4,5-Dimethoxy-2-nitrobenzohydrazides and 1-(1-Benzylpiperidin-4-yl)ethan-1-ones as Potential Antioxidant/Cholinergic Endowed Small Molecule Leads
Abstract
:1. Introduction
2. Materials and Methods
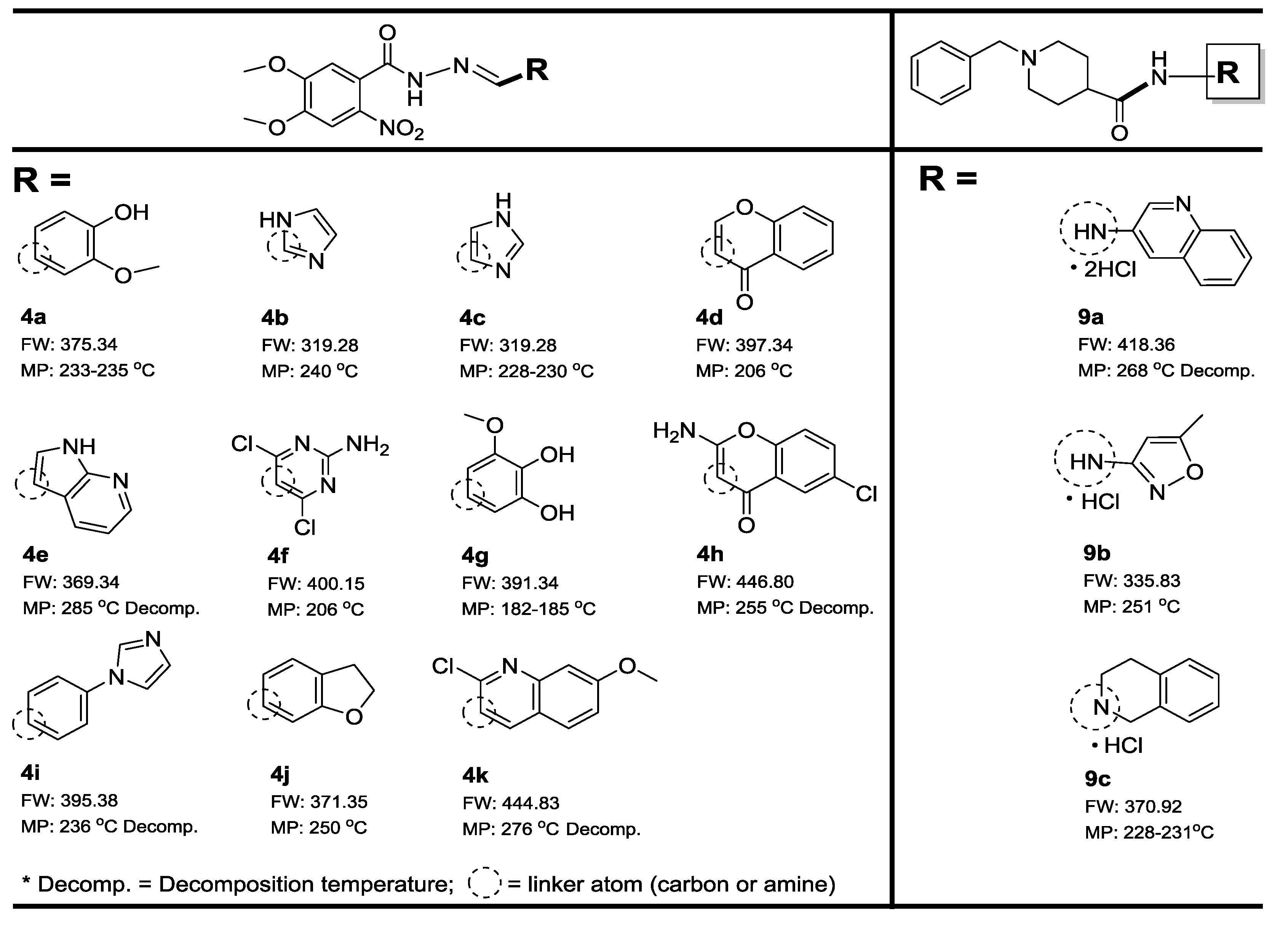
2.1. Synthesis
2.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
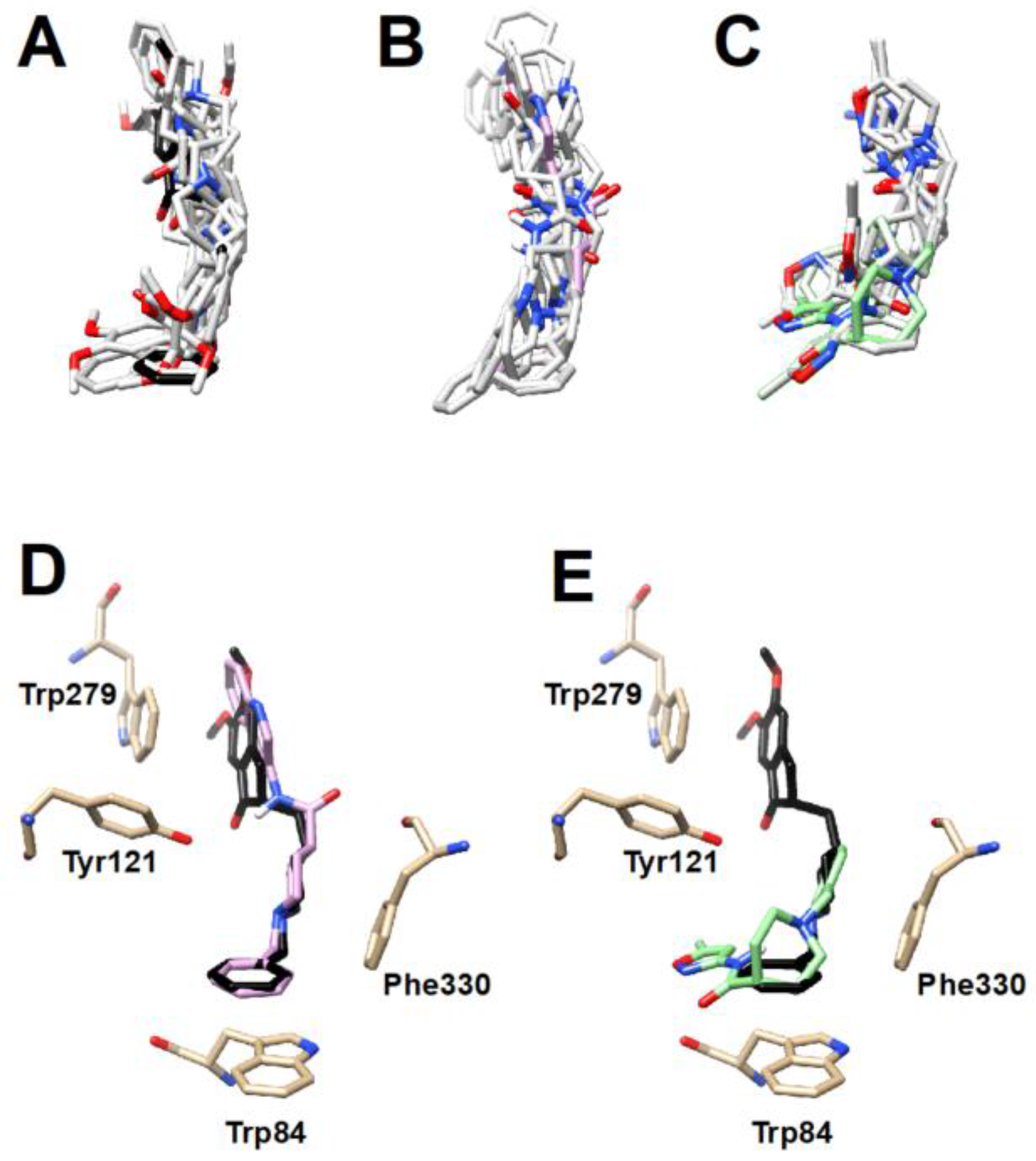
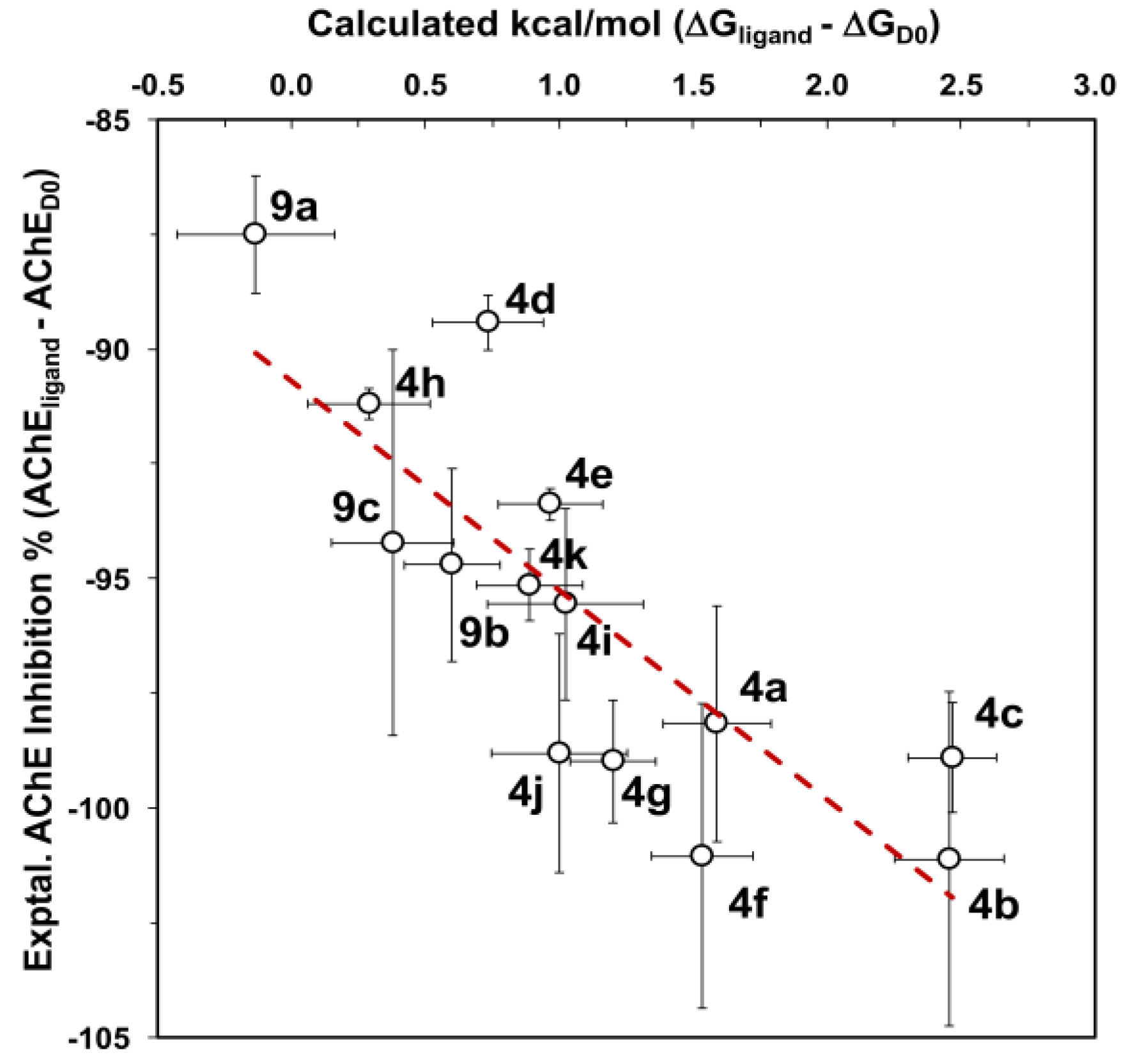
2.3. In Silico AChE Inhibition
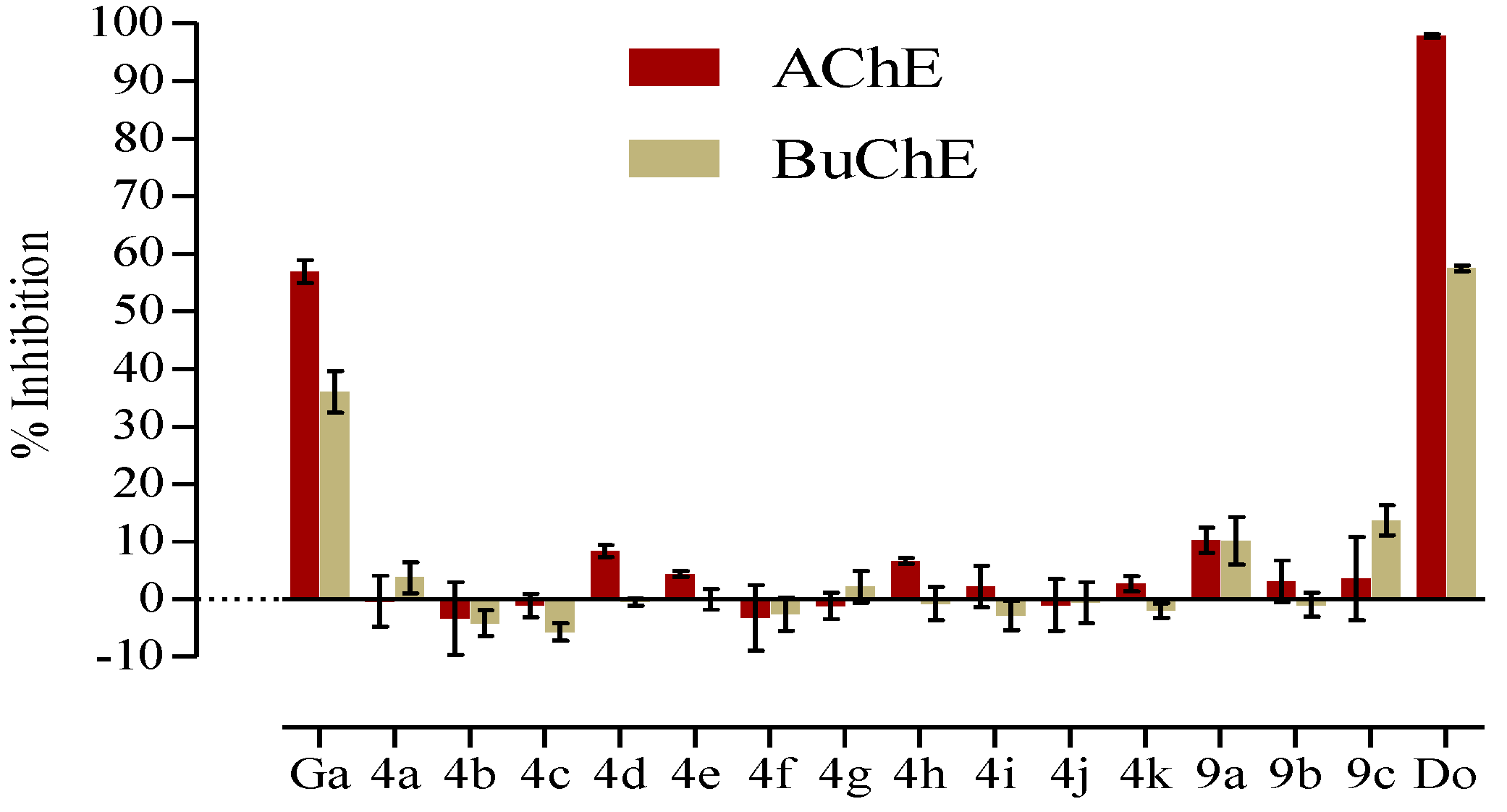
2.4. In Vitro AChE/BuChE Inhibition
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Padmini, K.; Preethi, P.J.; Divya, M.; Rohini, P.; Lohita, M.; Swetha, K.; Kaladar, P. A review on biological importance of hydrazones. Int. J. Pharm. Res. Rev. 2013, 2, 43–58. [Google Scholar]
- Rollas, S.; Kucukguzel, S.G. Biological activities of hydrazine derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, T.; Höfner, G.; Wein, T.; Wanner, K.T. Synthesis of 4-substituted nipecotic acid derivatives and their evaluation as potential GABA uptake inhibitors. Bioorg. Med. Chem. 2016, 24, 2072–2096. [Google Scholar] [CrossRef] [PubMed]
