Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes
Abstract
:1. Atherosclerosis
2. Flavonoids and Atherosclerosis
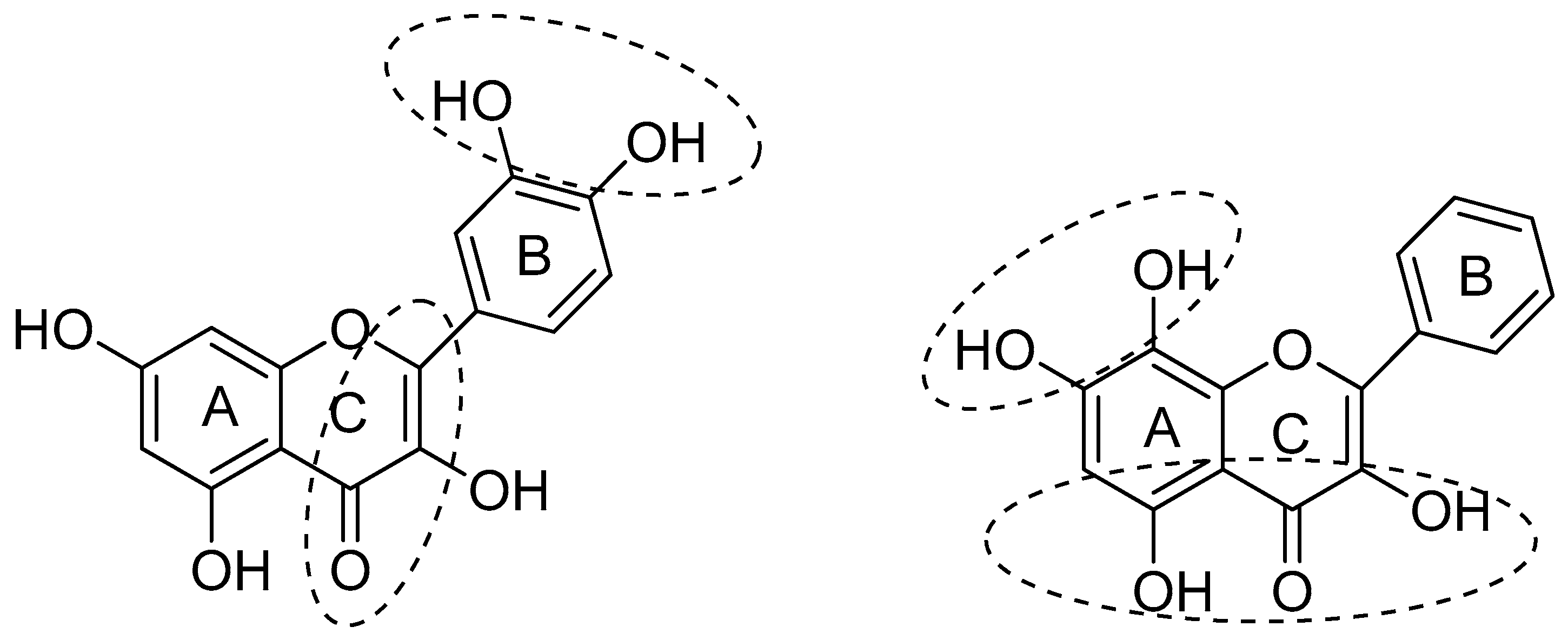
2.1. Direct Antioxidant Effects of Flavonoids
2.2. Indirect Antioxidant Effects of Flavonoids: The Involvment of ET-1 and NADPH Oxidase
2.3. Indirect Antioxidant Effects of Flavonoids: The Involvement of Myeloperoxidase (MPO) and HOCl Scavange
2.4. Flavonoids and Atherosclerosis: Clinical Studies
3. Flavonoids and Hypercholesterolemia
3.1. Flavonoids and Hypercholesterolemia: Effects on HMGCoA Reductase and LDL Receptors Expression
3.2. Flavonoids and Hypercholesterolemia: Effects on ACAT Proteins
3.3. Flavonoids and Cholesterol: Clinical Studies
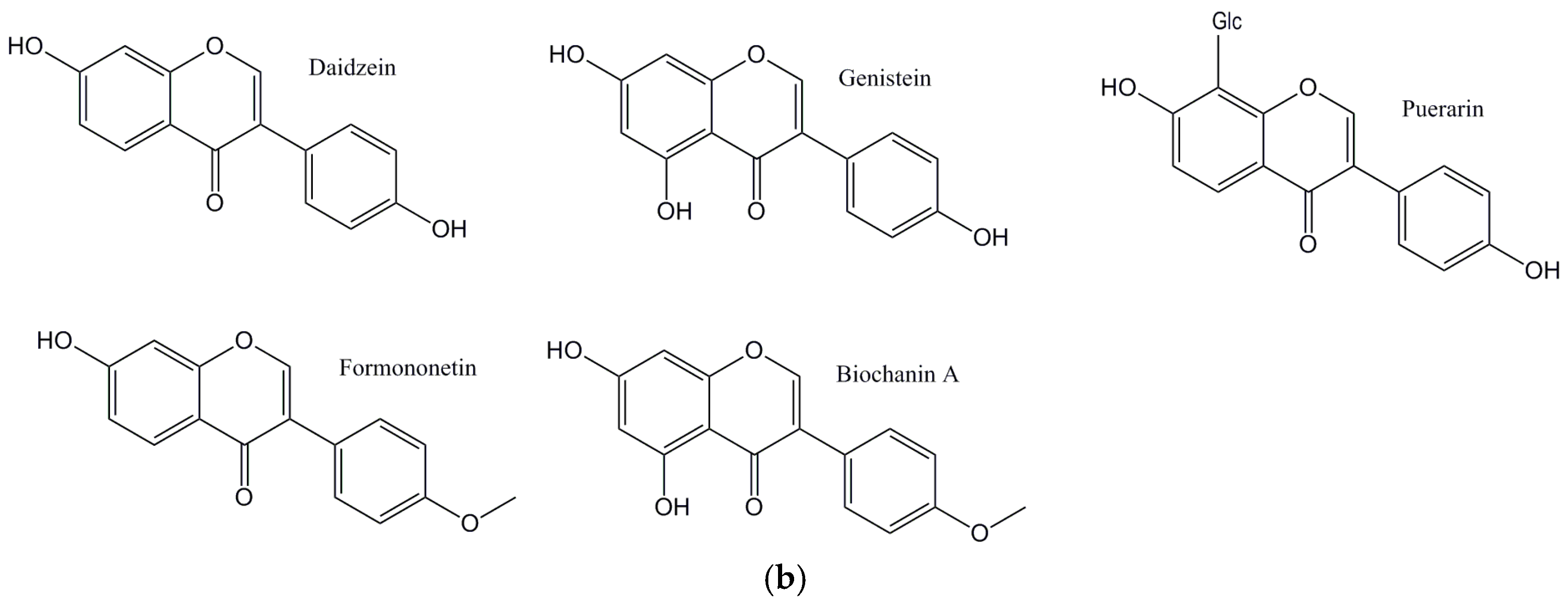
4. Flavonoids and Diabetes: A Snapshot
4.1. Flavonoids and Diabetes: Effects on α-glucosidase
4.2. Flavonoids and Diabetes: Effects on Kidney Function
4.3. Flavonoids and Diabetes: Effects on Pancreas and Insulin Secretion
4.4. Flavonoids and Diabetes: Clinical Studies
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tschudi, M.R.; Mesaros, S.; Luscher, T.F.; Malinski, T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries Increased decomposition by superoxide in hypertension. Hypertension 1996, 27, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NADPHoxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Berk, B.C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ. Res. 1992, 70, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Corda, S.; Zweier, J.L.; Capoggrossi, M.C.; Ziegelstein, R.C. Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation 1998, 97, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.L.; Avantaggiati, M.L.; Burgio, V.L.; Chirillo, P.; Collepardo, D.; Natoli, G.; Balsano, C.; Levrero, M. Reactive oxygen intermediates mediate angiotensin II-induced c-Jun, c-Fos heterodimer DNA binding activity and proliferative hypertrophic responses in myogenic cells. J. Biol. Chem. 1995, 270, 22129–22134. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Byron, K.L.; Samarel, A.M.; Bell, J.; Lucchesi, P.A. Hydrogen peroxide activates mitogen-activated protein kinases and Na1/H1 exchange in neonatal rat cardiac myocytes. Circ. Res. 1998, 82, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Alexander, R.W. Oxidative stress and cardiovascular disease. Circulation 1997, 96, 3264–3265. [Google Scholar] [PubMed]
- Fukai, T.; Ishiizaka, N.; Rajaggopalan, S.; Laursen, J.B.; Capers, Q.; Taylor, W.R.; Harrison, D.G.; de Leon, H.; Wilcox, J.N.; Griendling, K.K. p22phox mRNA expression and NADH/NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997, 80, 45–51. [Google Scholar]
- Abe, J.; Berk, B.C. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc. Med. 1998, 8, 59–64. [Google Scholar] [CrossRef]
- Laursen, J.B.; Rajagopalan, S.; Galis, Z.; Tarpet, M.; Freeman, B.A.; Harrison, D.G. Role of superoxide in angiotensin II–induced but not catecholamineinduced hypertension. Circulation 1997, 95, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Micucci, M.; Angeletti, A.; Cont, M.; Corazza, I.; Aldini, R.; Donadio, E.; Chiarini, A.; Budriesi, R. Hibiscus Sabdariffa L. Flowers and Olea Europea L. Leaves Extract-Based Formulation for Hypertension Care: In Vitro Efficacy and Toxicological Profile. J. Med. Food 2016, 19, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell. Longev. 2013, 2013, 471–790. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.L.; Joy, J.K.; Largent, R. Antioxidant and antiatherogenic effects of pomegranate. Am. J. Health Syst. Pharm. 