Application of Passive Sampling to Characterise the Fish Exometabolome
Abstract
:1. Introduction
2. Results
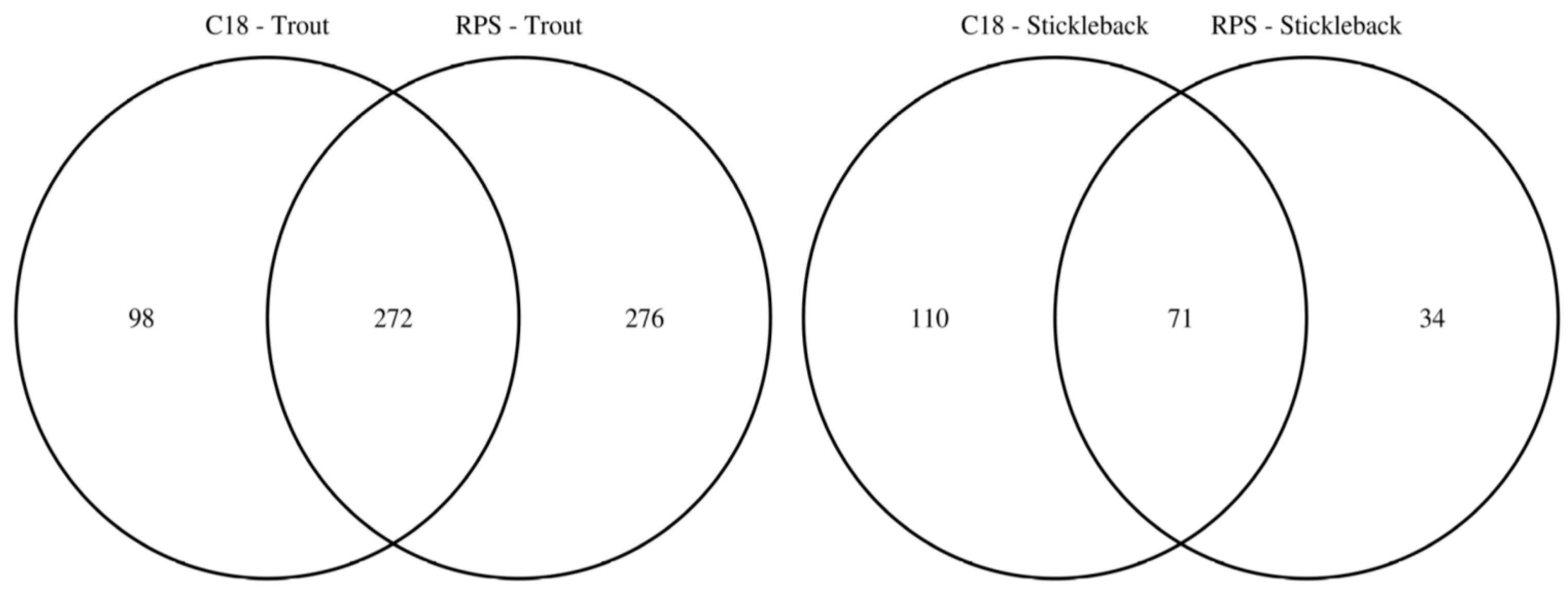
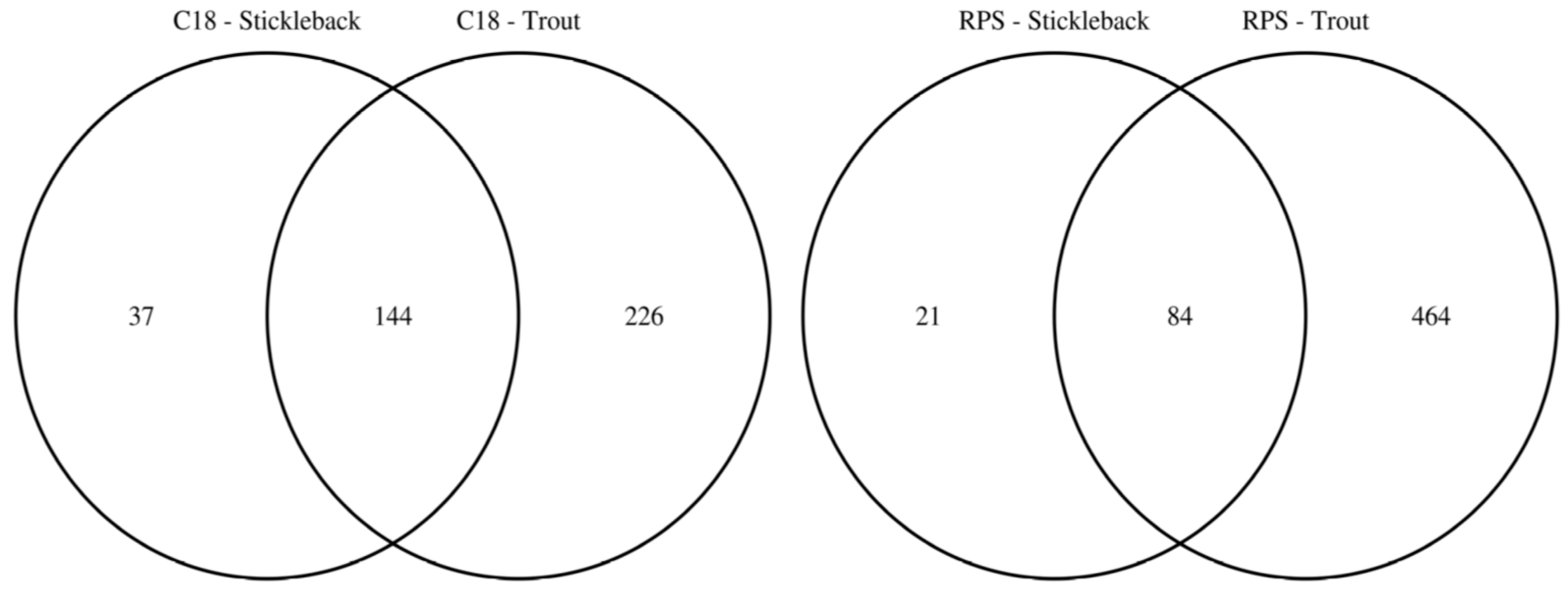
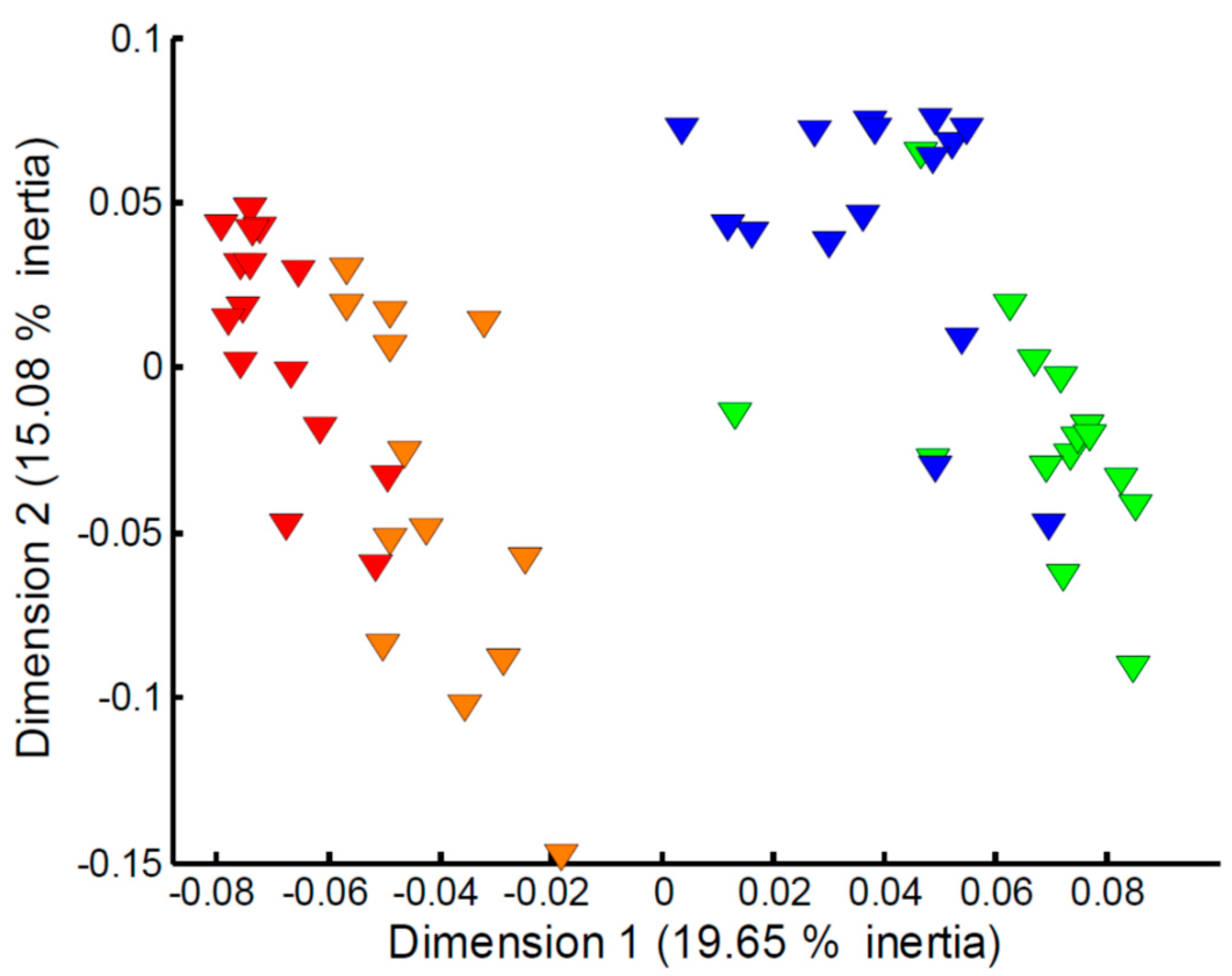
2.1. Effect of Empore™ Disk and Fish Species on the Measured Exometabolome
2.2. Comparison of Putatively Annotated Metabolites Captured on Empore™ Disks
2.3. Effect of Sampling Time on the Measured Fish Exometabolome
3. Discussion
4. Materials and Methods
4.1. Fish Husbandry
4.2. Capture of Fish Exometabolomes Using Chemcatcher® and Empore™ Disks
4.3. Extraction of Receiving Phase Empore™ Disks
4.4. Direct Infusion Mass Spectrometry Metabolomics
4.5. Data Processing and Peak Annotation
4.6. Statistical Analyses of Metabolomics Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, T.D.; Wu, H.F.; Santos, E.M.; Ball, J.; Katsiadaki, I.; Brown, M.M.; Baker, P.; Ortega, F.; Falciani, F.; Craft, J.A.; et al. Hepatic transcriptomic and metabolomic responses in the stickleback (gasterosteus aculeatus) exposed to environmentally relevant concentrations of dibenzanthracene. Environ. Sci. Technol. 2009, 43, 6341–6348. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.; Ball, J.S.; Williams, T.D.; Wu, H.F.; Ortega, F.; van Aerle, R.; Katsiadaki, I.; Falciani, F.; Viant, M.R.; Chipman, J.K.; et al. Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a fish model. Environ. Sci. Technol. 2010, 44, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Lange, A.; Hines, A.; Hill, E.M.; Katsu, Y.; Iguchi, T.; Tyler, C.R.; Viant, M.R. Metabolomics reveals target and off-target toxicities of a model organophosphate pesticide to roach (rutilus rutilus): Implications for biomonitoring. Environ. Sci. Technol. 2011, 45, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Zare, A.; Jackson, L.J.; Habibi, H.R.; Weljie, A.M. Environmental contaminant mixtures at ambient concentrations invoke a metabolic stress response in goldfish not predicted from exposure to individual compounds alone. J. Proteom. Res. 2012, 11, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Schock, T.B.; Newton, S.; Brenkert, K.; Leffler, J.; Bearden, D.W. An nmr-based metabolomic assessment of cultured cobia health in response to dietary manipulation. Food Chem. 2012, 133, 90–101. [Google Scholar] [CrossRef]
