Graphene Ink Film Based Electrochemical Detector for Paracetamol Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wei, D.; Andrew, P.; Yang, H.; Jiang, Y.; Li, F.; Shan, C.; Ruan, W.; Han, D.; Li, N.; Bower, C. Flexible solid state lithium batteries based on graphene inks. J. Mater. Chem. 2011, 21, 9762–9767. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V. A lithium-ion battery based on a graphene nanoflakes ink anode and a lithium iron phosphate cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Ervin, M.H.; Le, L.T.; Lee, W.Y. Inkjet-printed flexible graphene-based supercapacitor. Electrochim. Acta 2014, 147, 610–616. [Google Scholar] [CrossRef]
- Xu, Y.; Schwab, M.G.; Strudwick, A.J.; Hennig, I.; Feng, X.; Wu, Z.; Müllen, K. Screen-printable thin film supercapacitor device utilizing graphene/polyaniline inks. Adv. Energy Mater. 2013, 3, 1035–1040. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, D.Z. Photoluminescent graphene oxide ink to print sensors onto microporous membranes for versatile visualization bioassays. Angew. Chem. 2012, 124, 5700–5704. [Google Scholar] [CrossRef]
- Radoi, A.; Dragoman, M.; Cismaru, A.; Konstantinidis, G.; Dragoman, D. Self-powered microwave devices based on graphene ink decorated with gold nanoislands. J. Appl. Phys. 2012, 112, 611–621. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Lee, W.G.; Sultan, S.; Yousuf, M.; Harzandi, A.M.; Vij, V.; Kim, K.S. High-affinity-assisted nanoscale alloys as remarkable bifunctional catalyst for alcohol oxidation and oxygen reduction reactions. ACS Nano 2017, 11, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Kemp, K.C.; Nath, K.; Tiwari, R.N.; Nam, H.-G.; Kim, K.S. Interconnected Pt-nanodendrite/DNA/reduced-graphene-oxide hybrid showing remarkable oxygen reduction activity and stability. ACS Nano 2013, 7, 9223–9231. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, H.; Han, D.; Zhang, Q.; Niu, L.; Yang, H.; Bower, C.; Andrew, P.; Ryhänen, T. Properties of graphene inks stabilized by different functional groups. Nanotechnology 2011, 22, 245702. [Google Scholar] [CrossRef] [PubMed]