2011, 68, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Courbon, D.; Leone, N.; Gariepy, J.; Tzourio, C.; Dartigues, J.F.; Barberger-Gateau, P.; Ritchie, K.; Alperovitch, A.; Amouyel, P.; et al. Tea consumption is inversely associated with carotid plaques in women. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Nurmi, T.; Tuomainen, T.P.; Ruusunen, A.; Salonen, J.T.; Voutilainen, S. The intake of flavonoids and carotid atherosclerosis: The Kuopio ischaemic heart disease risk factor study. Br. J. Nutr. 2007, 98, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Bousette, N.; Giaid, A. Endothelin-1 in atherosclerosis and other vasculopathies. Can. J. Physiol. Pharmacol. 2003, 81, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Cernacek, P.; Stewart, D.J.; Monge, J.C.; Rouleau, J.L. The endothelin system and its role in acute myocardial infarction. Can. J. Physiol. Pharmacol. 2003, 81, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.N.; Fiebeler, A.; Park, J.K.; Dechend, R.; Luft, F.C. Angiotensin II and endothelin induce inflammation and thereby promote hypertensioninduced end-organ damage. Clin. Nephrol. 2003, 60, S2–S12. [Google Scholar] [PubMed]
- Schiffrin, E.L. Vascular endothelin in hypertension. Vascul. Pharmacol. 2005, 43, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; McMullan, D.M.; Bekker, J.M.; Fineman, J.R.; Black, S.M. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ. Res. 2001, 89, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fink, G.D.; Watts, S.W. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 2003, 107, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Loomis, E.D.; Sullivan, J.C.; Osmond, D.A.; Pollock, D.M.; Pollock, J.S. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J. Pharmacol. Exp. Ther. 2005, 315, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Lyle, A.N.; Griendling, K.K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 2006, 21, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Ushio-Fukai, M.; Lassègue, B.; Alexander, R.W. Angiotensin II signaling in vascular smooth muscle: New concepts. Hypertension 1997, 29, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Corson, M.A. Angiotensin II signal transduction in vascular smooth muscle: Role of tyrosine kinases. Circ. Res. 1997, 80, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Irani, K. A Review of the Roles of Reactive Oxygen Species in Smooth Muscle and Endothelial Cell Mitogenic and Apoptotic Signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Baas, A.S.; Berk, B.C. Differential activation of mitogen-activated protein kinases by H2O2 and O2 in vascular smooth muscle cells. Circ. Res. 1995, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Zafari, A.M.; Fukui, T.; Ishizaka, N.; Griendling, K.K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996, 271, 23317–23321. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Shah, A. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J. Biol. Chem. 2003, 278, 12094–12100. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Yao, G.; Schiffrin, E.L. c-Src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.A.; de Champlain, J. The interrelation of the angiotensin and endothelin systems on the modulation of NAD(P)H oxidase. Can. J. Physiol. Pharmacol. 