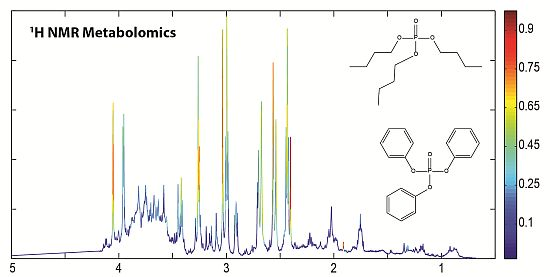
1H Nuclear Magnetic Resonance (NMR) Metabolomic Study of Chronic Organophosphate Exposure in Rats
Abstract
:1. Introduction
2. Results and Discussion
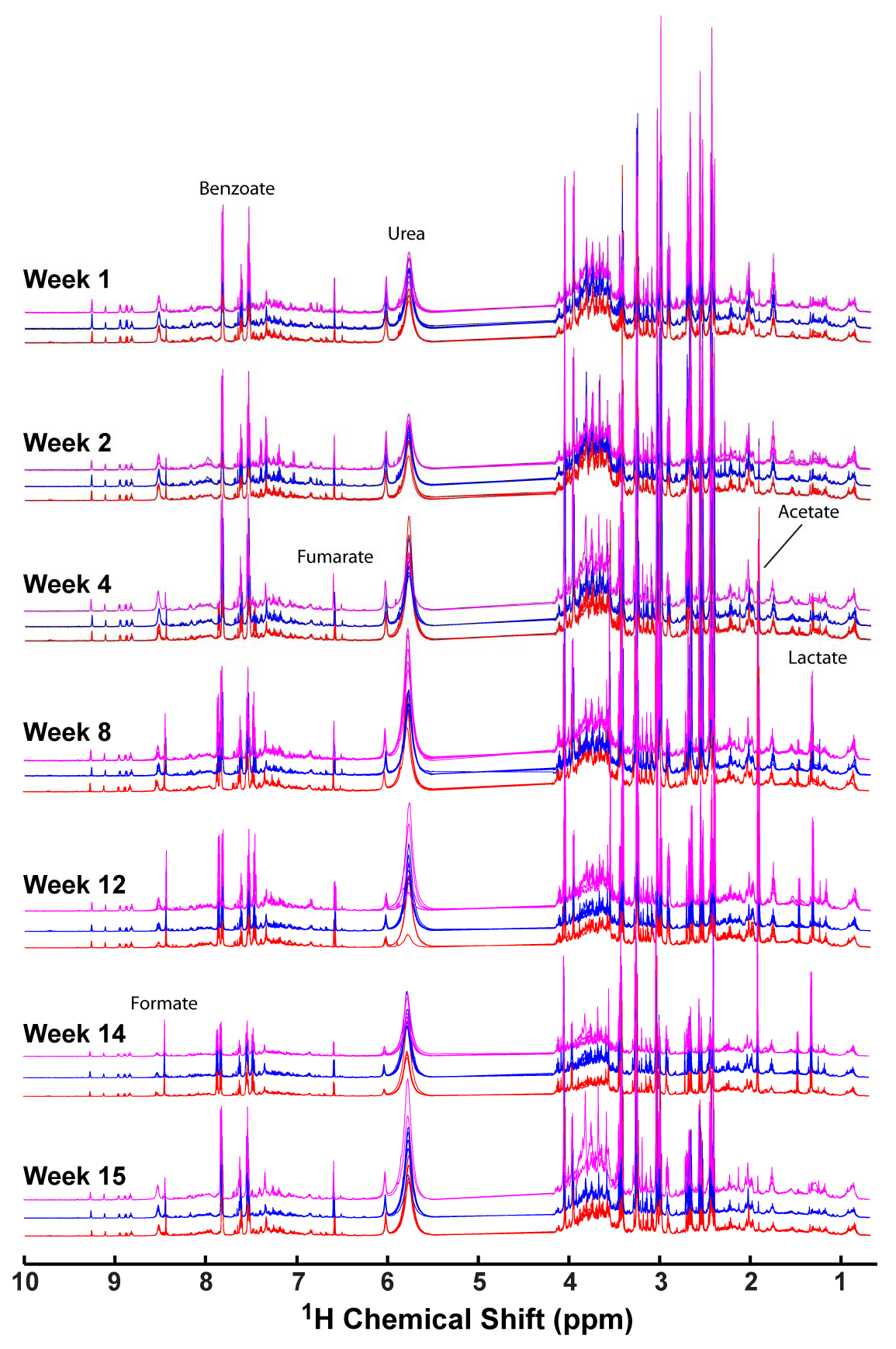
2.1. 1H NMR of Urine Following Chronic TPP and TBP Exposure
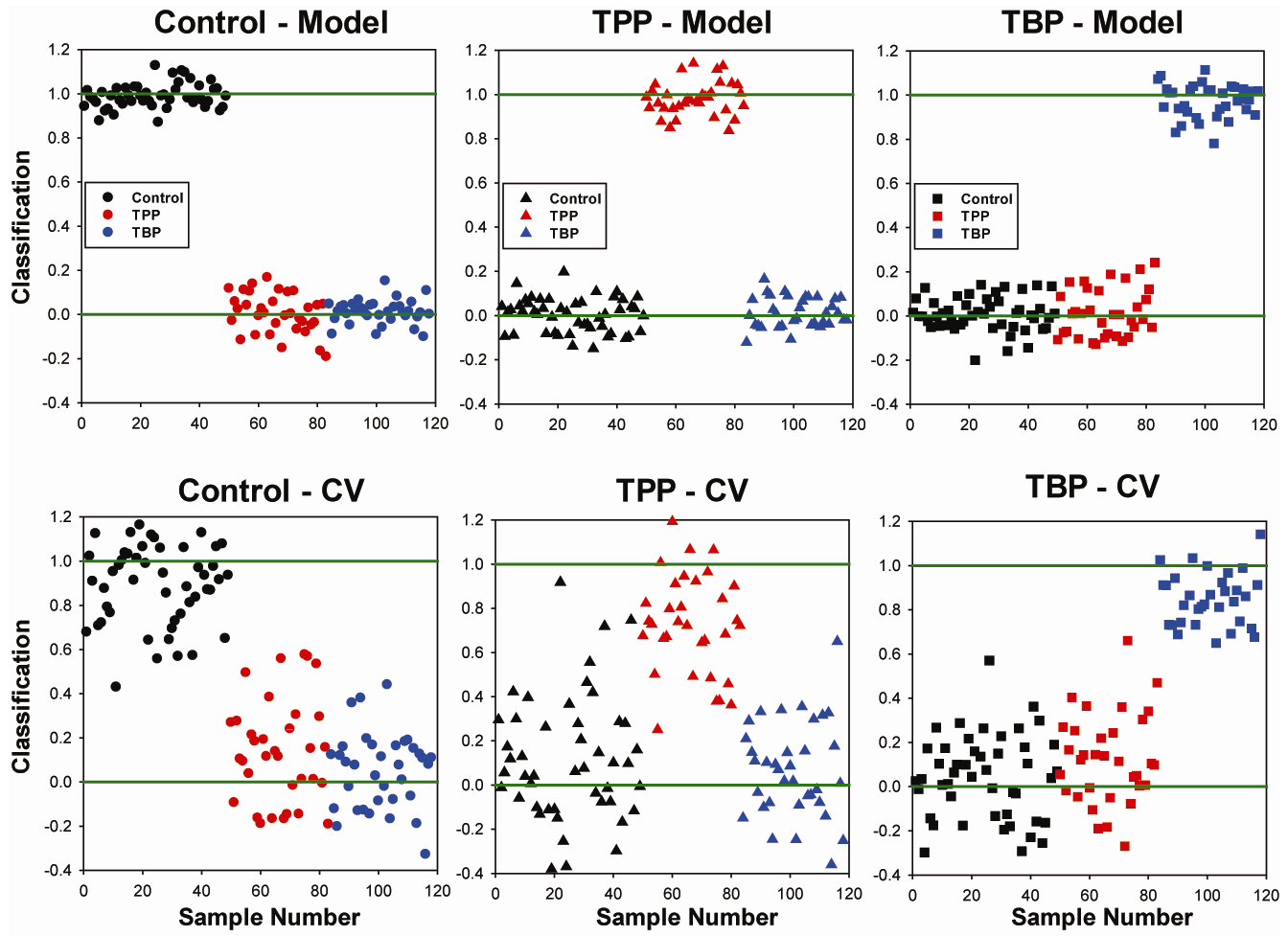
2.2. OSC-PLSDA Model Development for Chronic TPP and TBP Exposure
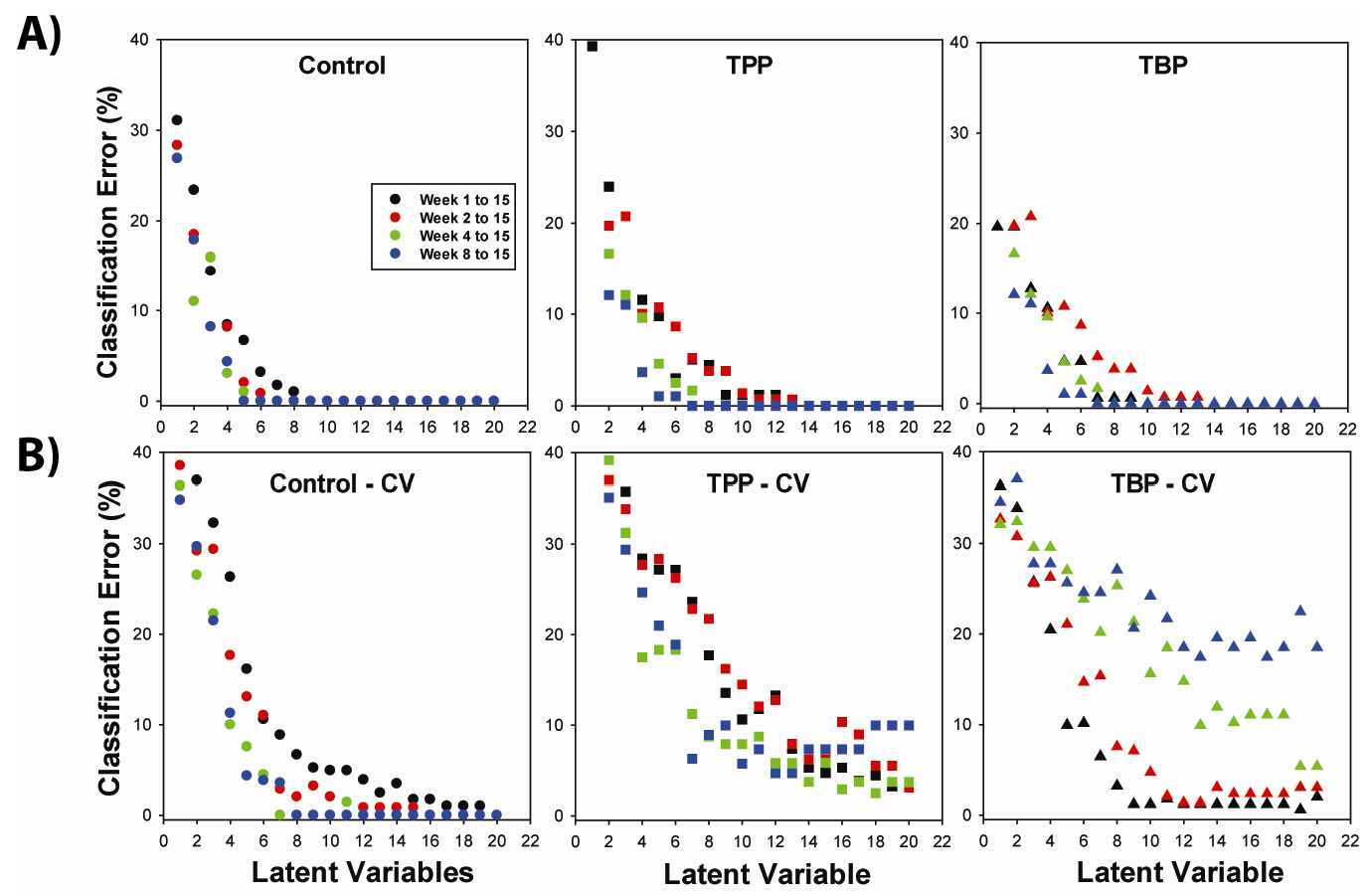
| System | % Error in Prediction (Model) | % Error in Prediction (Cross Validation) | |||||
|---|---|---|---|---|---|---|---|
| Control | TPP | TBP | Control | TPP | TBP | ||
| 6 LV | 3.2 | 3.0 | 4.7 | 10.6 | 27.2 | 10.2 | |
| 8 LV | 1.7 | 4.4 | 0.6 | 6.7 | 17.7 | 3.2 | |
| 10 LV | < 0.5 | 1.2 | < 0.1 | 4.9 | 10.6 | 1.2 | |
| 12 LV(Week 1 – 15) | < 0.1 | 1.2 | < 0.1 | 3.9 | 13.3 | 1.2 | |
| 12 LV(Week 2 – 15) | < 0.1 | 0.7 | < 0.1 | 0.8 | 12.8 | 1.4 | |
| 12 LV(Week 4 – 15) | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 5.8 | 14.8 | |
| 12 LV(Week 8-15) | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 4.7 | 18.5 | |
2.3. Important Metabolite Identification Using VIP Scores
2.3.1. Comparison of Metabolites for Acute and Chronic Exposure

| Resonance (ppm) | Metabolite | Scaled VIP Coefficients | ||||||
|---|---|---|---|---|---|---|---|---|
| Acute Exposure[22] | Chronic Exposure | |||||||
| Control | TBP | TPP | Control | TBP | TPP | |||
| 0.91 | Dibutyl Phosphate | <0.01 | 0.03 | 0.02 | <0.01 | <0.01 | <0.01 | |
| 1.31,1.32 | Lactate | -- | -- | -- | 0.19 | 0.18 | 0.05 | |