- Softeland, L.; Kirwan, J.A.; Hori, T.S.F.; Storseth, T.R.; Sommer, U.; Berntssen, M.H.G.; Viant, M.R.; Rise, M.L.; Waagbo, R.; Torstensen, B.E.; et al. Toxicological effect of single contaminants and contaminant mixtures associated with plant ingredients in novel salmon feeds. Food Chem. Toxicol. 2014, 73, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Easton, J.M.; Stentiford, G.D.; Ludwig, C.; Arvanitis, T.N.; Viant, M.R. Metabolic changes in flatfish hepatic tumours revealed by nmr-based metabolomics and metabolic correlation networks. J. Proteom. Res. 2008, 7, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Mirbahai, L.; Southam, A.D.; Sommer, U.; Williams, T.D.; Bignell, J.P.; Lyons, B.P.; Viant, M.R.; Chipman, J.K. Disruption of DNA methylation via s-adenosylhomocysteine is a key process in high incidence liver carcinogenesis in fish. J. Proteom. Res. 2013, 12, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Wise, D.; Greenway, T.; Khoo, L.; Griffin, M.J.; Jablonsky, M. Using 1-d h-1 and 2-d h-1 j-resolved nmr metabolomics to understand the effects of anemia in channel catfish (ictalurus punctatus). Metabolomics 2015, 11, 1131–1143. [Google Scholar] [CrossRef]
- Viant, M.R.; Bundy, J.G.; Pincetich, C.A.; de Ropp, J.S.; Tjeerdema, R.S. Nmr-derived developmental metabolic trajectories: An approach for visualizing the toxic actions of trichloroethylene during embryogenesis. Metabolomics 2005, 1, 149–158. [Google Scholar] [CrossRef]
- Huang, S.-M.; Xu, F.; Lam, S.H.; Gong, Z.; Ong, C.N. Metabolomics of developing zebrafish embryos using gas chromatography- and liquid chromatography-mass spectrometry. Mol. Biosyst. 2013, 9, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.D.M.; Leisen, J.; Zhou, M.; Byrne, J.J.; Lim-Hing, K.; Webb, H.D.; Gelbaum, L.; Viant, M.R.; Kubanek, J.; Fernandez, F.M. Biomarkers of whale shark health: A metabolomic approach. PLoS ONE 2012, 7, e49379. [Google Scholar] [CrossRef] [PubMed]
