A Fast and Validated Reversed-Phase HPLC Method for Simultaneous Determination of Simvastatin, Atorvastatin, Telmisartan and Irbesartan in Bulk Drugs and Tablet Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Chromatographic Conditions
2.3. Preparation of Solutions
2.3.1. Standard Stock Solutions
2.3.2. Working Standard Solutions
2.3.3. Sample Solutions
2.4. Method Validation
2.4.1. System Suitability
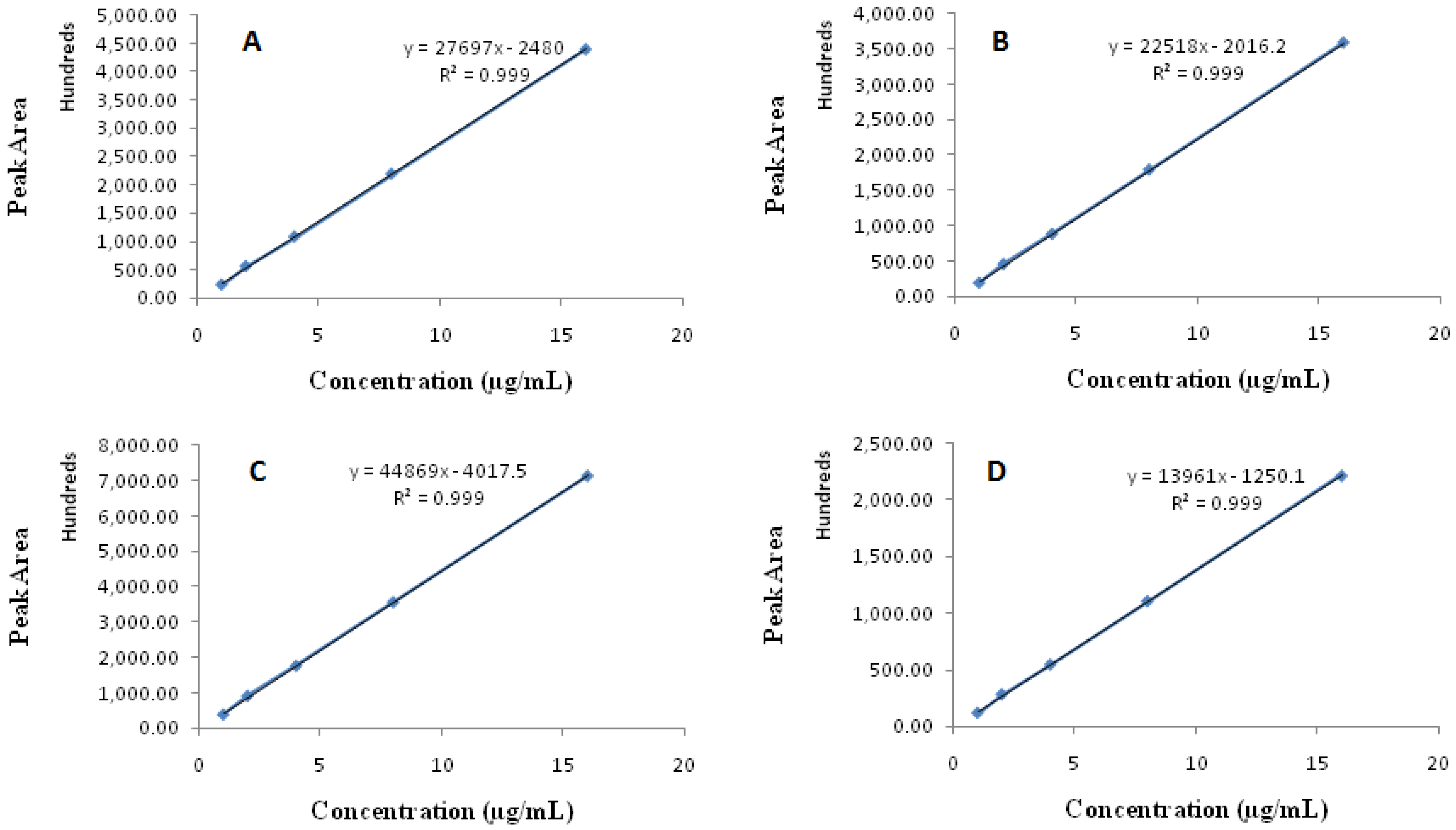
2.4.2. Linearity
2.4.3. Precision and Accuracy
2.4.4. Limit of Detection and Limit of Quantification
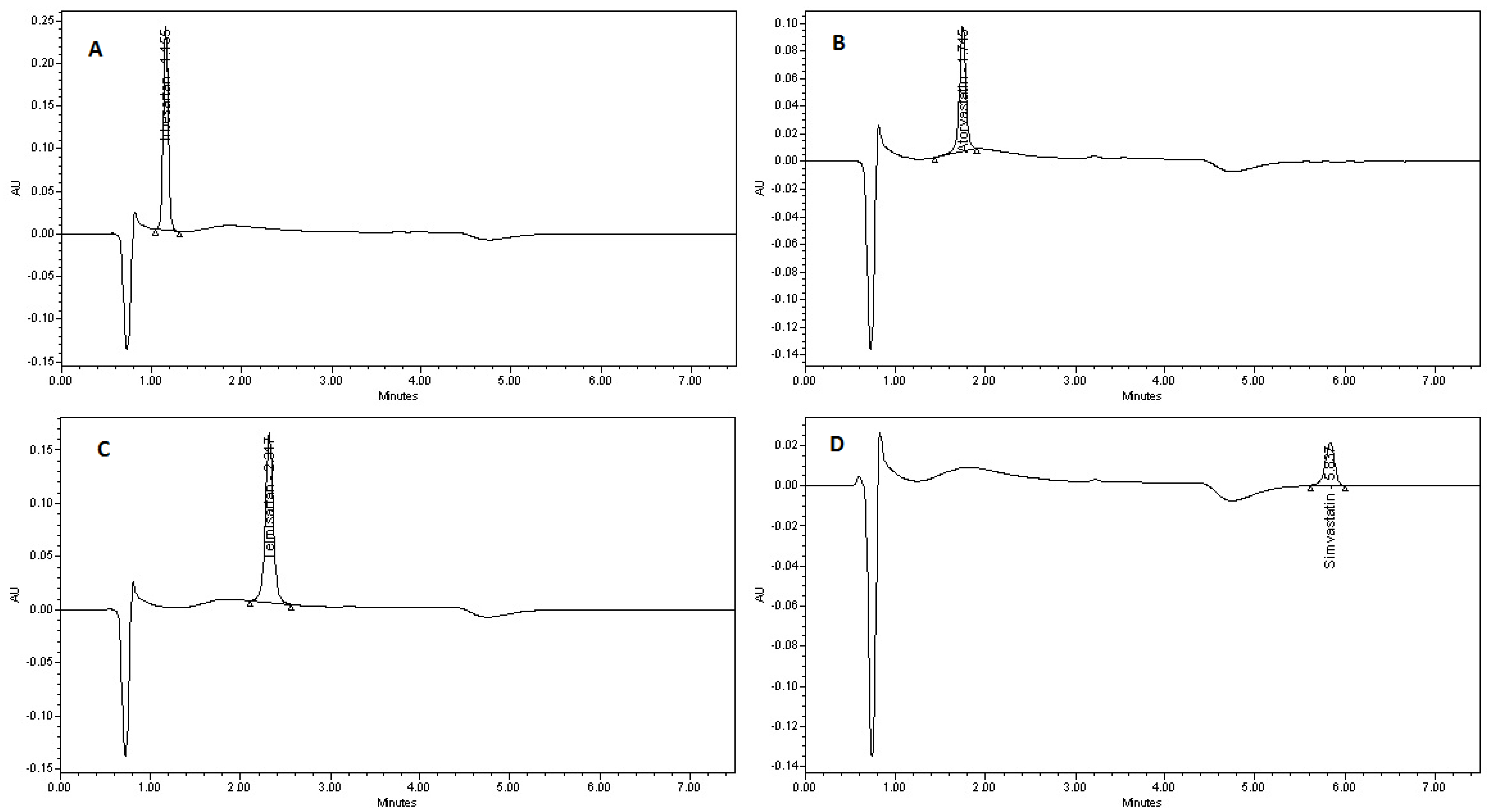
2.4.5. Specificity
2.4.6. Solution Stability
3. Results and Discussion
3.1. Method Development and Optimization
3.2. Method Validation
3.2.1. System Suitability
3.2.2. LOD and LOQ
3.2.3. Specificity
3.2.4. Linearity
3.2.5. Precision and Accuracy
3.2.6. Solution Stability
3.3. Application of the Developed Method in Determination of ATV, SMV, TLM and IRB in Tablet Dosage Forms
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Law, M.R.; Wald, N.J.; Rudnicka, A.R. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Bri. Med. J. 2003, 326, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Caballero, R.; Gomez, R.; Nunez, L.; Vaquero, M.; Delpon, E. Lipid-lowering therapy with statins, a new approach to antiarrhythmic therapy. Pharmacol. Ther. 2007, 114, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Jiang, H.; Sun, A.; Zou, Y.; Ge, J. Effects of statin treatment on cardiac function in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Clin. Cardiol. 2011, 34, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lennernas, H. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 2003, 42, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Vitale, G.; Seidita, A.; Guarneri, F.P.; Pepe, I.; Rinollo, C.; Rosa, S.D.; Rini, G.B.; Cillari, E.; Fede, G.D. Mevalonate pathway: Role of biphoslphonates and statins. Acta Med. Mediterr. 2011, 27, 97–107. [Google Scholar]