| 1.91 | Acetate | -- | -- | -- | 1.00 (#1) | 0.85 (#3) | 0.65 (#5) | |
| 2.05 | -- | 0.05 | 0.13 | 0.09 | 0.02 | 0.03 | <0.01 | |
| 2.40 | Succinate | 0.19 | 0.17 | 0.22 | 0.67 | 0.22 | 0.97 (#2) | |
| 2.41 | Succinate | <0.10 | 0.18 | 0.25 | 0.33 | 0.05 | 0.95 (#4) | |
| 2.43 | 2-Oxoglutarate | 0.37 (#5) | 0.73 (#3) | 0.52 (#4) | 0.81 (#5) | 0.94 (#2) | 0.32 | |
| 2.54, 2.56 | Citrate | 0.28 | 1.00 (#1) | 0.45 | 0.33 | 0.24 | 0.46 | |
| 2.68, 2.71 | Citrate | 0.23 | 0.80 (#2) | 0.51 (#5) | 0.17 | 0.20 | 0.20 | |
| 3.00 | 2-Oxoglutarate | 0.40 (#4) | 0.63 (#4) | 0.60 (#3) | 0.83 (#4) | 1.00 (#1) | 0.25 | |
| 3.04 | Creatine | 0.19 | 0.28 (#5) | 0.25 | 0.93 (#2) | 0.29 | 0.96 (#3) | |
| 3.25 | Betaine | 0.42 (#3) | 0.17 | 0.50 | 0.89 (#3) | 0.35 (#5) | 1.00 (#1) | |
| 3.27 | Taurine | 0.82 (#2) | 0.14 | 0.84 (#2) | 0.30 | 0.27 | 0.31 | |
| 3.41 | -- | 0.33 | <0.1 | 0.34 | 0.10 | 0.12 | 0.06 | |
| 3.42 | Taurine | 1.00 (#1) | <0.1 | 1.00 (#1) | 0.31 | 0.38 (#4) | 0.15 | |
| 3.43 | -- | 0.25 | <0.1 | 0.25 | 0.06 | 0.07 | <0.01 | |
| 3.67 | -- | 0.13 | 0.16 | 0.17 | <0.01 | <0.01 | <0.01 | |
| 3.81 | -- | 0.13 | 0.19 | 0.17 | <0.01 | <0.01 | <0.01 | |
| 4.06 | Creatine | 0.20 | 0.12 | 0.22 | 0.59 | 0.33 | 0.46 | |
| 5.78 | Urea | 0.03 | 0.01 | 0.03 | 0.01 | 0.01 | 0.02 | |
| 6.60 | Fumarate | 0.06 | 0.03 | 0.07 | 0.01 | 0.01 | 0.03 | |
| 7.54 | Benzoate | 0.01 | 0.09 | 0.04 | 0.10 | 0.07 | 0.05 | |
| 7.67 | Benzoate | 0.01 | 0.09 | 0.03 | 0.11 | 0.08 | 0.06 | |
| 7.82, 7.83 | Benzoate | 0.01 | 0.10 | 0.04 | 0.11 | 0.07 | 0.06 | |