- Ekman, D.R.; Teng, Q.; Jensen, K.M.; Martinovic, D.; Villeneuve, D.L.; Ankley, G.T.; Collette, T.W. Nmr analysis of male fathead minnow urinary metabolites: A potential approach for studying impacts of chemical exposures. Aquat. Toxicol. 2007, 85, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Date, Y.; Akama, M.; Kikuchi, J. Comparative metabolomic and ionomic approach for abundant fishes in estuarine environments of Japan. Sci. Rep. 2014, 4, 7005. [Google Scholar] [CrossRef] [PubMed]
- Al-Salhi, R.; Abdul-Sada, A.; Lange, A.; Tyler, C.R.; Hill, E.M. The xenometabolome and novel contaminant markers in fish exposed to a wastewater treatment works effluent. Environ. Sci. Technol. 2012, 46, 9080–9088. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Abdul-Sada, A.; Lange, A.; Tyler, C.R.; Hill, E.M. A new approach for plasma (xeno)metabolomics based on solid-phase extraction and nanoflow liquid chromatography-nanoelectrospray ionisation mass spectrometry. J. Chromatogr. A 2014, 1365, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Lange, A.; Al-Salhi, R.; Hill, E.M.; Tyler, C.R.; Viant, M.R. Distinguishing between the metabolome and xenobiotic exposome in environmental field samples analysed by direct-infusion mass spectrometry based metabolomics and lipidomics. Metabolomics 2014, 10, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Scott, A.P.; Siefkes, M.J.; Yan, H.G.; Liu, Q.; Yun, S.S.; Gage, D.A. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 2002, 296, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; James, J.D.; Stewart, C.; Scott, A.P. A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J. Fish Biol. 2004, 65, 1233–1252. [Google Scholar] [CrossRef]
- Ellis, T.; James, J.D.; Sundh, H.; Fridell, F.; Sundell, K.; Scott, A.P. Non-invasive measurement of cortisol and melatonin in tanks stocked with seawater atlantic salmon. Aquaculture 2007, 272, 698–706. [Google Scholar] [CrossRef]
