Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability
Abstract
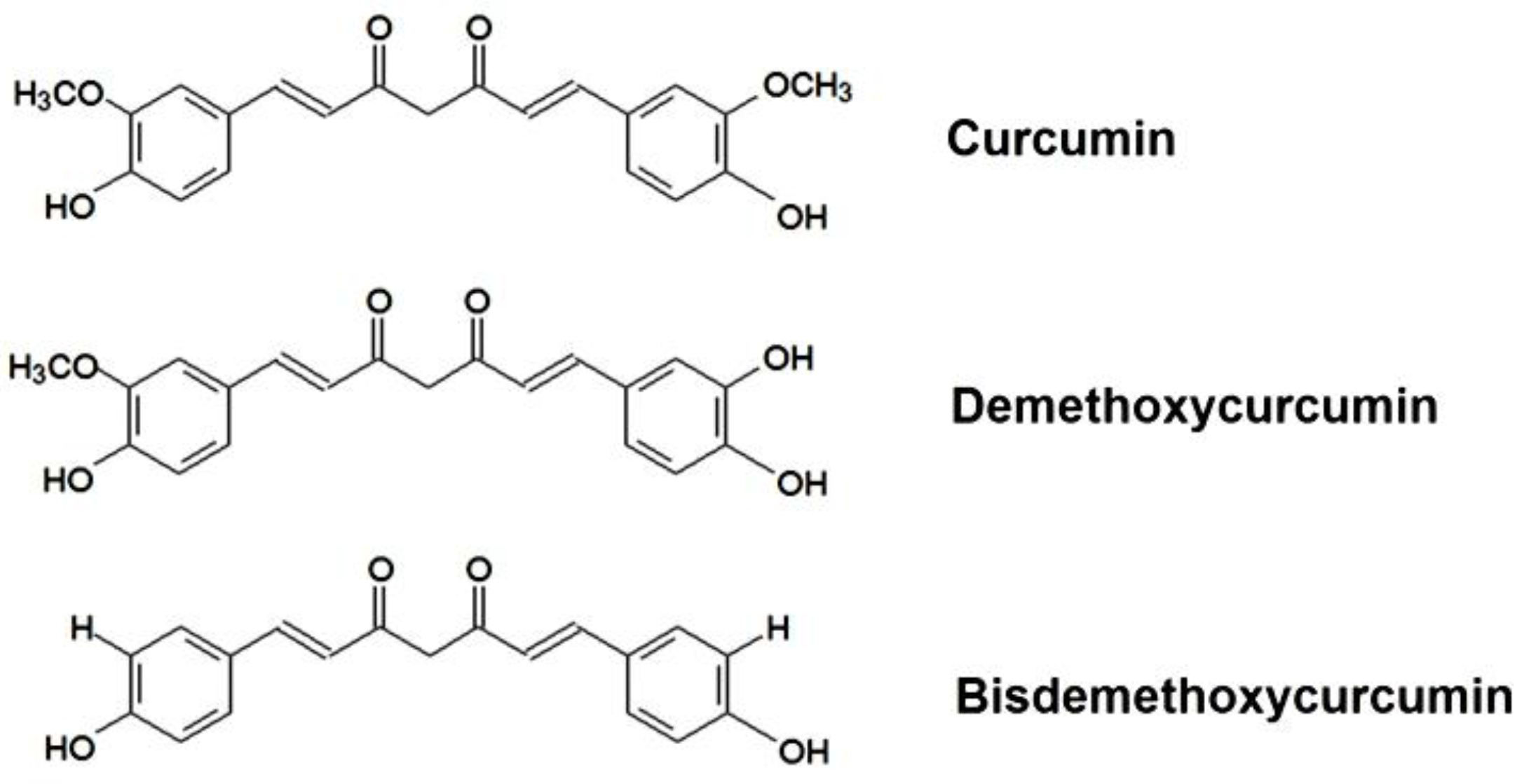
:1. Introduction
2. Materials and Methods
2.1. Chemicals
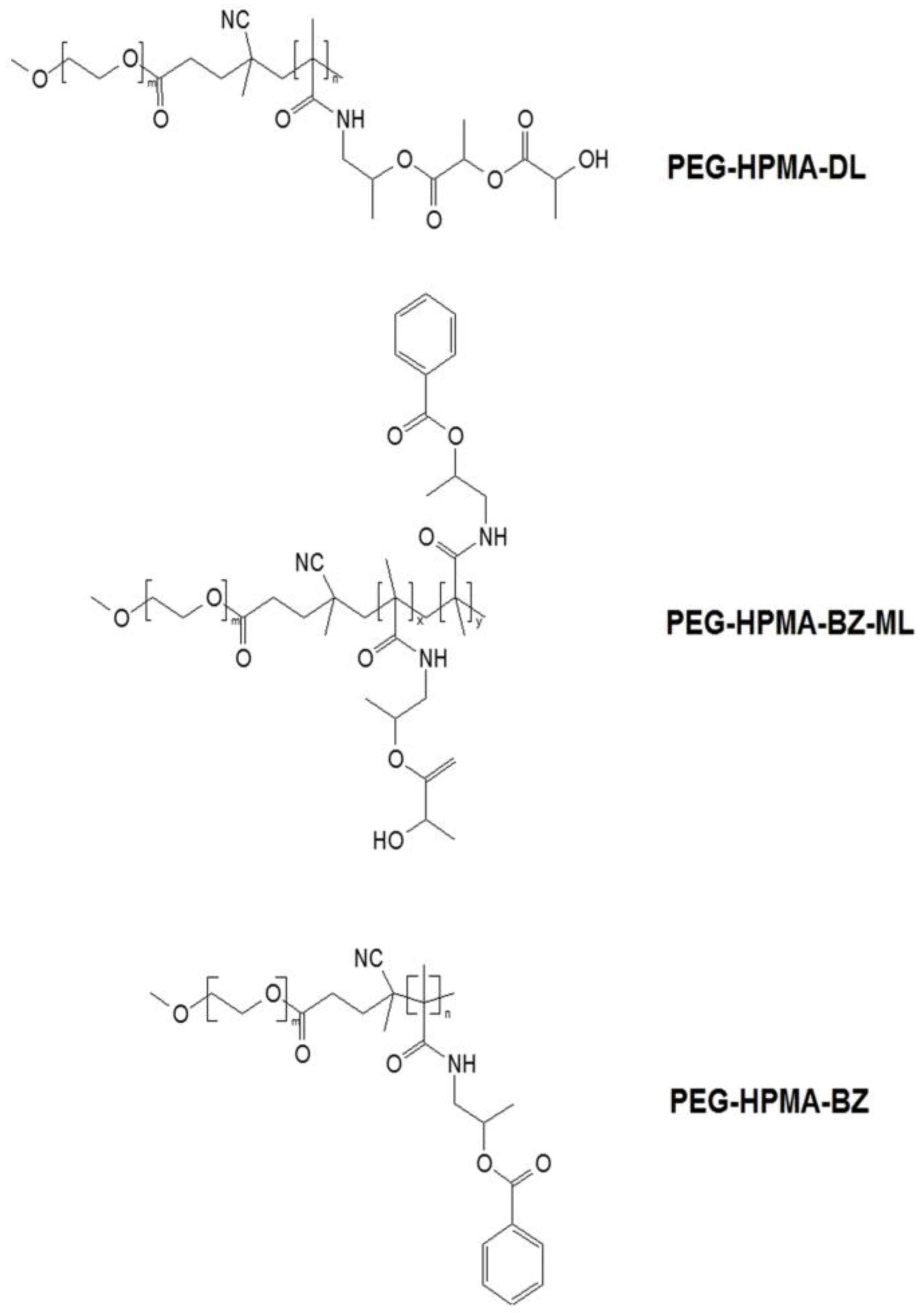
2.2. Preparation and Characterization of Cur-Nano
2.3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
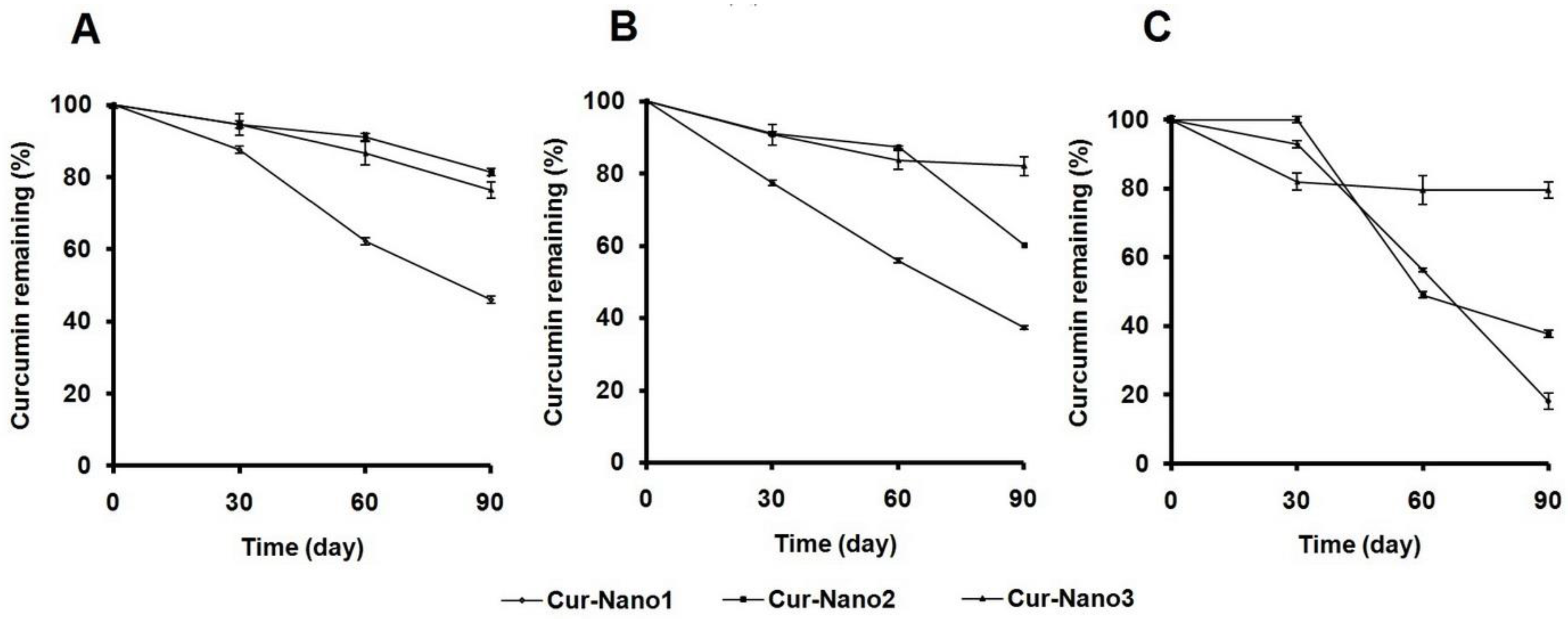
2.4. Stability Test under Storage Conditions
2.5. Particle Size Determination of Cur-Nano
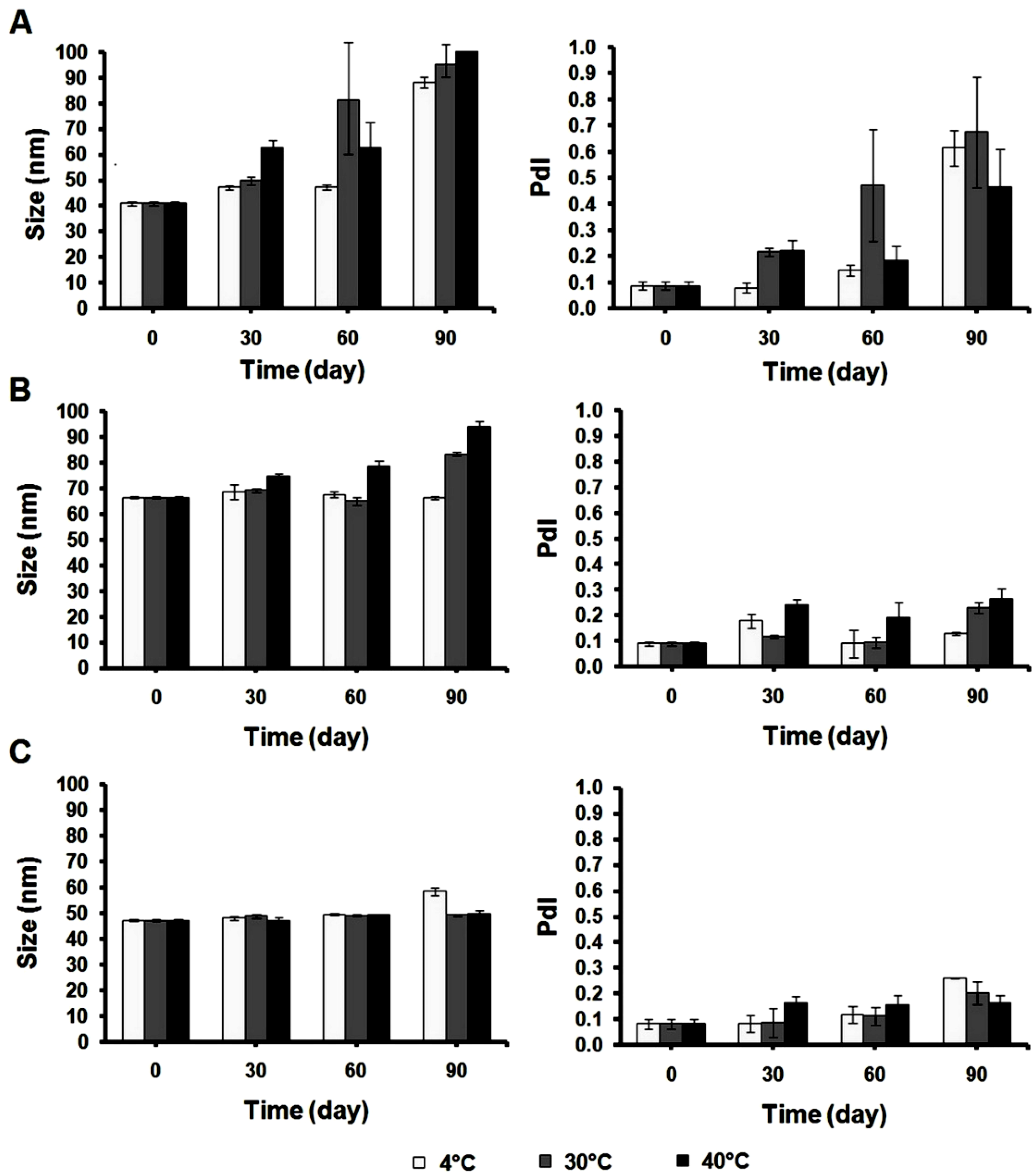
3. Results and Discussion
3.1. Characterization of Cur-Nano
3.2. Curcumin Stability under Storage Conditions
3.3. Particle Size Determination of Cur-Nano
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asai, A.; Miyazawa, T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr. 2001, 131, 2932–2935. [Google