2006, 84, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Wendt, M.; Szöcs, K.; Lassègue, B.; Schulz, E.; Oelze, M.; Tsilimingas, N. Effects of angiotensin II infusion on the expression and function of NAD (P) H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002, 90, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; Sorescu, D.; Szöcs, K.; Yin, Q.; Akers, M.; Zhang, Y.; Griendling, K.K. Novel gp91phox homologues in vascular smooth muscle cells. Circ. Res. 2001, 88, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Angeles Ocete, M.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Ross Watson, R.; Preedy, V.R. Bioactive Food as Dietary Interventions for Cardiovascular Disease: Bioactive Foods in Chronic Disease; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Jimenez, R.; Moreno, L.; Kroon, P.A.; Needs, P.W.; Hughes, D.A.; Duarte, J. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis 2009, 204, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Machha, A.; Mustafa, M.R. Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Forster, H.; Epstein, M. Protein kinase C and calcium channel activation as determinants of renal vasoconstriction by angiotensin II and endothelin. Circ. Res. 1993, 73, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Steffen, Y.; Schewe, T.; Sies, H. Myeloperoxidase-mediated LDL oxidation and endothelial cell toxicity of oxidized LDL: Attenuation by (−)-epicatechin. Free Radic. Res. 2006, 40, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Jung, T.; Klotz, L.O.; Schewe, T.; Grune, T.; Sies, H. Protein modification elicited by oxidized low-density lipoprotein (LDL) in endothelial cells: Protection by (–)-epicatechin. Free Radic. Biol. Med. 2007, 42, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Natsume, M.; Osakabe, N.; Oyama, M.; Sasaki, M.; Baba, S.; Nakamura, Y.; Terao, J. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: Differences between human and rat. Free Radic. Biol. Med. 2003, 34, 840–849. [Google Scholar] [CrossRef]
- Steffen, Y.; Schewe, T.; Sies, H. (–)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007, 359, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Kawai, Y. Flavonoids as substrates and inhibitors of myeloperoxidase: Molecular actions of aglycone and metabolites. Chem. Res. Toxicol. 2008, 21, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L.; Heinecke, J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Investig. 1997, 99, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; McHowat, J.; Hsu, F.F.; Brennan, M.L.; Hazen, S.L.; Ford, D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Kiyokawa, H.; Kimura, Y.; Kato, Y.; Tsuchiya, K.; Terao, J. Hypochlorous acid-derived modification of phospholipids: Characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry 2006, 45, 14201–14211. [Google Scholar] [CrossRef] [PubMed]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Geary, R.L. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef] [PubMed]
- Dillard, A.; Matthan, N.R.; Lichtenstein, A.H. Use of hamster as a model to study diet-induced atherosclerosis. Nutr. Metab. 2010, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Binsack, R.; Boersma, B.J.; Patel, R.P.; Kirk, M.; White, C.R.; Darley-Usmar, V.; Parks, D.A. Enhanced antioxidant activity after chlorination of quercetin by hypochlorous acid. Alcohol. Clin. Exp. Res. 2001, 25, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agric. 2006, 86, 2492–2498. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008, 28, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Yamamoto, H.; Uematsu, M.; Itoh, T.; Nakagawa, K.; Miyazawa, T.; Kangawa, K.; Miyatake, K. Green tea reverses endothelial dysfunction in healthy smokers. Heart 2004, 90, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jeong, M.H.; Cho, S.H.; Yun, J.H.; Chae, H.J.; Ahn, Y.K.; Lee, M.C.; Cheng, X.; Kondo, T.; Kang, J.C.; et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circ. J. 2006, 70, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.C.; Kim, H.S.; Jeong, T.S.; Bok, S.H.; Park, Y.B. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J. Cardiovasc. Pharmacol. 2001, 38, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E–knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J. Clin. Investig. 1983, 72, 743. [Google Scholar] [CrossRef] [PubMed]
- Diepeveen, S.H.; Wetzels, J.F.; Bilo, H.J.; Van Tits, L.J.H.; Stalenhoef, A.F.H. Cholesterol in end-stage renal disease: The good, the bad or the ugly. Neth. J. Med. 2008, 66, 53–61. [Google Scholar] [PubMed]
- Chen, Z.Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.I.; Chroni, A.; Krieger, M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 2006, 84, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Kuivenhoven, J.A.; Pritchard, H.; Hill, J.; Frohlich, J.; Assmann, G.; Kastelein, J. The molecular pathology of lecithin: Cholesterol acyltransferase (LCAT) deficiency syndromes. J. Lipid Res. 1997, 38, 191–205. [Google Scholar] [PubMed]
- Sikorski, J.A. Oral cholesteryl ester transfer protein (CETP) inhibitors: A potential new approach for treating coronary artery disease. J. Med. Chem. 2006, 49, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K.; Kearney, D.M.; Hobbs, H.H. Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J. Lipid Res. 1999, 40, 1384–1394. [Google Scholar] [PubMed]
- Soutar, A.K.; Naoumova, R.P. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Largis, E.E.; Wang, C.H.; DeVries, V.G.; Schaffer, S.A. CL 277,082: A novel inhibitor of ACAT-catalyzed cholesterol esterification and cholesterol absorption. J. Lipid Res. 1989, 30, 681–690. [Google Scholar] [PubMed]
- Prince, P.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- You, C.L.; Su, C.L.; Zhou, C.L. Study on Effect and mechanisms of Scutellaria baicalensis stem-leaf total flavonoid in regulating lipid metabolism. China J. Chin. Mater. Med. 2008, 33, 1064–1066. [Google Scholar]
- Borradaile, N.M.; Wilcox, L.J.; Edwards, J.Y.; Murray, W.H. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem. J. 2002, 366, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Whitehead, S.A. Phytoestrogens oestrogen synthesis and breast cancer. J. Steroid Biochem. Mol. Biol. 2008, 108, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Renouf, M.; Ye, Z.; Murphy, P.A.; Hendrich, S. Isoflavone glycitein diminished plasma cholesterol in female golden Syrian hamsters. J. Agric. Food Chem. 2007, 55, 11063–11067. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.A.; Sutherland, P.; Wang, S.A.; Chait, A.; LeBoeuf, R.C. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor–deficient mice. J. Nutr. 1998, 128, 954–959. [Google Scholar] [PubMed]
- Leopoldini, M.; Malaj, N.; Toscano, M.; Sindona, G.; Russo, N. On the inhibitor effects of bergamot juice flavonoids binding to the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) enzyme. J. Agric. Food Chem. 2010, 58, 10768–10773. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Sharma, C.; Adem, A.; Aburawi, E.; Ojha, S. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug Des. Dev. Ther. 2015, 9, 4943–4951. [Google Scholar] [PubMed]