| 8.44 | Formate | -- | -- | -- | 0.10 | 0.05 | 0.13 | |
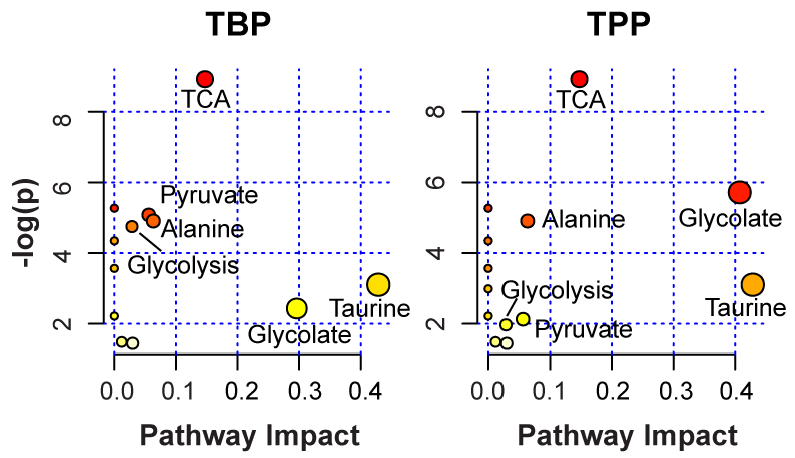
2.4. Identification of Impacted Metabolite Pathways
| TBP | TPP | ||||||
|---|---|---|---|---|---|---|---|
| Pathway Name | # | # | # | ||||
| Metabolites | Hits | -log(p) | Impact | Hits | -log(p) | Impact | |
| Citrate Cycle (TCA cycle) | 20 | 3 | 8.92 | 0.15 | 3 | 8.92 | 0.15 |
| Glyoxylate/Dicarboxylate | 16 | 1 | 2.41 | 0.30 | 2 | 5.72 | 0.41 |
| Taurine and Hypotaurine | 8 | 1 | 3.10 | 0.43 | 1 | 3.10 | 0.43 |
| Pyruvate | 22 | 2 | 5.08 | 0.06 | 1 | 2.13 | 0.06 |
| Alanine/Aspartate/Glutamate | 24 | 2 | 4.91 | 0.06 | 2 | 4.91 | 0.06 |
3. Experimental Section
3.1. Animal Studies
3.2. NMR Studies
3.3. Chemometric Analysis

 is equal to the squared PLS weight of that parameter multiplied by the percent explained sum of squares for that PLS dimension.
is equal to the squared PLS weight of that parameter multiplied by the percent explained sum of squares for that PLS dimension. 4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Poulsen, T.G.; Bester, K. Organic Micropollutant Degradation in Sewage Sludge During Composting Under Thermophilic Conditions. Environ. Sci. Technol. 2010, 44, 5086–5091. [Google Scholar] [CrossRef]
- Anderson, J.A.; Grundmann, A.; Bester, K. Organophosphrous Flame Retardants and Plasticisers in Surface Waters. Sci. Total Env. 2004, 332, 155–166. [Google Scholar] [CrossRef]