- Sebire, M.; Katsiadaki, I.; Scott, A.P. Non-invasive measurement of 11-ketotestosterone, cortisol and androstenedione in male three-spined stickleback (gasterosteus aculeatus). Gen. Comp. Endocrinol. 2007, 152, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Yildiz, H.Y.; Lopez-Olmeda, J.; Spedicato, M.T.; Tort, L.; Overli, O.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Davey, H.M.; Broadhurst, D.; Heald, J.K.; Rowland, J.J.; Oliver, S.G.; Kell, D.B. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat. Biotechnol. 2003, 21, 692–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kell, D.B.; Brown, M.; Davey, H.M.; Dunn, W.B.; Spasic, I.; Oliver, S.G. Metabolic footprinting and systems biology: The medium is the message. Nat. Rev. Microbiol. 2005, 3, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Dittmar, T.; Bondarev, V.; Weber, R.J.M.; Viant, M.R.; Schulz-Vogt, H.N. Exo-metabolome of pseudovibrio sp fo-beg1 analyzed by ultra-high resolution mass spectrometry and the effect of phosphate limitation. PLoS ONE 2014, 9, e96038. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Heuschele, J.; Nylund, G.M.; Pohnert, G.; Pavia, H.; Bjaerke, O.; Pender-Healy, L.A.; Tiselius, P.; Kiorboe, T. Solid phase extraction and metabolic profiling of exudates from living copepods. PeerJ 2016, 4, e1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingston, J.K.; Greenwood, R.; Mills, G.A.; Morrison, G.M.; Persson, L.B. Development of a novel passive sampling system for the time-averaged measurement of a range of organic pollutants in aquatic environments. J. Environ. Monit. 2000, 2, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Vrana, B.; Mills, G.A.; Allan, I.J.; Dominiak, E.; Svensson, K.; Knutsson, J.; Morrison, G.; Greenwood, R. Passive sampling techniques for monitoring pollutants in water. Trac-Trends Anal. Chem. 2005, 24, 845–868. [Google Scholar] [CrossRef]
- Vrana, B.; Mills, G.A.; Kotterman, M.; Leonards, P.; Booij, K.; Greenwood, R. Modelling and field application of the chemcatcher passive sampler calibration data for the monitoring of hydrophobic organic pollutants in water. Environ. Pollut. 2007, 145, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, E.L.M.; Bramaz, N.; Hollender, J.; Singer, H.; Escher, B.I. Passive sampling combined with ecotoxicological and chemical analysis of pharmaceuticals and biocides—Evaluation of three chemcatcher™ configurations. Water Res. 2009, 43, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.J.; Knutsson, J.; Guigues, N.; Mills, G.A.; Fouillac, A.