- Crouse, J.R.; Morgan, T.; Terry, J.G.; Ellis, J.; Vitolins, M.; Burke, G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999, 159, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Newell, K.A.; Cherin, R.; Haskell, W.L. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 728–735. [Google Scholar] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Jackson, C.J.C.; Connelly, P.W.; Parker, T.; Faulkner, D.; Josse, R.G. Effects of high-and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am. J. Clin. Nutr. 2002, 76, 365–372. [Google Scholar] [PubMed]
- Lichtenstein, A.H.; Jalbert, S.M.; Adlercreutz, H.; Goldin, B.R.; Rasmussen, H.; Schaefer, E.J.; Ausman, L.M. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Merz-Demlow, B.E.; Duncan, A.M.; Wangen, K.E.; Xu, X.; Carr, T.P.; Phipps, W.R.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am. J. Clin. Nutr. 2000, 71, 1462–1469. [Google Scholar] [PubMed]
- Wangen, K.E.; Duncan, A.M.; Xu, X.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 225–231. [Google Scholar] [PubMed]
- Steinberg, F.M.; Guthrie, N.L.; Villablanca, A.C.; Kumar, K.; Murray, M.J. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am. J. Clin. Nutr. 2003, 78, 123–130. [Google Scholar] [PubMed]
- Zhuo, X.G.; Melby, M.K.; Watanabe, S. Soy isoflavone intake lowers serum LDL cholesterol: A meta-analysis of 8 randomized controlled trials in humans. J. Nutr. 2004, 134, 2395–2400. [Google Scholar] [PubMed]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [PubMed]
- Wildman, R.E.C. Handbook of Nutraceuticals and Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kuhn, D.J.; Burns, A.C.; Kazi, A.; Dou, Q.P. Direct inhibition of the ubiquitin–proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2004, 1682, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.; Roach, P.D.; Bottema, C.D.; Pal, S. Green tea upregulates the low-density lipoprotein receptor through the sterol-regulated element binding protein in HepG2 liver cells. J. Agric. Food Chem. 2001, 49, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Roach, P.D. Modulation of cholesterol metabolism by the green tea polyphenol (−)-epigallocatechin gallate in cultured human liver (HepG2) cells. J. Agric. Food Chem. 2006, 54, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Roach, P.D. A green tea catechin extract upregulates the hepatic low-density lipoprotein receptor in rats. Lipids 2007, 42, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Abbey, M.; Roach, P.D. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 2007, 193, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Fong, W.P.; Cheung, Y.L.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J. Nutr. 1999, 129, 1094–1101. [Google Scholar] [PubMed]
- Yang, T.T.; Koo, M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 1999, 66, 411–423. [Google Scholar] [CrossRef]
- Maron, D.J.; Lu, G.P.; Cai, N.S.; Wu, Z.G.; Li, Y.H.; Chen, H.; Zhao, J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Van het Hof, K.H.; De Boer, H.S.; Wiseman, S.A.; Lien, N.; Westrate, J.A.; Tijburg, L.B. Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 1997, 66, 1125–1132. [Google Scholar] [PubMed]