- Anderson, J.; Bester, K. Elimination of Organophosphate Ester Flame Retardants and Plasticisers in Drinking Water Purification. Water Res. 2006, 40, 621–629. [Google Scholar] [CrossRef]
- Fries, E.; Puttmann, W. Occurance of Organophosphate Esters in Surface Water and Ground Water in Germany. J. Environ. Monit. 2001, 3, 621–626. [Google Scholar] [CrossRef]
- Fries, E.; Puttmann, W. Monitoring of the Three Organophosphate Esters TBP, TCEP and TBEP in River and Ground Water (Oder, Germany). J. Environ. Monit. 2003, 5, 346–352. [Google Scholar] [CrossRef]
- Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Sitka, A.; Scharf, S.; Gans, O. Determination of Selected Organophosphate Esters in the Aquatic Environment of Austria. Sci. Total Env. 2007, 388, 290–299. [Google Scholar] [CrossRef]
- Reemtsma, T.; Quintana, J.B.; Rodil, R.; Garcia-Lopez, M.; Rodriguez, I. Organophosphorous Flame Retardants and Plasticizers in Water and Air I. Ocuurance and Fate. Trends Anal. Chem. 2008, 27, 727–737. [Google Scholar] [CrossRef]
- Schindler, B.K.; Forster, K.; Angerer, J. Quantification of Two Urinary Metabolites of Organophsophorous Flame Retardants by Solid-Phase Extractiona on Gas Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2009, 395, 1167–1171. [Google Scholar] [CrossRef]
- Laham, S.; Szabo, J.; Long, G. Effects of Tri-n-Butyl Phosphate on the Peripheral Nervous System of the Sprague-Dawley Rat. Drug Chem.Toxicol. 1983, 6, 363–377. [Google Scholar] [CrossRef]
- Auletta, C.S. A Dietary Oncogenicity Study of Tributyl Phosphate in the CD-1 Mouse. Toxicology 1998, 128, 135–141. [Google Scholar] [CrossRef]