- Ciofu, C. The statins as anticancer agents. Maedica 2012, 7, 337. [Google Scholar]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Abeles, A.M.; Pillinger, M.H. Statins as anti-inflammatory and immunomodulatory agents: Afuture in rheumatologic therapy. Arthritis Rheumatol. 2006, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Walter, M.F.; Day, C.A.; Jacob, R.F. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J. Biol. Chem. 2006, 281, 9337–9345. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.P.M.; Davis, T.M.E. Statins as potential antimalarial drugs: Low relative potency and lack of synergy with conventional antimalarial drugs. Antimicrob. Agents Chemother. 2009, 53, 2212–2214. [Google Scholar] [CrossRef] [PubMed]
- Galgóczy, L.; Nyilasi, I.; Papp, T.; Vágvölgyi, C. Statins as antifungal agents. World J. Clin. Infect. Dis. 2011, 1, 4–10. [Google Scholar] [CrossRef]
- Hafez, H.M.; Elshanawane, A.A.; Abdelaziz, L.M.; Kamal, M.M. Quantitative determination of three angiotensin-II-receptor antagonists in presence of hydrochlorothiazide by RP-HPLC in their tablet preparations. Iran. J. Pharm. Res. 2013, 12, 635–643. [Google Scholar] [PubMed]
- Wani, T.A.; Khalil, N.Y.; Abdel-Rahman, H.M.; Darwish, I.A. Novel microwell-based spectrophotometric assay for determination of atorvastatin calcium in its pharmaceutical formulations. Chem. Cent. J. 2011, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Mathew, M. Spectrophotometric method of estimation of atorvastatin calcium using sulfo-phospho-vanillin reaction. J. Appl. Pharm. Sci. 2012, 2, 150–154. [Google Scholar]
- Maher, H.M.; Youssef, R.M.; Hassan, E.M.; El-Kimary, E.I.; Barary, M.A. Enhanced spectrophotometric determination of two antihyperlipidemic mixtures containing ezetimibe in pharmaceutical preparations. Drug Test. Anal. 2011, 3, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Al-Adl, S.M.; Abdel-Aziz, L.M.; Mohamed, M.A.M. Spectrophotometric determination of atorvastatin calcium and rosuvastatin calcium in bulk and dosage form using p-dimethyl aminobenzaldehyde. J. Appl. Pharm. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Erturk, S.; Aktas, S.E.; Ersoy, L.; Ficicioglu, S. An HPLC method for the determination of atorvastatin and its impurities in bulk drug and tablets. J. Pharm. Biomed. Anal. 2003, 33, 1017–1023. [Google Scholar] [CrossRef]
