NMR Profiling of Metabolites in Larval and Juvenile Blue Mussels (Mytilus edulis) under Ambient and Low Salinity Conditions
Abstract
:1. Introduction
2. Results
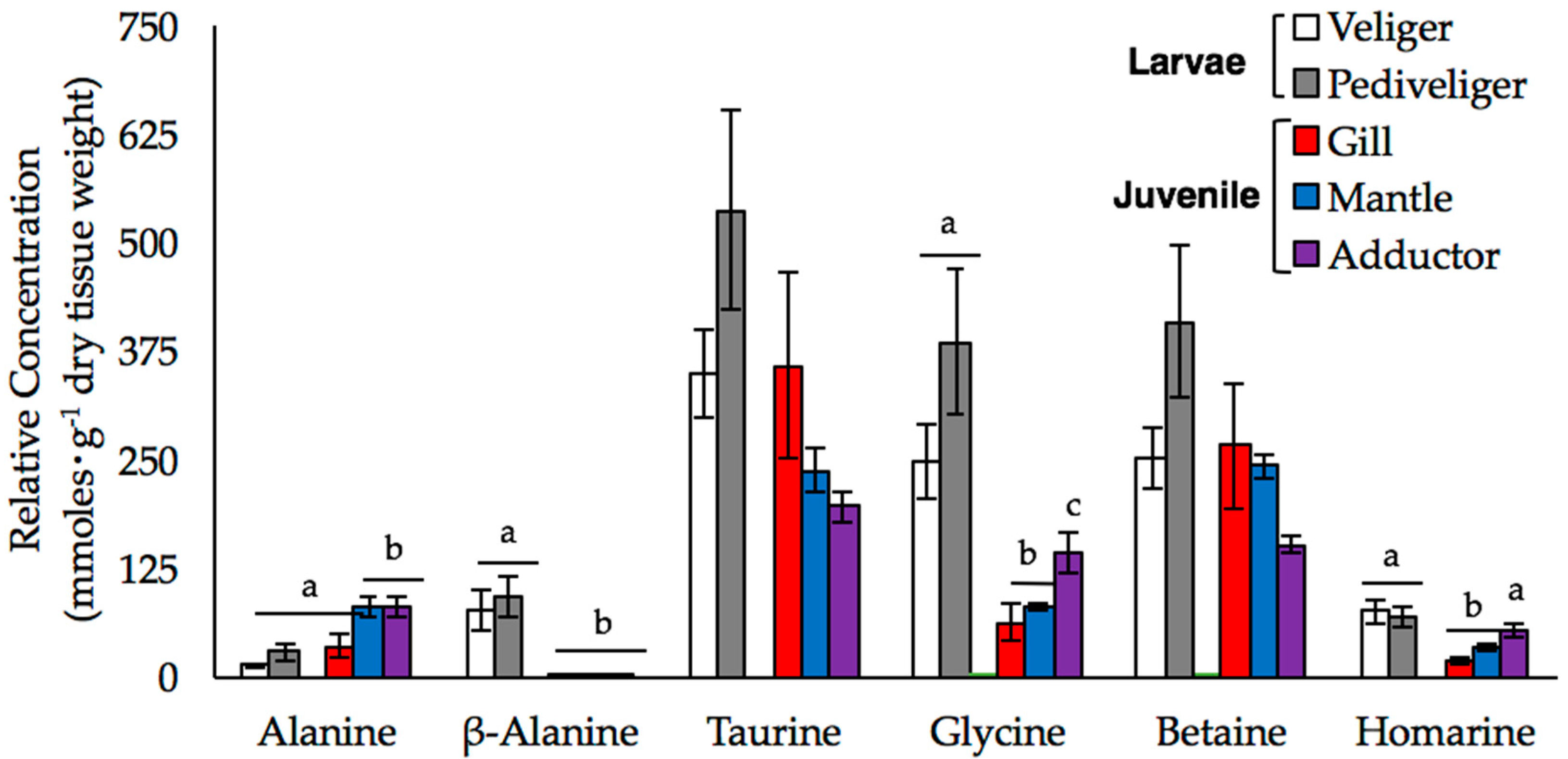
2.1. Baseline Metabolic Profiles
2.2. Metabolite Concentrations during Hypoosmotic Expsoure
3. Discussion
3.1. Baseline Metabolic Profiles
3.2. Metabolite Concentrations During Hypoosmotic Exposure
4. Materials and Methods
4.1. Sample Collection
4.2. NMR Spectroscopy
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arribas, L.P.; Donnarumma, L.; Gabriela Palomo, M.; Scrosati, R.A. Intertidal mussels as ecosystem engineers: Their associated invertebrate biodiversity under contrasting wave exposures. Mar. Biodivers. 2014, 44, 203–211. [Google Scholar] [CrossRef]
- Goldberg, E.D. The mussel watch—A first step in global marine monitoring. Mar. Pollut. Bull. 1975, 6, 111. [Google Scholar] [CrossRef]
- Fasulo, S.; Iacono, F.; Cappello, T.; Corsaro, C.; Maisano, M.; D’Agata, A.; Giannetto, A.; De Domenico, E.; Parrino, V.; Lo Paro, G.; et al. Metabolomic investigation of Mytilus galloprovincialis (Lamarck 1819) caged in aquatic environments. Ecotoxicol. Environ. Saf. 2012, 84, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Maisano, M.; D’Agata, A.; Natalotto, A.; Mauceri, A.; Fasulo, S. Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Mar. Environ. Res. 2013, 91, 52–60. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, A.; Cappello, T.; Maisano, M.; Parrino, V.; Giannetto, A.; Brundo, M.V.; Ferrante, M.; Mauceri, A. Cellular biomarkers in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Lake Faro (Sicily, Italy). Ital. J. Zool. 2014, 81, 43–54. [Google Scholar] [CrossRef]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-inducible factor α and Hif-prolyl hydroxylase characterization and gene expression in short-time air-exposed Mytilus galloprovincialis. Mar. Biotechnol. 2015, 17, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Maisano, M.; Cappello, T.; Natalotto, A.; Vitale, V.; Parrino, V.; Giannetto, A.; Oliva, S.; Mancini, G.; Cappello, S.; Mauceri, A.; et al. Effects of petrochemical contamination on caged marine mussels using a multi-biomarker approach: Histological changes, neurotoxicity and hypoxic stress. Mar. Environ. Res. 2017, 128, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Tuffnail, W.; Mills, G.A.; Cary, P.; Greenwood, R. An environmental 1H NMR metabolomics study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia, and starvation. Metabolomics 2009, 5, 33–43. [Google Scholar] [CrossRef]
