The Brain–Intestinal Mucosa–Appendix– Microbiome–Brain Loop
Abstract
:1. Commentary
1.1. Macrophages and Intestinal Tissue
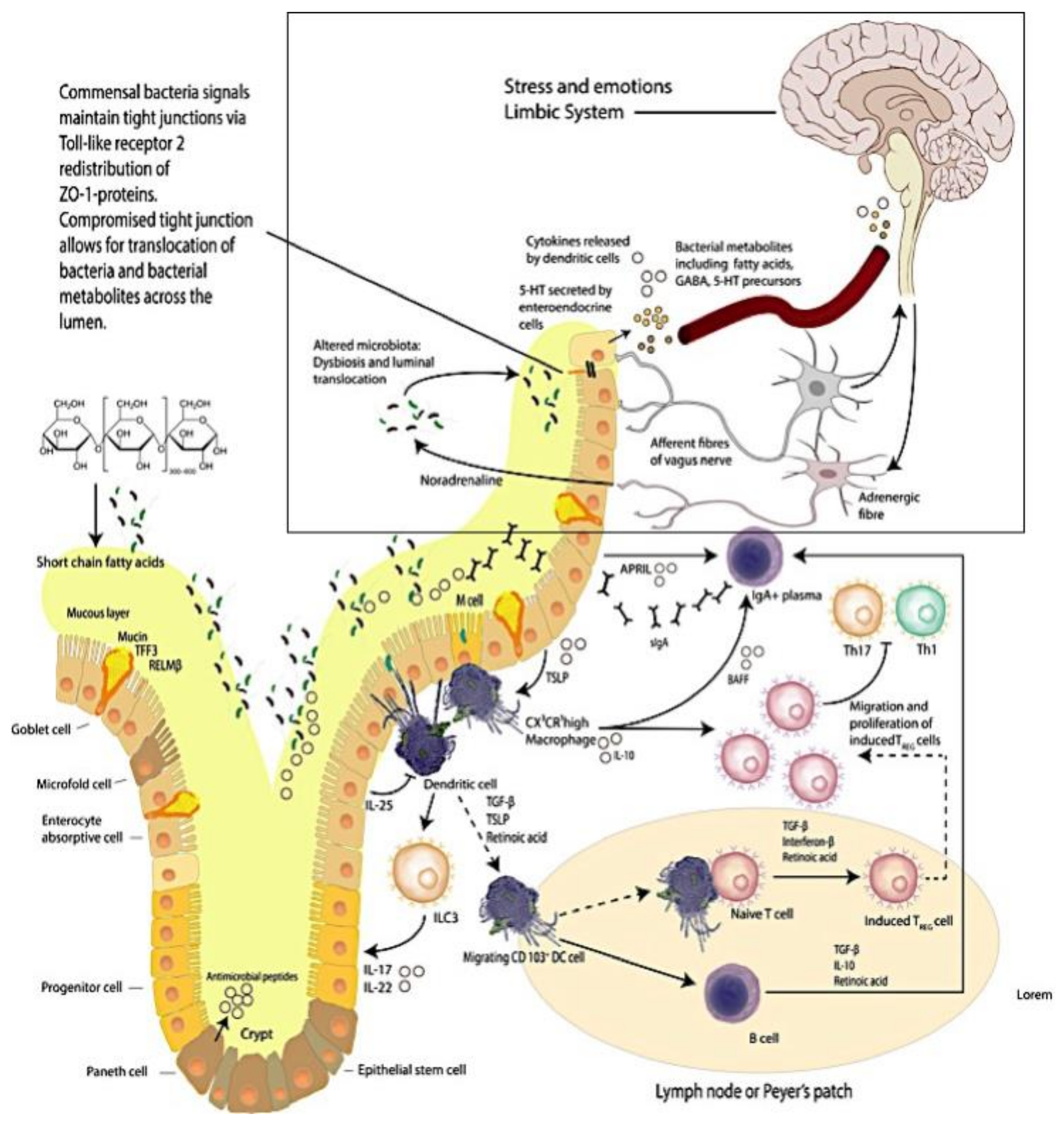
1.2. The Brain–Intestinal Microbiome/Epithelia–Mucosa–Brain Loop
1.3. The Vermiform Appendix
1.4. Probiotics
2. Reprise
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, M.L.; Solnica-Krezel, L. Regulation of gastrulation movements by emergent cell and tissue interactions. Curr. Opin. Cell Biol. 2017, 48, 33–39. [Google Scholar] [CrossRef] [PubMed]
- De Santa Barbara, P.; van den Brink, G.R.; Roberts, D.J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. CMLS 2003, 60, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Garay, P.A.; McAllister, A.K. Novel roles for immune molecules in neural development: Implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2010, 2, 136. [Google Scholar] [CrossRef] [PubMed]
- Lantz, O.; Legoux, F. Mait cells: An historical and evolutionary perspective. Immunol. Cell Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Napier, R.J.; Adams, E.J.; Gold, M.C.; Lewinsohn, D.M. The role of mucosal associated invariant t cells in antimicrobial immunity. Front. Immunol. 2015, 6, 344. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.B.; Ndung’u, T.; Kasprowicz, V.O. The role of mucosal-associated invariant t cells in infectious diseases. Immunology 2017, 150, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Caricilli, A.M.; Castoldi, A.; Camara, N.O. Intestinal barrier: A gentlemen’s agreement between microbiota and immunity. World J. Gastrointest. Pathophysiol. 2014, 5, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflug. Arch. Eur. J. Physiol. 2017, 469, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P. Host-pathogen interactions: The seduction of molecular cross talk. Gut 2002, 50 (Suppl. 3), iii2–iii8. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Saurer, L.; Mueller, C. Intestinal macrophages: Differentiation and involvement in intestinal immunopathologies. Semin. Immunopathol. 2009, 31, 171–184. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, S.; Stakenborg, N.; Matteoli, G.; Verheijden, S.; Boeckxstaens, G.E. Muscularis macrophages: Key players in intestinal homeostasis and disease. Cell. Immunol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Kreiner, G.; Nalepa, I. Macrophages and depression—A misalliance or well-arranged marriage? Pharmacol. Rep. 2013, 65, 1663–1672. [Google Scholar] [CrossRef]
