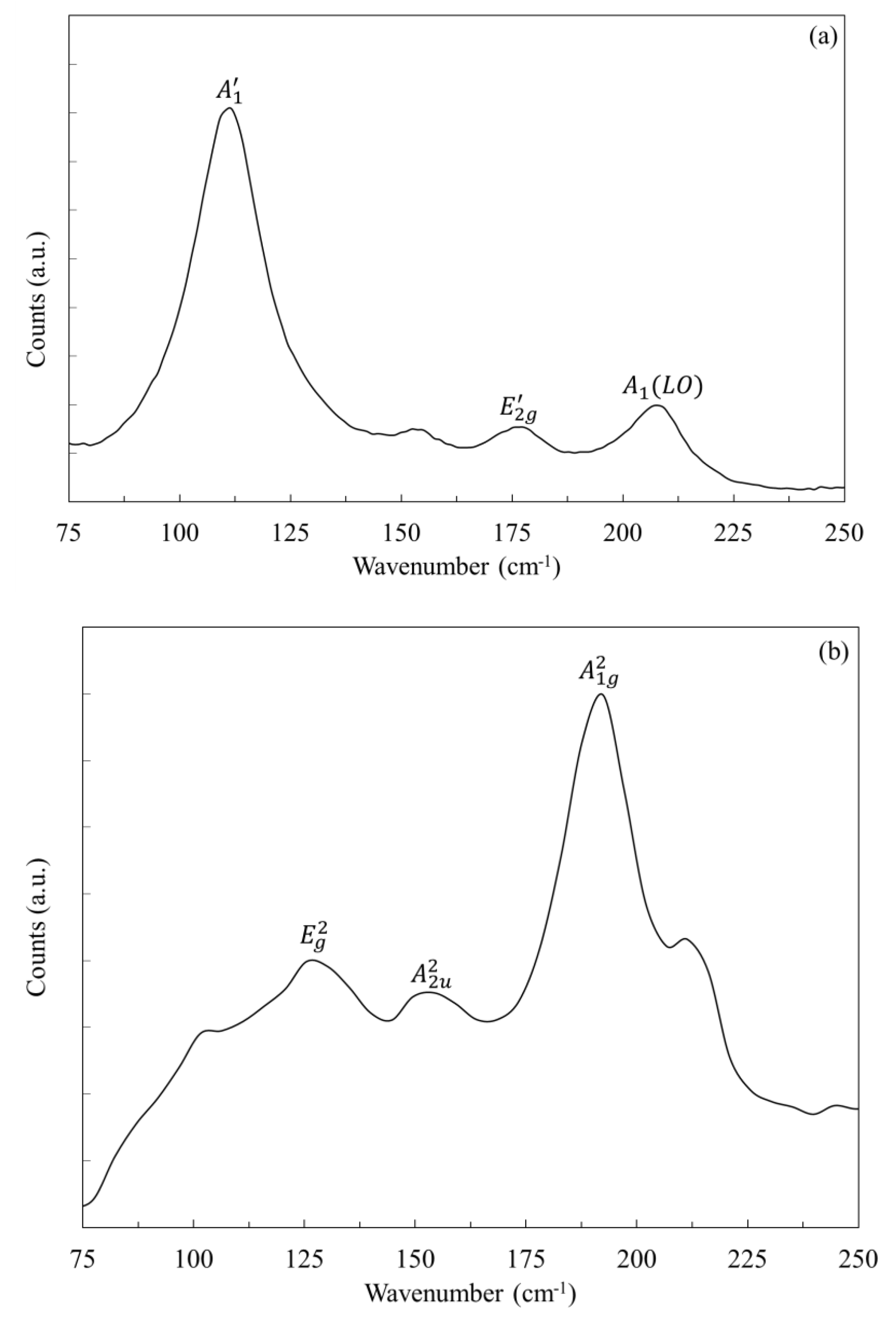
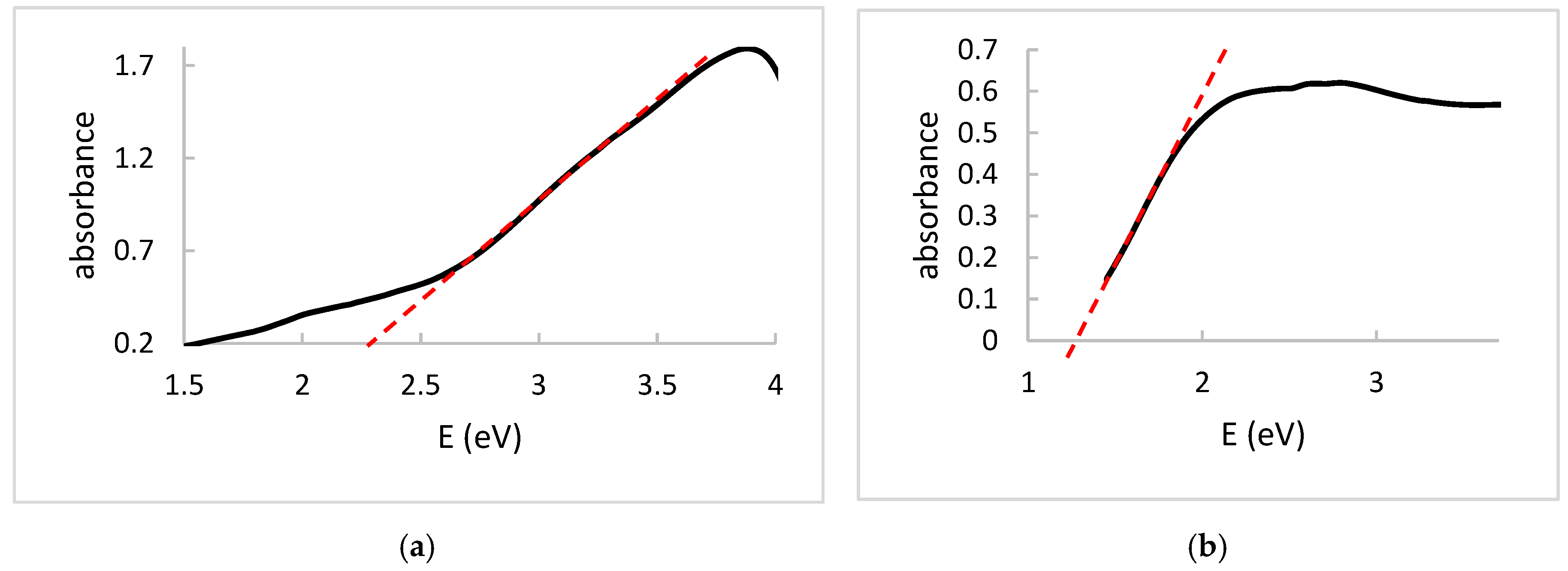
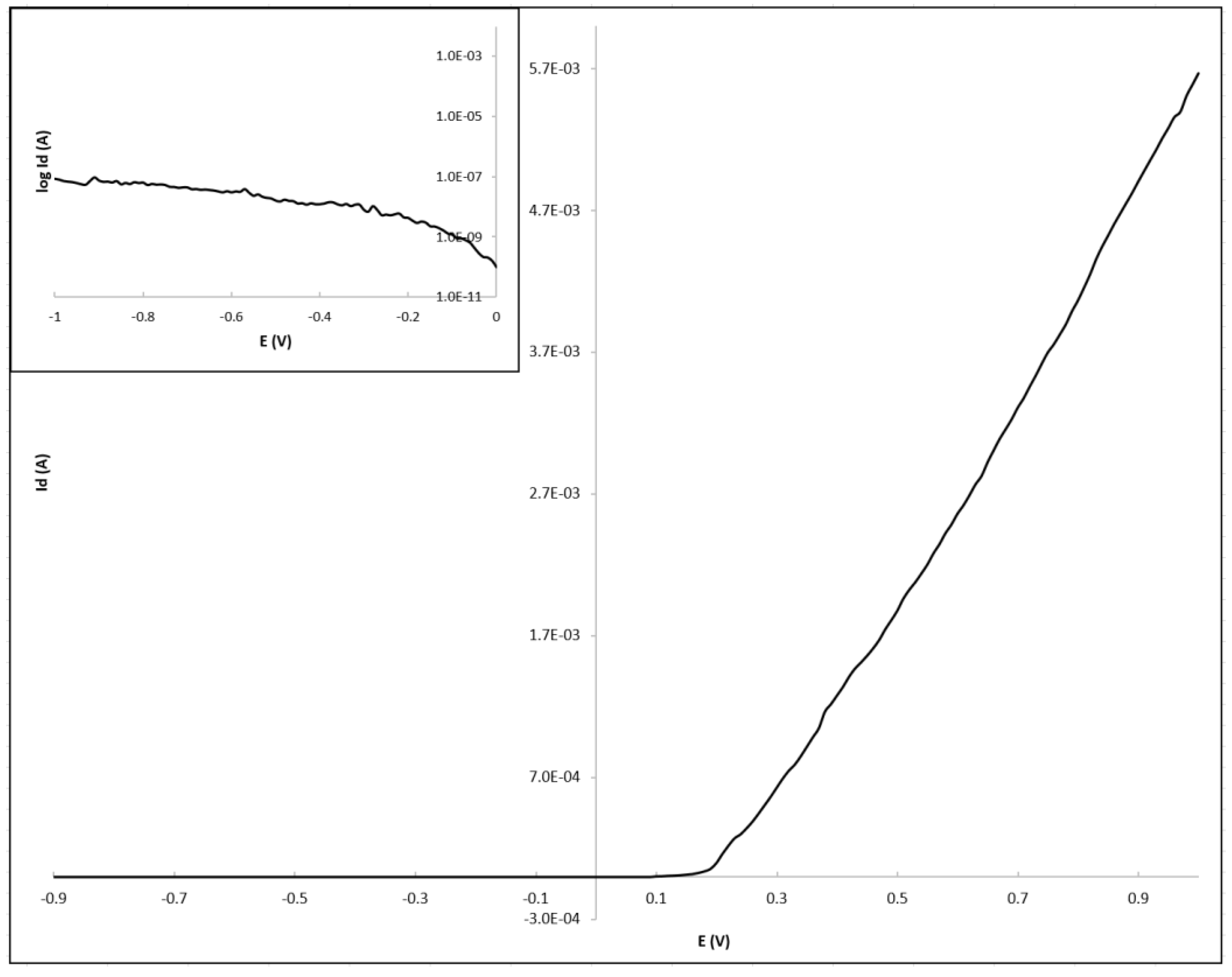
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.; Plachinda, P.; Padigi, P.; Solanki, R.; Rouvimov, S. Growth of multiple ws2/sns layered semiconductor heterojunctions. Nanoscale 2016, 8, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chen, H.Y.; Penumatcha, A.V.; Appenzeller, J. High performance multilayer mos2 transistors with scandium contacts. Nano Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer mos2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, M.W.; Puretzky, A.A.; Idrobo, J.C.; Ma, C.; Chi, M.; Yoon, M.; Rouleau, C.M.; Kravchenko, I.I.; Geohegan, D.B.; et al. Controlled vapor phase growth of single crystalline, two-dimensional gase crystals with high photoresponse. Sci. Rep. 2014, 4, 5497. [Google Scholar] [PubMed]
- Luxa, J.; Wang, Y.; Sofer, Z.; Pumera, M. Layered post-transition-metal dichalcogenides (x-m-m-x) and their properties. Chemistry 2016, 22, 18810–18816. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zheng, W.; Cao, W.; Hu, P. Back gated multilayer inse transistors with enhanced carrier mobilities via the suppression of carrier scattering from a dielectric interface. Adv. Mater. 2014, 26, 6587–6593. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wen, F.; Li, B.; Wang, Q.; Huang, Y.; Gong, Y.; He, Y.; Dong, P.; Bellah, J.; George, A.; et al. Optoelectronic memory using two-dimensional materials. Nano Lett. 2015, 15, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Backes, C.; Gholamvand, Z.; Hanlon, D.; McAteer, D.; Nerl, H.C.; McGuire, E.; Seral-Ascaso, A.; Ramasse, Q.M.; McEvoy, N.; et al. Preparation of gallium sulfide nanosheets by liquid exfoliation and their application as hydrogen evolution catalysts. Chem. Mater. 2015, 27, 3483–3493. [Google Scholar] [CrossRef]