- Auletta, C.S.; Weiner, M.L.; Richter, W.R. A Dietary Toxicity/Oncogenicity Study of Tributyl Phosphate in the Rat. Toxicology 1998, 128, 125–143. [Google Scholar] [CrossRef]
- Noda, T.; Yamano, T.; Shimizu, M.; Morita, S. Effects of Tri-n-Butyl Phosphate on Pregnancy in Rats. Fund. Chem. Toxic. 1994, 32, 1031–1036. [Google Scholar]
- Hinton, D.M.; Jessop, J.J.; Arnold, A.; Albert, R.H.; Hines, F.A. Evaluation of Immunotoxicity in a Subchronic Feeding Study of Triphenyl Phosphate. Toxicol. Ind. Health 1987, 3, 71–89. [Google Scholar]
- Hinton, D.M.; Jessop, J.J.; Arnold, A.; Albert, R.H.; Hines, F.A. Evaluation of Immunotoxicity in a Subchronic Feeding Study of Triphenyl Phosphate. Intl. J. Occup. Med. Immunol. Toxicol. 1996, 5, 43–60. [Google Scholar]
- Welsh, J.J.; Collins, T.F.X.; Whitby, K.E.; Black, T.N.; Arnold, A. Teratogenic Potential of Triphenyl Phosphate in Sprague-Dawly (Spartan) Rats. Toxicol. Ind. Health 1987, 3, 357–369. [Google Scholar]
- International Programme on Chemical Safety (IPCS). Environmental Health Criteria 111—Triphenyl Phosphate. Available online: www.inchem.org (accessed on March 25 2012).
- International Programme on Chemical Safety (IPCS). Environmental Health Criteria 112—Tributyl Phosphate. Available online: www.inchem.org (accessed on March 25 2012).
- Suzuki, T.; Sasaki, K.; Eakeda, M.; Uchiyama, M. Metabolism of Tributyl Phosphate in Male Rats. J. Agric. Food Chem. 1984, 32, 603–610. [Google Scholar] [CrossRef]