- Simionato, L.D.; Ferello, L.; Stamer, S.G.; Repetto, M.F.; Zubata, P.D.; Segall, A.I. A validated reversed-phase HPLC method for the determination of atorvastatin calcium in tablets. Austin Chromatogr. 2014, 1, 1–4. [Google Scholar]
- Seshachalam, U.; Kothapally, C.B. HPLC analysis for simultaneous determination of atorvastatin and ezetimibe in pharmaceutical formulations. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 714–721. [Google Scholar] [CrossRef]
- Hassan, S.A.; Elzanfaly, E.S.; El-Zeany, S.B.; Salem, M.Y. Development and validation of HPLC and CE methods for simultaneous determination of amlodipine and atorvastatin in the presence of their acidic degradation products in tablets. Acta Pharm. 2016, 66, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, V.; Reddy, K.; Ahhirao, K. Development of UV spectrophotometric methods and validation for estimation of simvastatin in bulk and tablet dosage form by absorbance maxima and area under the curve method. J. Appl. Pharm. 2014, 6, 55–64. [Google Scholar]
- Balaji, S.; Sunitha, A. Development and validation of spectrophotometeric method for simultaneous determination of simvastatin and ezetimibe in tablet formulations. Pak. J. Pharm. Sci. 2010, 23, 375–378. [Google Scholar] [PubMed]
- Sharma, S.; Manocha, N. Estimation of sitagliptin phosphate and simvastatin and validation of the proposed method in a combined marketed tablet dosage form by simultaneous equation method. Int. J. Curr. Pharm. Res. 2012, 4, 144–147. [Google Scholar]
- Sreelakshmi, V.; Rao, U.M.V.; Pugazhendhy, S.; Shrivastava, S.K.; Sunitha, M. Development and validation of simvastatin by RP-HPLC method in bulk drug and pharmaceutical dosage forms. Am. J. PharmTech Res. 2013, 3, 370–377. [Google Scholar]