- Mayavan, S.; Siva, T.; Sathiyanarayanan, S. Graphene ink as a corrosion inhibiting blanket for iron in an aggressive chloride environment. RSC Adv. 2013, 3, 24868–24871. [Google Scholar] [CrossRef]
- Benwadih, M.; Aliane, A.; Jacob, S.; Bablet, J.; Coppard, R.; Chartier, I. Integration of a graphene ink as gate electrode for printed organic complementary thin-film transistors. Org. Electron. 2014, 15, 614–621. [Google Scholar] [CrossRef]
- Arapov, K.; Bex, G.; Hendriks, R.; Rubingh, E.; Abbel, R.; De With, G.; Friedrich, H. Conductivity enhancement of binder-based graphene inks by photonic annealing and subsequent compression rolling&thinsp. Adv. Eng. Mater. 2016, 18, 1234–1239. [Google Scholar]
- Tiwari, J.N.; Seo, Y.-K.; Yoon, T.; Lee, W.G.; Cho, W.J.; Yousuf, M.; Harzandi, A.M.; Kang, D.-S.; Kim, K.-Y.; Suh, P.-G. Accelerated bone regeneration by two-photon photoactivated carbon nitride nanosheets. ACS Nano 2017, 11, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Seema, H.; Kemp, K.C.; Ahmed, N.; Tiwari, J.N.; Park, S.; Kim, K.S. Solution-processable conductive micro-hydrogels of nanoparticle/graphene platelets produced by reversible self-assembly and aqueous exfoliation. J. Mater. Chem. A 2013, 1, 12900–12908. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Nath, K.; Kumar, S.; Tiwari, R.N.; Kemp, K.C.; Le, N.H.; Youn, D.H.; Lee, J.S.; Kim, K.S. Stable platinum nanoclusters on genomic DNA–graphene oxide with a high oxygen reduction reaction activity. Nat. Commun. 2013, 4, 2221. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2015, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, A.; Lai, G.; Su, W.; Malherbe, F.; Yu, J.; Lin, C.-T.; Yu, A. Defects regulating of graphene ink for electrochemical determination of ascorbic acid, dopamine and uric acid. Talanta 2018, 180, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, K.; Zhang, H.; Zheng, Y.; Su, W.; Liu, Z. Multi-walled carbon nanotube-assisted electrodeposition of silver dendrite coating as a catalytic film. Coatings 2017, 7, 232. [Google Scholar] [CrossRef]
- Guo, S.; Wang, W.; Ozkan, C.S.; Ozkan, M. Assembled graphene oxide and single-walled carbon nanotube ink for stable supercapacitors. J. Mater. Res. 2013, 28, 918–926. [Google Scholar] [CrossRef]