- Hu, P.; Wen, Z.; Wang, L.; Tan, P.; Xiao, K. Synthesis of few-layer gase nanosheets for high performance photodetectors. ACS Nano 2012, 6, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Amory, C.; Bernède, J.C.; Marsillac, S. Study of a growth instability of γ-in2se3. J. Appl. Phys. 2003, 94, 6945–6948. [Google Scholar] [CrossRef]
- Sánchez-Royo, J.F.; Muñoz-Matutano, G.; Brotons-Gisbert, M.; Martínez-Pastor, J.P.; Segura, A.; Cantarero, A.; Mata, R.; Canet-Ferrer, J.; Tobias, G.; Canadell, E.; et al. Electronic structure, optical properties, and lattice dynamics in atomically thin indium selenide flakes. Nano Res. 2014, 7, 1556–1568. [Google Scholar] [CrossRef]
- Mudd, G.W.; Svatek, S.A.; Ren, T.; Patane, A.; Makarovsky, O.; Eaves, L.; Beton, P.H.; Kovalyuk, Z.D.; Lashkarev, G.V.; Kudrynskyi, Z.R.; et al. Tuning the bandgap of exfoliated inse nanosheets by quantum confinement. Adv. Mater. 2013, 25, 5714–5718. [Google Scholar] [CrossRef] [PubMed]
- Shigeru, S.; Tesuo, I. Crystalline inse films prepared by rf-sputtering technique. Jpn. J. Appl. Phys. 1991, 30, L2127. [Google Scholar]
- Lei, S.; Ge, L.; Najmaei, S.; George, A.; Kappera, R.; Lou, J.; Chhowalla, M.; Yamaguchi, H.; Gupta, G.; Vajtai, R.; et al. Evolution of the electronic band structure and efficient photo-detection in atomic layers of inse. ACS Nano 2014, 8, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.L.; Barron, A.R. Metal− organic chemical vapor deposition of indium selenide thin films. Chem. Mater. 1998, 10, 650–657. [Google Scholar] [CrossRef]
- Ho, C.H.; Lin, C.H.; Wang, Y.P.; Chen, Y.C.; Chen, S.H.; Huang, Y.S. Surface oxide effect on optical sensing and photoelectric conversion of alpha-in2se3 hexagonal microplates. ACS Appl. Mater. Interfaces 2013, 5, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G. The sb-se (antimony-selenium) system. J. Phase Equilibria 1993, 14, 753–763. [Google Scholar] [CrossRef]