- Varma, R.A.; Shanmukha-Kumar, J.V.; Reddy, M.S. Estimation of simvastatin and ezetimibe in combined tablet formulation by stability indicating high performance liquid chromatography. Pharm. Lett. 2015, 7, 204–212. [Google Scholar]
- Oliveira, P.R.; Barth, T.; Todeschini, V.; Dalmora, S. Simultaneous liquid chromatographic determination of ezetimibe and simvastatin in pharmaceutical products. J. AOAC Int. 2007, 90, 1566–1572. [Google Scholar] [PubMed]
- Sonali, R.; Pallavi, P.; Vittal, C. Development and validation of HPTLC method for the estimation of sitagliptin phosphate and simvastatin in bulk and marketed formulation. Int. J. Drug Dev. Res. 2012, 4, 292–297. [Google Scholar]
- Palled, M.S.; Chatter, M.; Rajesh, P.M.N.; Bhat, A.R. Difference spectrophotometric determination of telmisartan in tablet dosage forms. Indian J. Pharm. Sci. 2006, 68, 685–686. [Google Scholar]
- Ilango, K.; Kumar, P.S. Validated spectrophotometric methods for the simultaneous determination of telmisartan and atorvastatin in bulk and tablets. Pharm. Methods 2012, 3, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Nalwade, S.; Reddy, V.R.; Rao, D.D.; Rao, I.K. Rapid simultaneous determination of telmisartan, amlodipine besylate and hydrochlorothiazide in a combined poly pill dosage form by stability-indicating ultra performance liquid chromatography. Sci. Pharm. 2011, 79, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Rai, M.; Kawde, P.B.; Farooqui, M. Simultaneous determination of telmisartan, amlodipine besylate and hydrochlorothiazide in tablet dosage form by using stability indicating HPLC method. Pharm. Sin. 2015, 6, 13–18. [Google Scholar]
- Kurade, V.P.; Pai, M.G.; Gude, R. RP-HPLC estimation of ramipril and telmisartan in tablets. Indian J. Pharm. Sci. 2009, 71, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Simultaneous estimation of telmisartan and ramipril in combined dosage form by using HPTLC. Pharm. Lett. 2012, 4, 509–514. [Google Scholar]