- Min, J.Y.; Yoon, S.; Ji-Hoon, L.; Sung-Jun, P.; Ryong, K.S.; In, I. Submillimeter-scale graphene patterning through ink-jet printing of graphene oxide ink. Chemistry Lett. 2011, 40, 54–55. [Google Scholar]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T. Graphene oxide-based electrode inks for 3d-printed lithium-ion batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Pan, F.-M.; Chen, T.-M.; Tiwari, R.N.; Lin, K.-L. Electrocatalytic activity of Pt nanoparticles electrodeposited on amorphous carbon-coated silicon nanocones. J. Power Sources 2010, 195, 729–735. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Chang, Y.M.; Lin, K.L. A promising approach to the synthesis of 3d nanoporous graphitic carbon as a unique electrocatalyst support for methanol oxidation. ChemSusChem 2010, 3, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Q.; Wang, N.; Chen, Z.; Tian, Q.; Yang, H.; Wang, K. Reverse-micelle-induced exfoliation of graphite into graphene nanosheets with assistance of supercritical CO2. Chem. Mater. 2015, 27, 3262–3272. [Google Scholar] [CrossRef]
- Iguchi, H.; Higashi, C.; Funasaki, Y.; Fujita, K.; Mori, A.; Nakasuga, A.; Maruyama, T. Rational and practical exfoliation of graphite using well-defined poly(3-hexylthiophene) for the preparation of conductive polymer/graphene composite. Sci. Rep. 2017, 7, 39937. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ahn, S.I.; Choi, K.C. Simultaneous synthesis and patterning of graphene electrodes by reactive inkjet printing. Carbon 2014, 66, 172–177. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Fei, T.; Zhang, T. Template-free synthesized hollow NiO–SnO2 nanospheres with high gas-sensing performance. Sens. Actuators B Chem. 2012, 164, 90–95. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.-Z. Probing the electrochemical properties of graphene nanosheets for biosensing applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Hong, S. Graphene ink fabricated screen printed electrode for Cd2+ and Pd2+ determination in Xiangjiang river. Int. J. Electrochem. Sci. 2016, 11, 7430–7439. [Google Scholar]