- Chang, H.-W.; Sarkar, B.; Liu, C.W. Synthesis of sb2se3 nanowires via a solvothermal route from the single source precursor sb[se2p(oipr)2]3. Cryst. Growth Des. 2007, 7, 2691–2695. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, S.; Qin, S.; Liu, X.; Chen, J.; Xue, D.-J.; Luo, M.; Cao, Y.; Cheng, Y.; et al. Thin-film sb2se3 photovoltaics with oriented one-dimensional ribbons and benign grain boundaries. Nat. Photonics 2015, 9, 409–415. [Google Scholar] [CrossRef]
- Kutasov, V.A. Shifting the maximum figure-of-merit of (bi, sb)2(te, se)3 thermoelectrics to lower temperatures. In Thermoelectrics Handbook; CRC Press: Boca Raton, FL, USA, 2005; pp. 37-18–37-31. [Google Scholar]
- Liu, X.; Chen, J.; Luo, M.; Leng, M.; Xia, Z.; Zhou, Y.; Qin, S.; Xue, D.J.; Lv, L.; Huang, H.; et al. Thermal evaporation and characterization of sb2se3 thin film for substrate sb2se3/cds solar cells. ACS Appl. Mater. Interfaces 2014, 6, 10687–10695. [Google Scholar] [CrossRef] [PubMed]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Rajpure, K.; Bhosale, C. Effect of se source on properties of spray deposited sb 2 se 3 thin films. Mater. Chem. Phys. 2000, 62, 169–174. [Google Scholar] [CrossRef]
- Rodriguez-Lazcano, Y.; Peña, Y.; Nair, M.; Nair, P. Polycrystalline thin films of antimony selenide via chemical bath deposition and post deposition treatments. Thin Solid Films 2005, 493, 77–82. [Google Scholar] [CrossRef]
- Fernandez, A.; Merino, M. Preparation and characterization of sb 2 se 3 thin films prepared by electrodeposition for photovoltaic applications. Thin Solid Films 2000, 366, 202–206. [Google Scholar] [CrossRef]