- Monforte, M.T.; Trovato, A.; Kirjavainen, S.; Forestieri, A.M.; Galati, E.M.; Lo, C.R. Biological effects of hesperidin, a Citrus flavonoid.(note II): Hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco (Soc. Chim. Ital. 1989) 1995, 50, 595–599. [Google Scholar]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Vigil Portales, P. Delphinol® standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical trial. Panminerva. Med. 2016, 58 (Suppl. S1), 1–6. [Google Scholar] [PubMed]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Rho, M.C.; Lee, S.W.; Choi, J.N.; Kim, K.; Song, G.Y.; Kim, Y.K. Bavachin and isobavachalcone, acyl-coenzyme A: Cholesterol acyltransferase inhibitors from Psoralea corylifolia. Arch. Pharm. Res. 2008, 31, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Rho, M.C.; Lee, S.W.; Kwon, O.E.; Park, H.R.; Kang, J.Y.; Kim, Y.K. Glabrol, an acyl-coenzyme A: Cholesterol acyltransferase inhibitor from licorice roots. J. Ethnopharmacol. 2007, 110, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Zamansoltani, F.; Nassiri-Asl, M.; Abbasi, E. Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin. Pharmacol. Toxicol. 2009, 104, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.H.; Lee, S.H.; Park, Y.B.; Bae, K.H.; Son, K.H.; Jeong, T.S.; Choi, M.S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar] [PubMed]
- Wilcox, L.J.; Borradaile, N.M.; de Dreu, L.E.; Huff, M.W. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J. Lipid Res. 2001, 42, 725–734. [Google Scholar] [PubMed]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeon, S.M.; Lee, M.K.; Cho, Y.Y.; Kwon, E.Y.; Lee, J.H.; Choi, M.S. Comparison of hesperetin and its metabolites for cholesterol-lowering and antioxidative efficacy in hypercholesterolemic hamsters. J. Med. Food 2010, 13, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, E.J.; Cook, N.R.; Manson, J.E.; Moorthy, M.V.; Buring, J.E.; Rimm, E.B.; Lee, I.M. Strength Training and the Risk of Type 2 Diabetes and Cardiovascular Disease. Med. Sci. Sports Exerc. 2017, 49, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Khavandi, K.; Khavandi, A.; Asghar, O.; Greenstein, A.; Withers, S.; Heagerty, A.M.; Malik, R.A. Diabetic cardiomyopathy—A distinct disease? Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Wachter, R. Impact of diabetes and hypertension on the heart. Curr. Opin. Cardiol. 2008, 23, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Stump, C.S. Insights into the biology of diabetic vascular disease: What’s new? Am. J. Hypertens. 2004, 17, 2S–6S. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, R. Epidemiology of diabetes. Adv. Drug Deliv. Rev. 2002, 54, 1165–1172. [Google Scholar] [CrossRef]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Ishizuka, M.; Terasawa, M.; Wu, J.B.; Sasaoka, T.; Kimura, I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabu, M.C.; Smitha, K.; Kuttan, R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar] [PubMed]
- MacKenzie, T.; Leary, L.; Brooks, W.B. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: Double-blind randomized study. Metabolism 2007, 56, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Blade, M.C.; Salvado, M.J.; Arola, L.; Ardevol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006, 79, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Jeong, J.C. Anti-adipogenic effect of kaempferol, a component of Polygonati rhizoma. J. Korean Oriental Med. 2010, 31, 158–166. [Google Scholar]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [PubMed]