- Ilango, K.; Kumar, P.S.S. Development and validation of stability indicating HPTLC and HPLC methods for simultaneous determination of telmisartan and atorvastatin in their formulations. J. Chem. 2013, 2013, 725385. [Google Scholar] [CrossRef]
- Mehta, S.; Singh, S.; Chikhalia, K.; Mehta, P.; Dadhania, T. Determination of assay and uniformity of content of ramipril and telmisartan in their multiple dosage forms by a developed and validated supercritical fluid chromatographic technique. Anal. Methods 2014, 6, 7068–7074. [Google Scholar] [CrossRef]
- Albero, I.; Rodenas, V.; García, S.; Sanchez-Pedreno, C. Determination of irbesartan in the presence of hydrochlorothiazide by derivative spectrophotometry. J. Pharm. Biomed. Anal. 2002, 29, 299–305. [Google Scholar] [CrossRef]
- Erk, N. Three new spectrophotometric methods applied to the simultaneous determination of hydrochlorothiazide and irbesartan. Pharmazie 2003, 58, 543–548. [Google Scholar] [PubMed]
- Kumar, J.S.P.; Annapurna, M.M. New spectrophotometric methods for the simultaneous determination of irbesartan and hydrochlorothiazide in combined dosage forms. Pharm. Methods 2015, 6, 120–125. [Google Scholar] [CrossRef]
- Rane, V.P.; Patil, K.R.; Sangshetti, J.N.; Yeole, R.D.; Shinde, D.B. Stability indicating LC method for simultaneous determination of irbesartan and hydrochlorothiazide in pharmaceutical preparations. J. Chromatogr. Sci. 2010, 48, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Abdelhameed, A.S.; Khalil, N.Y.; Khan, A.A.; Darwish, I.A. HPLC method with monolithic column for simultaneous determination of irbesartan and hydrochlorothiazide in tablets. Acta Pharm. 2014, 64, 187–198. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology, Q2 (R1). November 2005. Available online: http://www.ipqpubs.com/wp-content/uploads/2011/09/Q2_R1__Guideline.pdf (accessed on 17 August 2017).
- Center for Drug Evaluation and Research, US Food and Drug Administration. Reviewer Guidance, Validation of Chromatographic Methods; FDA: Rockville, MD, USA, 1994. Available online: https://www.fda.gov/downloads/Drugs/Guidances/UCM134409.pdf (accessed on 17 August 2017).