- Xue, M.-Z.; Fu, Z.-W. Pulsed laser deposited sb 2 se 3 anode for lithium-ion batteries. J. Alloys Compd. 2008, 458, 351–356. [Google Scholar] [CrossRef]
- Tabernor, J.; Christian, P.; O'Brien, P. A general route to nanodimensional powders of indium chalcogenides. J. Mater. Chem. 2006, 16, 2082. [Google Scholar] [CrossRef]
- Tideswell, N.W.; Kruse, F.H.; McCullough, J.D. The crystal structure of antimony selenide, sb2se3. Acta Crystallogr. 1957, 10, 99–102. [Google Scholar] [CrossRef]
- Chen, Z.; Gacem, K.; Boukhicha, M.; Biscaras, J.; Shukla, A. Anodic bonded 2d semiconductors: From synthesis to device fabrication. Nanotechnology 2013, 24, 415708. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, K.; Imai, K. Far-infrared reflection and raman scattering spectra in γ-inse. Phys. Status Solidi 1988, 149, K183–K186. [Google Scholar] [CrossRef]
- Marsillac, S.; Combot-Marie, A.M.; Bernède, J.C.; Conan, A. Experimental evidence of the low-temperature formation of γ-in2se3 thin films obtained by a solid-state reaction. Thin Solid Films 1996, 288, 14–20. [Google Scholar] [CrossRef]
- Weszka, J.; Daniel, P.; Burian, A.; Burian, A.M.; Nguyen, A.T. Raman scattering in in2se3 and inse2 amorphous films. J. Non·Cryst. Solids 2000, 265, 98–104. [Google Scholar] [CrossRef]
- Bera, A.; Pal, K.; Muthu, D.V.S.; Sen, S.; Guptasarma, P.; Waghmare, U.V.; Sood, A.K. Sharp raman anomalies and broken adiabaticity at a pressure induced transition from band to topological insulator in sb2se3. Phys. Rev. Lett. 2013, 110, 107401. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Z.G.; Cernoskova, E.; Vassilev, V.S.; Boycheva, S.V. Thermomechanical and structural characterization of gese2–sb2se3–znse glasses. Mater. Lett. 2003, 57, 1025–1028. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Z.; Li, Y. Synthesis and characterization of sb2se3 nanorods. Mater. Res. Bull. 2002, 37, 495–502. [Google Scholar] [CrossRef]