- Hanamura, T.; Mayama, C.; Aoki, H.; Hirayama, Y.; Shimizu, M. Antihyperglycemic effect of polyphenols from Acerola (Malpighia emarginata DC.) fruit. Biosci. Biotechnol. Biochem. 2006, 70, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kobayashi, M.; Hayashida, S.; Matsumoto, K. Luteolin, a flavone, does not suppress postprandial glucose absorption through an inhibition of α-glucosidase action. Biosci. Biotechnol. Biochem. 2002, 66, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Velussi, M.; Cernigoi, A.M.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (23 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T.; Oya, T.; Okubo, T.; Juneja, L.R. Green tea polyphenols and dietary fibre protect against kidney damage in rats with diabetic nephropathy. J. Pharm. Pharmacol. 2005, 57, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.P.; Gu, Z.L. Puerarin reduces increased c-fos, c-jun, and type IV collagen expression caused by high glucose in glomerular mesangial cells. Acta Pharmacol. Sin. 2005, 26, 982. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, H.J.; Pouli, A.E.; Ainscow, E.K.; Jouaville, L.S.; Rizzuto, R.; Rutter, G.A. Glucose Generates Sub-plasma Membrane ATP Microdomains in Single Islet β-Cells potential role for strategically located mitochondria. J. Biol. Chem. 1999, 274, 13281–13291. [Google Scholar] [CrossRef] [PubMed]
- El Latif, M.A.A.; Mohamed, N.H.; Zaki, N.L.; Abbas, M.S.; Sobhy, H.M. Effects of Soybean Isoflavone on Lipid Profiles and Antioxidant Enzyme Activity in Streptozotocin Induced Diabetic Rats. Glob. J. Pharmacol. 2014, 8, 378–384. [Google Scholar]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Roghani, M.; Baluchnejadmojarad, T. Hypoglycemic and hypolipidemic effect and antioxidant activity of chronic epigallocatechin-gallate in streptozotocin-diabetic rats. Pathophysiology 2010, 17, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.C.; He, H.; Wei, X.; Ge, S.; Lu, Y.H. Comparison of Regulation Mechanisms of Five Mulberry Ingredients on Insulin Secretion under Oxidative Stress. J. Agric. Food Chem. 2016, 64, 8763–8772. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Tian, W.; Huan, M.; Chen, J.; Fu, H. Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med. (Maywood) 2017, 242, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kittl, M.; Beyreis, M.; Tumurkhuu, M.; Fürst, J.; Helm, K.; Pitschmann, A.; Gaisberger, M.; Glasl, S.; Ritter, M.; Jakab, M. Quercetin Stimulates Insulin Secretion and Reduces the Viability of Rat INS-1 Beta-Cells. Cell. Physiol. Biochem. 2016, 39, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zeka, K.; Arroo, R.R.J. Saffron Crocus (Crocus sativus L.) as a Source of Kaempferol. In Kaempferol: Biosynthesis, Food Sources and Therapeutic Uses; Garde-Cerdán, T., Gonzalo-Diago, A., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2016; pp. 197–215. [Google Scholar]
- Zeka, K.; Ruparelia, K.C.; Wilson, P.B.; Sousa, M.C.; Juma, N.; Desai, U.; Grootveld, M.; Arroo, R.J. Determination of Heavy Metals Present in the Hypoglycemic Karela Powder: An Analytical Assay. EC Pharmacol. Toxicol. 2017, 4, 4–11. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeka, K.; Ruparelia, K.; Arroo, R.R.J.; Budriesi, R.; Micucci, M. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases 2017, 5, 19. https://doi.org/10.3390/diseases5030019
Zeka K, Ruparelia K, Arroo RRJ, Budriesi R, Micucci M. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases. 2017; 5(3):19. https://doi.org/10.3390/diseases5030019
Chicago/Turabian StyleZeka, Keti, Ketan Ruparelia, Randolph R. J. Arroo, Roberta Budriesi, and Matteo Micucci. 2017. "Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes" Diseases 5, no. 3: 19. https://doi.org/10.3390/diseases5030019