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2015, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.L.; Solomon, B.D.; Hsieh, C.S. T-cell selection and intestinal homeostasis. Immunol. Rev. 2014, 259, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Kurts, C. The debate about dendritic cells and macrophages in the kidney. Front. Immunol. 2015, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A. Macrophages as apc and the dendritic cell myth. J. Immunol. 2008, 181, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Ueda, Y.; Sawa, Y.; Jeon, S.G.; Ma, J.S.; Okumura, R.; Kubo, A.; Ishii, M.; Okazaki, T.; Murakami, M.; et al. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent t-cell-dependent colitis. Proc. Natl. Acad. Sci. USA 2012, 109, 5010–5015. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar] [PubMed]
- Zheng, S.G. Regulatory T cells vs Th17: Differentiation of Th17 versus treg, are the mutually exclusive? Am. J. Clin. Exp. Immunol. 2013, 2, 94–106. [Google Scholar] [PubMed]
- Wahl, S.M. Transforming growth factor-beta: Innately bipolar. Curr. Opin. Immunol. 2007, 19, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Oda, C.; Udayanga, K.G.; Nakamura, Y.; Nakazawa, Y.; Totsuka, N.; Miki, H.; Iino, S.; Tahara-Hanaoka, S.; Honda, S.; Shibuya, K.; et al. Apoptotic epithelial cells control the abundance of treg cells at barrier surfaces. Nat. Immunol. 2016, 17, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Nakamura, K.; Ihara, E.; Akiho, H.; Takayanagi, R. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig. Dis. Sci. 2011, 56, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Benoist, C.; Bluestone, J.A.; Campbell, D.J.; Ghosh, S.; Hori, S.; Jiang, S.; Kuchroo, V.K.; Mathis, D.; Roncarolo, M.G.; et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat. Immunol. 2013, 14, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Essential exercise: Physical and mental health “inextricably” linked. Major disease prevention is shown to be an additional benefit. DukeMed. Healthnews 2010, 16, 4–5.