- Secor, E.B.; Gao, T.Z.; Islam, A.E.; Rao, R.; Wallace, S.G.; Zhu, J.; Putz, K.W.; Maruyama, B.; Hersam, M.C. Enhanced conductivity, adhesion, and environmental stability of printed graphene inks with nitrocellulose. Chem. Mater. 2017, 29, 2332–2340. [Google Scholar] [CrossRef]
- Secor, E.B.; Ahn, B.Y.; Gao, T.Z.; Lewis, J.A.; Hersam, M.C. Rapid and versatile photonic annealing of graphene inks for flexible printed electronics. Adv. Mater. 2015, 27, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Del, S.K.; Bornemann, R.; Bablich, A.; Schäfer-Eberwein, H.; Li, J.; Kowald, T.; Östling, M.; Bolívar, P.H.; Lemme, M.C. Optimizing the optical and electrical properties of graphene ink thin films by laser-annealing. 2D Mater. 2015, 2, 011003. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, L.; Dai, Y.; Zhong, M.; Lu, X.; Kan, X. Electrochemical sensor for paracetamol recognition and detection based on catalytic and imprinted composite film. Biosens. Bioelectron. 2015, 71, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zidan, M.; Tee, T.W.; Abdullah, A.H.; Zainal, Z.; Kheng, G.J. Electrochemical oxidation of paracetamol mediated by nanoparticles bismuth oxide modified glassy carbon electrode. Int. J. Electrochem. Sci. 2011, 6, 279–288. [Google Scholar]
- Wei, R. Biosynthesis of Au–Ag alloy nanoparticles for sensitive electrochemical determination of paracetamol. Int. J. Electrochem. Sci. 2017, 12, 9131–9140. [Google Scholar] [CrossRef]
- Gee, C.M.; Tseng, C.C.; Wu, F.Y.; Chang, H.P.; Li, L.J.; Hsieh, Y.P.; Lin, C.T.; Chen, J.C. Flexible transparent electrodes made of electrochemically exfoliated graphene sheets from low-cost graphite pieces. Displays 2013, 34, 315–319. [Google Scholar] [CrossRef]
- Skaltsas, T.; Ke, X.; Bittencourt, C.; Tagmatarchis, N. Ultrasonication induces oxygenated species and defects onto exfoliated graphene. J. Phys. Chem. C 2016, 117, 23272–23278. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Cheng, H.-T.; Wu, P.-X.; Weng, C.-J.; Santiago, K.S.; Yeh, J.-M. Electrochemical sensor constructed using a carbon paste electrode modified with mesoporous silica encapsulating PANI chains decorated with GNPs for detection of ascorbic acid. Electrochim. Acta 2017, 238, 246–256. [Google Scholar] [CrossRef]