-M.; Greenwood, R. Evaluation of the chemcatcher and dgt passive samplers for monitoring metals with highly fluctuating water concentrations. J. Environ. Monit. 2007, 9, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southam, A.D.; Payne, T.G.; Cooper, H.J.; Arvanitis, T.N.; Viant, M.R. Dynamic range and mass accuracy of wide-scan direct infusion nanoelectrospray fourier transform ion cyclotron resonance mass spectrometry-based metabolomics increased by the spectral stitching method. Anal. Chem. 2007, 79, 4595–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, R.J.M.; Southam, A.D.; Sommer, U.; Viant, M.R. Characterization of isotopic abundance measurements in high resolution ft-icr and orbitrap mass spectra for improved confidence of metabolite identification. Anal. Chem. 2011, 83, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.A.; Weber, R.J.M.; Broadhurst, D.I.; Viant, M.R. Direct infusion mass spectrometry metabolomics dataset: A benchmark for data processing and quality control. Sci. Data 2014, 1, 140012. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.S.; Weber, R.J.M.; Southam, A.D.; Payne, T.G.; Hrydziuszko, O.; Arvanitis, T.N.; Viant, M.R. A new approach to toxicity testing in daphnia magna: Application of high throughput ft-icr mass spectrometry metabolomics. Metabolomics 2009, 5, 44–58. [Google Scholar] [CrossRef]
- Denton, J.E.; Yousef, M.K.; Yousef, I.M.; Kuksis, A. Bile acid compostion of rainbow trout (salmo gairdneri). Lipids 1974, 9, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.J.M.; Li, E.; Bruty, J.; He, S.; Viant, M.R. Maconda: A publicly accessible mass spectrometry contaminants database. Bioinformatics 2012, 28, 2856–2857. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, P.C.; Hara, T.J. Discrimination of bile acids by the rainbow trout olfactory system: Evidence as potential pheromone. Biol. Res. 2008, 41, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.G.; Southam, A.D.; Arvanitis, T.N.; Viant, M.R. A signal filtering method for improved quantification and noise discrimination in fourier transform ion cyclotron resonance mass spectrometry-based metabolomics data. J. Am. Soc. Mass Spectrom. 