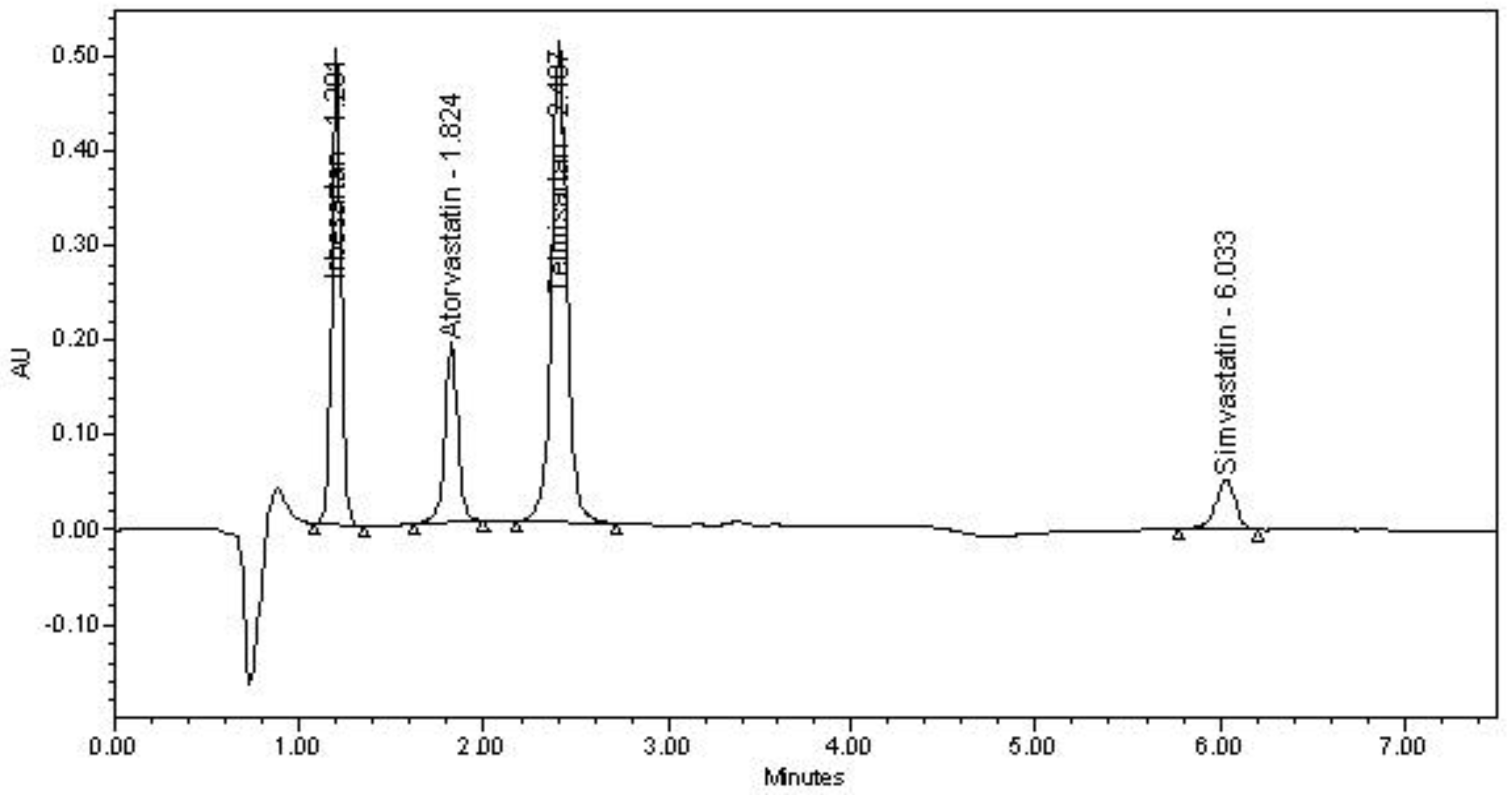
| Parameters | IRB | ATV | TLM | SMV |
|---|---|---|---|---|
| Retention time (min) | 1.20 | 1.82 | 2.40 | 6.03 |
| USP Tailing factor | 1.12 | 0.98 | 1.18 | 1.08 |
| USP Resolution | 5.92 | 4.21 | 20.63 | |
| USP Plate count | 2701 | 3621 | 4299 | 15122 |
| Percent RSD of peak area (n = 6) | 0.41 | 0.82 | 0.36 | 0.89 |
| Concentration Levels (µg/mL) | ATV | SMV | TLM | IRB | |
|---|---|---|---|---|---|
| Intra-day Precision and Accuracy | |||||
| %RSD of peak area (Average % recovery) | 2 | 2.10 (101.99) | 1.41 (100.75) | 1.39 (102.14) | 1.89 (100.41) |
| 8 | 1.61 (100.21) | 0.46 (100.30) | 1.58 (100.26) | 0.38 (100.75) | |
| 16 | 1.39 (100.07) | 1.30 (100.32) | 1.265 (100.34) | 1.07 (100.83) | |
| Inter-day Precision and Accuracy | |||||
| %RSD of peak area (Average % recovery) | 2 | 2.06 (101.95) | 1.52 (102.78) | 1.39 (102.02) | 1.52 (102.64) |
| 8 | 1.60 (100.32) | 0.97 (99.99) | 0.69 (99.98) | 0.38 (101.38) | |
| 16 | 1.35 (100.10) | 1.61 (101.34) | 0.36 (100.80) | 1.07 (101.20) | |
| Analytes | Storage Conditions | Average %Recovery * |
|---|---|---|
| Atorvastatin | Normal laboratory temperature (25 °C) for 12 h | 99.86 |
| Refrigerator temperature (4 °C) for 14 days | 99.12 | |
| −20 °C for 30 days | 100.12 | |
| Simvastatin | Normal laboratory temperature (25 °C) for 12 h | 99.74 |
| Refrigerator temperature (4 °C) for 14 days | 99.87 | |
| −20 °C for 30 days | 99.84 | |
| Telmisartan | Normal laboratory temperature (25 °C) for 12 h | 100.69 |
| Refrigerator temperature (4 °C) for 14 days | 100.98 | |
| −20 °C for 30 days | 101.59 | |
| Irbesartan | Normal laboratory temperature (25 °C) for 12 h | 100.96 |
| Refrigerator temperature (4 °C) for 14 days | 101.56 | |
| −20 °C for 30 days | 101.26 |