Scholar] [PubMed]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Jawień, J.; Gajda, M.; Mateuszuk, L.; Gebska, A.; Korabiowska, M.; Chłopicki, S.; Korbut, R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 2005, 56, 627–635. [Google Scholar] [PubMed]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor-and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Kurien, B.; Singh, A.; Matsumoto, H.; Scofield, R. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J.; Kopečková, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Šubr, V. Structural and chemical aspects of HPMA copolymers as drug carriers. Adv. Drug Deliv. Rev. 2010, 62, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Novo, L.; van Gaal, E.V.B.; Mastrobattista, E.; van Nostrum, C.F.; Hennink, W.E. Decationized crosslinked polyplexes for redox-triggered gene delivery. J. Control. Release 2013, 169, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Yang, J.; Ray, A.; Cheney, D.; Ghandehari, H.; Kopeček, J. Biodegradable multiblock poly (N-2-hydroxypropyl) methacrylamide gemcitabine and paclitaxel conjugates for ovarian cancer cell combination treatment. Int. J. Pharm. 2013, 454, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 2005, 103, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van Steenbergen, M.J.; Teunissen, E.A.; Novo, L.; Gradmann, S.; Baldus, M.; van Nostrum, C.F.; Hennink, W.E. π-π stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Khonkarn, R.; Mankhetkorn, S.; Talelli, M.; Hennink, W.; Okonogi, S. Cytostatic effect of xanthone-loaded mPEG-b-p(HPMAm-Lac2) micelles towards doxorubicin sensitive and resistant cancer cells. Colloids Surf. B 2012, 94, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Shi, Y.; van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Khonkarn, R.; Mankhetkorn, S.; Hennink, W.E.; Okonogi, S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2011, 79, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Neradovic, D.; van Steenbergen, M.; Vansteelant, L.; Meijer, Y.; van Nostrum, C.; Hennink, W. Degradation mechanism and kinetics of thermosensitive polyacrylamides containing lactic acid side chains. Macromolecules 2003, 36, 7491–7498. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A Medicinal Chemist’s Guide to Molecular Interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
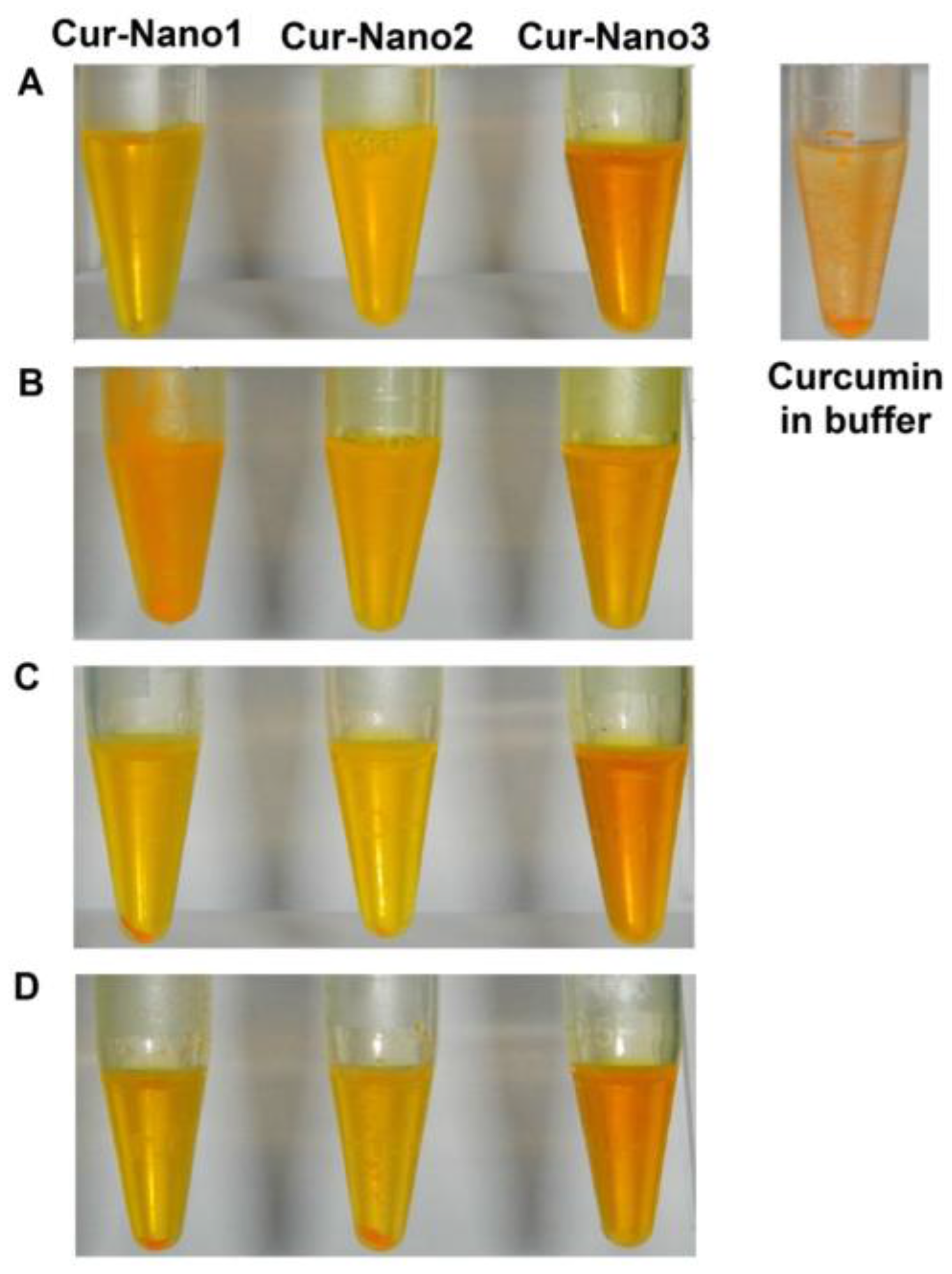
| Formulation | Aromatic Content in Polymer (%) | EE (%) | LC (%) |
|---|---|---|---|
| Cur-Nano1 | 0 | 61.3 ± 0.5 | 6.8 ± 0.1 |
| Cur-Nano2 | 25 | 61.3 ± 0.1 | 6.8 ± 0.0 |
| Cur-Nano3 | 100 | 113.8 ± 3.4 | 12.6 ± 0.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonogi, S.; Naksuriya, O.; Charumanee, S.; Sirithunyalug, J. Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability. Sci. Pharm. 2016, 84, 625-633. https://doi.org/10.3390/scipharm84040625
Okonogi S, Naksuriya O, Charumanee S, Sirithunyalug J. Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability. Scientia Pharmaceutica. 2016; 84(4):625-633. https://doi.org/10.3390/scipharm84040625
Chicago/Turabian StyleOkonogi, Siriporn, Ornchuma Naksuriya, Suporn Charumanee, and Jakkapan Sirithunyalug. 2016. "Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability" Scientia Pharmaceutica 84, no. 4: 625-633. https://doi.org/10.3390/scipharm84040625