- Bonina, F.P.; Arenare, L.; Palagiano, F.; Saija, A.; Nava, F.; Trombetta, D.; De Caprariis, P. Synthesis, stability, and pharmacological evaluation of nipecotic acid prodrugs. J. Pharm. Sci. 1999, 88, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Török, B.; Sood, A.; Bag, S.; Tulsan, R.; Ghosh, S.; Borkin, D.; Kennedy, A.R.; Melanson, M.; Madden, R.; Zhou, W.; et al. Diaryl hydrazones as multifunctional inhibitors of amyloid self-assembly. Biochemistry 2013, 52, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Zapata-Sudo, G.; Kummerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T. Synthesis and vasodilatory activity of new N-acylhydrazones derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. 2005, 13, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.C.; Lima, L.M.; Da Silva, K.C.; Léda, P.H.; De Miranda, A.L.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Belskaya, N.P.; Dehaen, W.; Bakuleva, V.A. Synthesis and properties of hydrazones bearing amide, thioamide and amidine functions. ARKIVOK 2010, 2010, 275–332. [Google Scholar]
- Ismail, M.M.; Kamel, M.M.; Mohamed, L.W.; Faggal, S.I. Synthesis of new indole derivatives structurally related to donepezil and their biological evaluation as acetylcholinesterase inhibitors. Molecules 2012, 17, 4811–4823. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Martorana, A.; Giacalone, V.; Bonsignore, R.; Pace, A.; Gentile, C.; Pibiri, I.; Buscemi, S.; Lauria, A.; Palumbo Piccionello, A. Heterocyclic scaffolds for the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2016, 22, 3971–3995. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Christoffer, T.; Zhang, Q.; Ming, C. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol. Behav. 2007, 92, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lipton, S.A. Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ. 2011, 18, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate flourescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, B.