- Suzuki, T.; Sasaki, K.; Takeda, M.; Uchiyama, M. Some S-Containing Metabolites of Tributyl Phosphate in the Rat. J. Agric. Food Chem. 1984, 32, 1278–1283. [Google Scholar] [CrossRef]
- Sasaki, K.; Suzuki, T.; Takeda, M.; Uchiyama, M. Metabolism of Phosphoric Acid Triesters by Rat Liver Homogenate. Bull. Environ. Contam. Toxicol. 1984, 33, 281–288. [Google Scholar]
- Sudakin, D.L.; Stone, D.L. Dialkyl Phosphates as Biomarkers of Organophosphates: The Current Divide Between Epidemiology and Clinical Toxicology. Clin. Toxicol. 2011, 49, 771–781. [Google Scholar] [CrossRef]
- Alam, T.M.; Alam, M.K.; Neerathilingam, M.; Volk, D.E.; Sarkar, S.; Ansari, G.A.S.; Luxon, B.A. 1H NMR Metabonomic Study of Rat Response to Triphenyl Phosphate and Tri-butyl Phosphate Exposure. Metabolomics 2010, 6, 386–394. [Google Scholar] [CrossRef]
- Neerathilingam, M.; Volk, D.E.; Sarkar, S.; Alam, T.M.; Alam, M.K.; Ansari, G.A.S.; Luxon, B.A. 1H NMR-Based Metabonomic Investigation of Tributyl Phosphate Exposure in Rats. Toxicol. Lett. 2010, 199, 10–16. [Google Scholar] [CrossRef]
- Lindon, J.C.; Holmes, E.; Nicholson, J.K. Pattern Recognition Methods and Application in Biomedical Magnetic Resonance. Prog. NMR Spectroscopy 2001, 39, 1–40. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K. Spectroscopic and Statistical Techniques for Information Recovery in Metabonomics and Metabolomics. Annu. Rev. Anal. Chem. 2008, 1, 45–69. [Google Scholar] [CrossRef]
- The Handbook of Metabonomics and Metabolomics; Lindon, J.C.; Nicholson, J.K.; Holmes, E. (Eds.) Elsevier: Amsterdam, the Netherland, 2007; p. 561.