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M.; Geffard, M.; Bosmans, E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [PubMed]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Kohler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Cl. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Bambling, M.; Alford, H. The gastrointestinal tract microbiome, probiotics, and mood. Inflammopharmacology 2014, 22, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota's effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Wakeley, C.P. The position of the vermiform appendix as ascertained by an analysis of 10,000 cases. J. Anat. 1933, 67, 277–283. [Google Scholar] [PubMed]
- Gebbers, J.O.; Laissue, J.A. Bacterial translocation in the normal human appendix parallels the development of the local immune system. Ann. N. Y. Acad. Sci. 2004, 1029, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Im, G.Y.; Modayil, R.J.; Lin, C.T.; Geier, S.J.; Katz, D.S.; Feuerman, M.; Grendell, J.H. The appendix may protect against clostridium difficile recurrence. Clin. Gastroenterol. Hepatol. 2011, 9, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Van Wey, A.S.; Cookson, A.L.; Roy, N.C.; McNabb, W.C.; Soboleva, T.K.; Shorten, P.R. Bacterial biofilms associated with food particles in the human large bowel. Mol. Nutr. Food Res. 2011, 55, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Kooij, I.A.; Sahami, S.; Meijer, S.L.; Buskens, C.J.; Te Velde, A.A. The immunology of the vermiform appendix: A review of the literature. Clin. Exp. Immunol. 2016, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Angenent, L.T.; Gordon, J.I. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 2004, 5, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, R.R.; Everett, M.L.; Wahl, S.D.; Lee, Y.H.; Orndorff, P.E.; Parker, W. Secretory iga and mucin-mediated biofilm formation by environmental strains of escherichia coli: Role of type 1 pili. Mol. Immunol. 2006, 43, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nature reviews. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bazar, K.A.; Lee, P.Y.; Joon Yun, A. An “eye” in the gut: The appendix as a sentinel sensory organ of the immune intelligence network. Med. Hypotheses 2004, 63, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE 2012, 7, e41009. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.A.; Simmons, K.; Norman, E. Bacterial flora of the appendix fossa in appendicitis and postoperative wound infection. J. Clin. Pathol. 1974, 27, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Tadrous, A.; Fouhy, F.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Andrews, E.; Cotter, P.D.; Stanton, C.; Ross, R.P. Microbial composition of human appendices from patients following appendectomy. MBio 2013, 4, e00366-12. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Chen, W.T.; Muo, C.H.; Ke, T.W.; Fang, C.W.; Sung, F.C. Association between appendectomy and subsequent colorectal cancer development: An asian population study. PLoS ONE 2015, 10, e0118411. [Google Scholar] [CrossRef] [PubMed]
- Enblad, M.; Birgisson, H.; Ekbom, A.; Sandin, F.; Graf, W. Increased incidence of bowel cancer after non-surgical treatment of appendicitis. Eur. J. Surg. Oncol. 2017, 43, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Canton, G.; Santonastaso, P.; Fraccon, I.G. Life events, abnormal illness behavior, and appendectomy. Gen. Hosp. Psychiatry 1984, 6, 191–195. [Google Scholar] [CrossRef]
- Beaurepaire, J.E.; Jones, M.; Eckstein, R.P.; Smith, R.C.; Piper, D.W.; Tennant, C. The acute appendicitis syndrome: Psychological aspects of the inflamed and non-inflamed appendix. J. Psychosom. Res. 1992, 36, 425–437. [Google Scholar] [CrossRef]
- Creed, F. Life events and appendicectomy. Lancet 1981, 1, 1381–1385. [Google Scholar] [CrossRef]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small ssri resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lemme-Dumit, J.M.; Polti, M.A.; Perdigon, G.; Galdeano, C.M. Probiotic bacteria cell walls stimulate the activity of the intestinal epithelial cells and macrophage functionality. Benefic. Microbes 2018, 9, 153–164. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Vitetta, G.; Hall, S. The Brain–Intestinal Mucosa–Appendix– Microbiome–Brain Loop. Diseases 2018, 6, 23. https://doi.org/10.3390/diseases6020023
Vitetta L, Vitetta G, Hall S. The Brain–Intestinal Mucosa–Appendix– Microbiome–Brain Loop. Diseases. 2018; 6(2):23. https://doi.org/10.3390/diseases6020023
Chicago/Turabian StyleVitetta, Luis, Gemma Vitetta, and Sean Hall. 2018. "The Brain–Intestinal Mucosa–Appendix– Microbiome–Brain Loop" Diseases 6, no. 2: 23. https://doi.org/10.3390/diseases6020023