- Lim, D.-H.; Wilcox, J. Dft-based study on oxygen adsorption on defective graphene-supported Pt nanoparticles. J. Phys. Chem. C 2011, 115, 22742–22747. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Meng, C.; Han, Y. Palladium nanoparticles/defective graphene composites as oxygen reduction electrocatalysts: A first-principles study. J. Phys. Chem. C 2012, 116, 2710–2719. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Strano, M.S.; Pumera, M.; Gras, S.L. Two-dimensional transition metal dichalcogenides in biosystems. Adv. Funct. Mater. 2015, 25, 5086–5099. [Google Scholar] [CrossRef]
- Alsaif, M.M.Y.A.; Chrimes, A.F.; Daeneke, T.; Balendhran, S.; Bellisario, D.O.; Son, Y.; Field, M.R.; Zhang, W.; Nili, H.; Nguyen, E.P. High-performance field effect transistors using electronic inks of 2d molybdenum oxide nanoflakes. Adv. Funct. Mater. 2016, 26, 91–100. [Google Scholar] [CrossRef]
- Babaei, A.; Garrett, D.J.; Downard, A.J. Selective simultaneous determination of paracetamol and uric acid using a glassy carbon electrode modified with multiwalled carbon nanotube/chitosan composite. Electroanalysis 2011, 23, 417–423. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.; Yu, A. Preparation of β-cyclodextrin functionalized reduced graphene oxide: Application for electrochemical determination of paracetamol. RSC Adv. 2015, 5, 76973–76978. [Google Scholar] [CrossRef]
- Tefera, M.; Geto, A.; Tessema, M.; Admassie, S. Simultaneous determination of caffeine and paracetamol by square wave voltammetry at poly(4-amino-3-hydroxynaphthalene sulfonic acid)-modified glassy carbon electrode. Food Chem. 2016, 210, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Baccarin, M.; Santos, F.A.; Vicentini, F.C.; Zucolotto, V.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical sensor based on reduced graphene oxide/carbon black/chitosan composite for the simultaneous determination of dopamine and paracetamol concentrations in urine samples. J. Electroanal. Chem. 2017, 799, 436–443. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Liu, L.; Zhang, J. Simultaneous electrochemical determination of paracetamol and diclofenac based on poly(diallyldimethylammonium chloride) functionalized graphene. Electroanalysis 2016, 28, 76–82. [Google Scholar] [CrossRef]