- Wishaert, D.S. Quantitative Metabolomics Using NMR. Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K. Analytical Technologies for Metabonomics and Metabolomics, and Mulit-Omic Information Recovery. Trends Anal. Chem. 2008, 27, 194–204. [Google Scholar] [CrossRef]
- Coen, M.; Homes, E.; Lindon, J.C.; Nicholson, J.K. NMR-Based Metabolic Profiling and Metabonomic Approaches to Problems in Molecular Technology. Chem. Res. Toxicol. 2008, 21, 9–27. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in Metabonomics. Journal Proteome Research 2007, 6, 469–479. [Google Scholar] [CrossRef]
- Ebbels, T.M.D. Non-Linear Methods for the Analysis of Metabolic Profiles. In The Handbook of Metabonomics and Metabolomics; Lindon, J.C., Nicholson, J.K., Holmes, E., Eds.; Elsevier: Ansterdam, The Netherland, 2007; pp. 201–226. [Google Scholar]
- Gavaghan, C.L.; Wilson, I.D.; Nicholson, J.K. Physiological Variation in Metabolic Phenotyping and Functional Genomic Studies: Use of Orthogonal Signal Correction and PLS-DA. FEBS Letters 2002, 530, 191–196. [Google Scholar] [CrossRef]
- Southam, A.D.; Lange, A.; Hines, A.; Hill, E.M.; Katsu, Y.; Iguchi, T.; Tyler, C.R.; Viant, M.R. Metabolomics Reveals Target and Off-Target Toxicities of a Model Organophosphate Pesticide to Roach (Rutilus rutilus): Implications for Biomonitoring. Environ. Sci. Technol. 2011, 45, 3759–3767. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic Influence of Organophosphate,Carbamate, and Organochlorine Pesticides on Cellular Metabolism of Lipids, Proteins, and Carbohydrates: A Systematic Review. Hum. Exp. Toxicol. 2010, 30, 1119–1140. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probalistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Analytical Chemistry 2006, 78, 4281–4290. [Google Scholar]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and Normalization Effects in NMR Spectroscopic Metabonomic Data Sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar]
- Brindle, J.T.; et al. Rapid and Noninvasive Diagnosis of the Presence and Severity of Coronary Heart Disease Using 1H-NMR-Based Metabonomics. Nature Medicine 2002, 8, 1439–1444. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal Projections to Latent Structures (O-PLS). J. Chemometrics 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Trygg, J. O2-PLS for Qualitative and Quantitative Analysis in Multivariate Calibration. J. Chemometrics 2002, 16, 283–293. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of Some Variable Selection Methods When Multicollinearity is Present. Chemometr. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alam, T.M.; Neerathilingam, M.; Alam, M.K.; Volk, D.E.; Ansari, G.A.S.; Sarkar, S.; Luxon, B.A. 1H Nuclear Magnetic Resonance (NMR) Metabolomic Study of Chronic Organophosphate Exposure in Rats. Metabolites 2012, 2, 479-495. https://doi.org/10.3390/metabo2030479
Alam TM, Neerathilingam M, Alam MK, Volk DE, Ansari GAS, Sarkar S, Luxon BA. 1H Nuclear Magnetic Resonance (NMR) Metabolomic Study of Chronic Organophosphate Exposure in Rats. Metabolites. 2012; 2(3):479-495. https://doi.org/10.3390/metabo2030479
Chicago/Turabian StyleAlam, Todd M., Muniasamy Neerathilingam, M. Kathleen Alam, David E. Volk, G. A. Shakeel Ansari, Swapna Sarkar, and Bruce A. Luxon. 2012. "1H Nuclear Magnetic Resonance (NMR) Metabolomic Study of Chronic Organophosphate Exposure in Rats" Metabolites 2, no. 3: 479-495. https://doi.org/10.3390/metabo2030479