| Analytes | Recovery Sample Concentrations (μg/mL) | Percentage of Targeted Concentration | Amount of Drugs Recovered (μg/mL) | %Recovery ± %RSD * |
|---|---|---|---|---|
| Atorvastatin | 5 | 50% | 5.02 | 100.42 ± 0.57 |
| 10 | 100% | 10.18 | 101.81 ± 0.89 | |
| 15 | 150% | 15.14 | 100.98 ± 0.73 | |
| Simvastatin | 5 | 50% | 4.94 | 98.86 ± 1.09 |
| 10 | 100% | 9.98 | 99.80 ± 1.09 | |
| 15 | 150% | 14.95 | 99.67 ± 1.10 | |
| Telmisartan | 5 | 50% | 5.06 | 101.38 ± 1.58 |
| 10 | 100% | 10.12 | 101.20 ± 1.59 | |
| 15 | 150% | 15.16 | 101.07 ± 1.59 | |
| Irbesartan | 5 | 50% | 5.07 | 101.50 ± 0.38 |
| 10 | 100% | 10.12 | 101.20 ± 0.38 | |
| 15 | 150% | 15.15 | 101.05 ± 0.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazmi, H.A.; Alnami, A.M.; Arishi, M.A.A.; Alameer, R.K.; Al Bratty, M.; Rehman, Z.U.; Javed, S.A.; Arbab, I.A. A Fast and Validated Reversed-Phase HPLC Method for Simultaneous Determination of Simvastatin, Atorvastatin, Telmisartan and Irbesartan in Bulk Drugs and Tablet Formulations. Sci. Pharm. 2018, 86, 1. https://doi.org/10.3390/scipharm86010001
Alhazmi HA, Alnami AM, Arishi MAA, Alameer RK, Al Bratty M, Rehman ZU, Javed SA, Arbab IA. A Fast and Validated Reversed-Phase HPLC Method for Simultaneous Determination of Simvastatin, Atorvastatin, Telmisartan and Irbesartan in Bulk Drugs and Tablet Formulations. Scientia Pharmaceutica. 2018; 86(1):1. https://doi.org/10.3390/scipharm86010001
Chicago/Turabian StyleAlhazmi, Hassan A., Ahmed M. Alnami, Mohammed A. A. Arishi, Raad K. Alameer, Mohammed Al Bratty, Zia Ur Rehman, Sadique A. Javed, and Ismail A. Arbab. 2018. "A Fast and Validated Reversed-Phase HPLC Method for Simultaneous Determination of Simvastatin, Atorvastatin, Telmisartan and Irbesartan in Bulk Drugs and Tablet Formulations" Scientia Pharmaceutica 86, no. 1: 1. https://doi.org/10.3390/scipharm86010001