2009, 20, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1h nmr metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Hrydziuszko, O.; Viant, M.R. Missing values in mass spectrometry based metabolomics: An undervalued step in the data processing pipeline. Metabolomics 2012, 8, S161–S174. [Google Scholar] [CrossRef]
- Parsons, H.M.; Ludwig, C.; Gunther, U.L.; Viant, M.R. Improved classification accuracy in 1- and 2-dimensional nmr metabolomics data using the variance stabilising generalised logarithm transformation. BMC Bioinform. 2007, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.A.; Broadhurst, D.I.; Davidson, R.L.; Viant, M.R. Characterising and correcting batch variation in an automated direct infusion mass spectrometry (dims) metabolomics workflow. Anal. Bioanal. Chem. 2013, 405, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.J.M.; Viant, M.R. Mi-pack: Increased confidence of metabolite identification in mass spectra by integrating accurate masses and metabolic pathways. Chemom. Intell. Lab. Syst. 2010, 104, 75–82. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in kegg. Nucleic Acids Res 2006, 34, D354–D357. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viant, M.R.; Elphinstone Davis, J.; Duffy, C.; Engel, J.; Stenton, C.; Sebire, M.; Katsiadaki, I. Application of Passive Sampling to Characterise the Fish Exometabolome. Metabolites 2017, 7, 8. https://doi.org/10.3390/metabo7010008
Viant MR, Elphinstone Davis J, Duffy C, Engel J, Stenton C, Sebire M, Katsiadaki I. Application of Passive Sampling to Characterise the Fish Exometabolome. Metabolites. 2017; 7(1):8. https://doi.org/10.3390/metabo7010008
Chicago/Turabian StyleViant, Mark R., Jessica Elphinstone Davis, Cathleen Duffy, Jasper Engel, Craig Stenton, Marion Sebire, and Ioanna Katsiadaki. 2017. "Application of Passive Sampling to Characterise the Fish Exometabolome" Metabolites 7, no. 1: 8. https://doi.org/10.3390/metabo7010008