- Filip, M.R.; Patrick, C.E.; Giustino, F. Gw quasiparticle band structures of stibnite, antimonselite, bismuthinite, and guanajuatite. Phys. Rev. B 2013, 87, 2450–2458. [Google Scholar] [CrossRef]
- Beck, K.M.; Wiley, W.R.; Venkatasubramanian, E.; Ohuchi, F. Vacancies ordered in screw form (vosf) and layered indium selenide thin film deposition by laser back ablation. Appl. Surf. Sci. 2009, 255, 9707–9711. [Google Scholar] [CrossRef]
- Suntola, T. Atomic layer epitaxy. Mater. Sci. Rep. 1989, 4, 261–312. [Google Scholar] [CrossRef]
- Browning, R.; Padigi, P.; Solanki, R.; Tweet, D.J.; Schuele, P.; Evans, D. Atomic layer deposition of mos2 thin films. Mater. Res. Express 2015, 2, 035006. [Google Scholar] [CrossRef]
- Browning, R.; Kuperman, N.; Solanki, R.; Kanzyuba, V.; Rouvimov, S. Large area growth of layered wse2 films. Semicond. Sci. Technol. 2016, 31, 095002. [Google Scholar] [CrossRef]
- Saiki, K.; Ueno, K.; Shimada, T.; Koma, A. Application of van der waals epitaxy to highly heterogeneous systems. J. Cryst. Growth 1989, 95, 603–606. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, R.; Kuperman, N.; Moon, B.; Solanki, R. Atomic Layer Growth of InSe and Sb2Se3 Layered Semiconductors and Their Heterostructure. Electronics 2017, 6, 27. https://doi.org/10.3390/electronics6020027
Browning R, Kuperman N, Moon B, Solanki R. Atomic Layer Growth of InSe and Sb2Se3 Layered Semiconductors and Their Heterostructure. Electronics. 2017; 6(2):27. https://doi.org/10.3390/electronics6020027
Chicago/Turabian StyleBrowning, Robert, Neal Kuperman, Bill Moon, and Raj Solanki. 2017. "Atomic Layer Growth of InSe and Sb2Se3 Layered Semiconductors and Their Heterostructure" Electronics 6, no. 2: 27. https://doi.org/10.3390/electronics6020027