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Cumming, G.; Fidler, F.; David, L.V. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H. Biological assay challenges from compound solubility: Strategies for bioassay optimization. Drug Discov. Today 2006, 11, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.B.; Gee, S.J.; Hammock, B.D. Use of a 96-well microplate reader for measuring routine enzyme activities. Anal. Biochem. 1987, 166, 353–360. [Google Scholar] [CrossRef]
- Bag, S.; Tulsan, R.; Sood, A.; Cho, H.; Redjeb, H.; Zhou, W.; LeVine, H., III; Török, B.; Török, M. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015, 25, 626–630. [Google Scholar]
- Bag, S.; Ghosh, S.; Tulsan, R.; Sood, A.; Zhou, W.; Schifone, C.; Foster, M.; LeVine, H., III; Török, B.; Török, M. Design, synthesis and biological activity of multifunctional α,β-unsaturated carbonyl scaffolds for Alzheimers disease. Bioorg. Med. Chem. Lett. 2013, 23, 2614–2618. [Google Scholar]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banu, R.; Gerding, J.; Franklin, C.; Sikazwe, D.; Horton, W.; Török, M.; Davis, J.; Cheng, K.H.; Nakazwe, M.; Mochona, B. 4,5-Dimethoxy-2-nitrobenzohydrazides and 1-(1-Benzylpiperidin-4-yl)ethan-1-ones as Potential Antioxidant/Cholinergic Endowed Small Molecule Leads. Sci. Pharm. 2018, 86, 2. https://doi.org/10.3390/scipharm86010002
Banu R, Gerding J, Franklin C, Sikazwe D, Horton W, Török M, Davis J, Cheng KH, Nakazwe M, Mochona B. 4,5-Dimethoxy-2-nitrobenzohydrazides and 1-(1-Benzylpiperidin-4-yl)ethan-1-ones as Potential Antioxidant/Cholinergic Endowed Small Molecule Leads. Scientia Pharmaceutica. 2018; 86(1):2. https://doi.org/10.3390/scipharm86010002
Chicago/Turabian StyleBanu, Rukhsar, Jason Gerding, Cynthia Franklin, Donald Sikazwe, William Horton, Marianna Török, Julian Davis, Kwan H. Cheng, Muziya Nakazwe, and Bereket Mochona. 2018. "4,5-Dimethoxy-2-nitrobenzohydrazides and 1-(1-Benzylpiperidin-4-yl)ethan-1-ones as Potential Antioxidant/Cholinergic Endowed Small Molecule Leads" Scientia Pharmaceutica 86, no. 1: 2. https://doi.org/10.3390/scipharm86010002