- Mao, A.; Li, H.; Jin, D.; Yu, L.; Hu, X. Fabrication of electrochemical sensor for paracetamol based on multi-walled carbon nanotubes and chitosan–copper complex by self-assembly technique. Talanta 2015, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tanuja, S.B.; Kumara Swamy, B.E.; Pai, K.V. Electrochemical determination of paracetamol in presence of folic acid at nevirapine modified carbon paste electrode: A cyclic voltammetric study. J. Electroanal. Chem. 2017, 798, 17–23. [Google Scholar] [CrossRef]

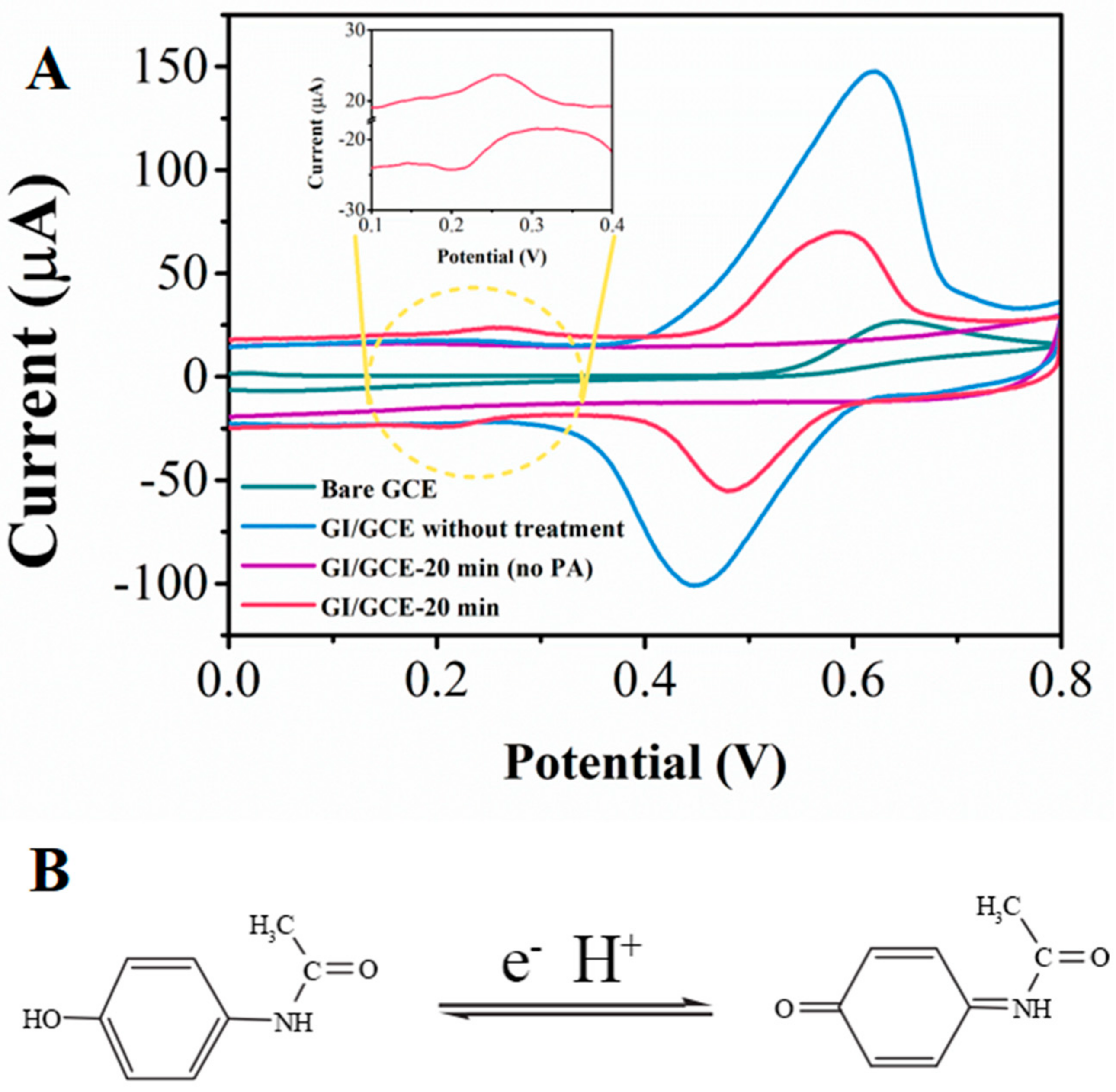
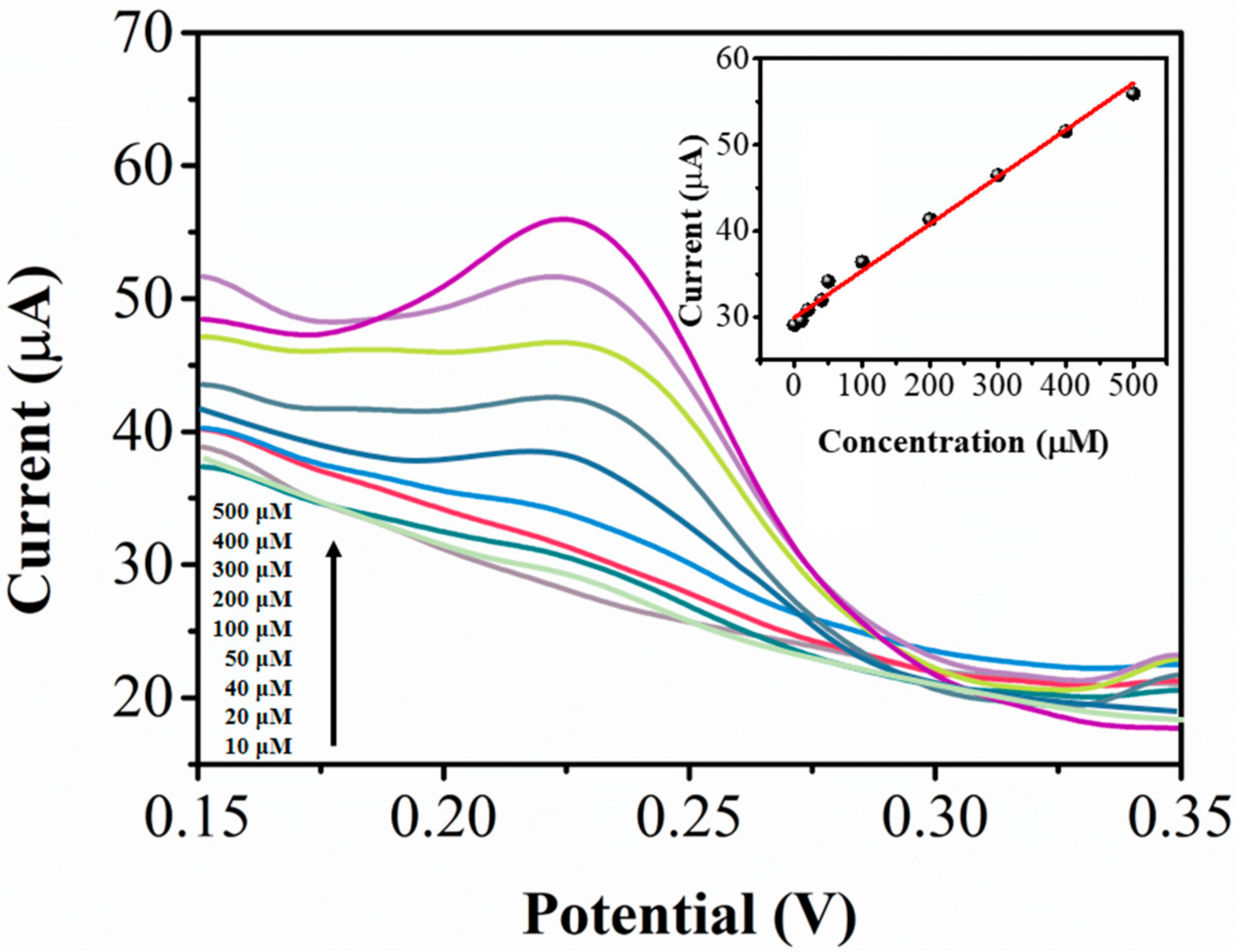
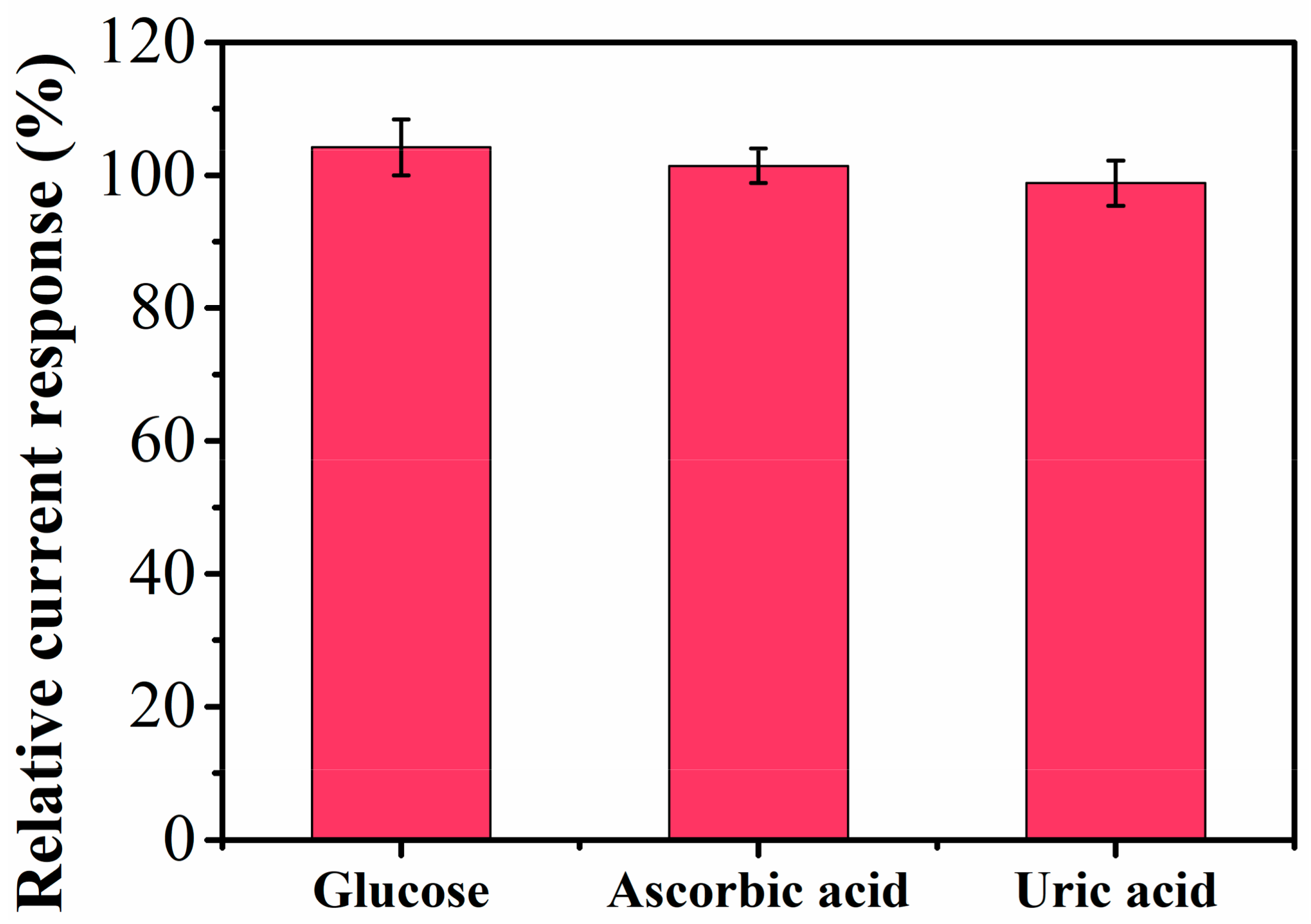
| Electrode | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|
| MWCNTs/GCE | 2–250 | 0.16 | [46] |
| β-cyclodextrin/RGO/GCE | 10–800 | 2.3 | [47] |
| Poly(AHNSA)/GCE | 10–125 | 0.45 | [48] |
| RGO/CB/CS/GCE | 2.8–190 | 0.053 | [49] |
| PDDA-graphene/GCE | 20–200 | 0.221 | [50] |
| MWCNTs/CTS–Cu/GCE | 0.1–200 | 0.024 | [51] |
| Nevirapine/CPE | 20–250 | 0.77 | [52] |
| GI/GCE | 10–500 | 2.7 | This work |
| Sample | Found (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 47.89 | 50 | 97.30 | 99.40 | 3.66 |
| 2 | 48.03 | 100 | 148.55 | 100.35 | 2.57 |
| 3 | 47.55 | 50 | 98.99 | 101.48 | 2.29 |
| 4 | 47.24 | 100 | 149.05 | 101.23 | 3.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Xie, K.; Zheng, Y.; Zhang, L.; Su, W. Graphene Ink Film Based Electrochemical Detector for Paracetamol Analysis. Electronics 2018, 7, 15. https://doi.org/10.3390/electronics7020015
Fu L, Xie K, Zheng Y, Zhang L, Su W. Graphene Ink Film Based Electrochemical Detector for Paracetamol Analysis. Electronics. 2018; 7(2):15. https://doi.org/10.3390/electronics7020015
Chicago/Turabian StyleFu, Li, Kefeng Xie, Yuhong Zheng, Luxi Zhang, and Weitao Su. 2018. "Graphene Ink Film Based Electrochemical Detector for Paracetamol Analysis" Electronics 7, no. 2: 15. https://doi.org/10.3390/electronics7020015