- Ellis, R.P.; Spicer, J.I.; Byrne, J.J.; Sommer, U.; Viant, M.R.; White, D.A.; Widdicombe, S. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environ. Sci. Technol. 2014, 48, 7044–7052. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Climate change stressors cause metabolic depression in the blue mussel, Mytilus edulis, from the Gulf of Maine. Limnol. Oceanogr. 2016, 61, 1705–1717. [Google Scholar] [CrossRef]
- Hines, A.; Oladiran, G.S.; Bignell, J.P.; Stentiford, G.D.; Viant, M.R. Direct sampling of organisms from the field and knowledge of their phenotype: Key recommendations for environmental metabolomics. Environ. Sci. Technol. 2007, 41, 3375–3381. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.H.; Dondero, F.; Viarengo, A.; Griffin, J.L. Metabolic profiling of Mytilus galloprovincialis and its potential applications for pollution assessment. Mar. Ecol. Prog. Ser. 2008, 369, 169–179. [Google Scholar] [CrossRef]
- Sprung, M. Physiological energetics of mussel larvae (Mytilus edulis). I. Shell growth and biomass. Mar. Ecol. Prog. Ser. 1984, 17, 283–293. [Google Scholar] [CrossRef]
- Sprung, M.; Widdows, J. Rate of heat dissipation by gamete and larval stages of Mytilus edulis. Mar. Biol. 1986, 91, 41–45. [Google Scholar] [CrossRef]
- Lange, R. The osmotic function of amino acids and taurine in the mussel, Mytilus edulis. Comp. Biochem. Physiol. 1963, 10, 173–179. [Google Scholar] [CrossRef]
- Bowlus, R.D.; Somero, G.N. Solute compatibility with enzyme function and structure: Rationales for the selection of osmotic agents and end-products of anaerobic metabolism in marine invertebrates. J. Exp. Zoolog. 1979, 208, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. Protons, osmolytes and fitness of internal milieu for protein function. Am. J. Physiol. 1986, 251, R197–R213. [Google Scholar] [PubMed]
- Shumway, S.E.; Gabbott, P.A.; Youngson, A. The effect of fluctuating salinity on the concentrations of free amino acids and ninhydrin-positive substances in the adductor muscles of eight species of bivalve molluscs. J. Exp. Mar. Biol. Ecol. 1977, 29, 131–150. [Google Scholar] [CrossRef]
- Davenport, J. Is Mytilus edulis a short term osmoregulator? Comp. Biochem. Physiol. 1979, 64A, 91–95. [Google Scholar] [CrossRef]
- Livingstone, D.R.; Widdows, J.; Fieth, P. Aspects of nitrogen metabolism of the common mussel Mytilus edulis: Adaptation to abrupt and fluctuating changes in salinity. Mar. Biol. 1979, 53, 41–55. [Google Scholar] [CrossRef]
- Deaton, L.E.; Hilbish, T.J.; Koehn, R.K. Hyperosmotic volume regulation in the tissues of the mussel Mytilus edulis. Comp. Biochem. Physiol. 1985, 80A, 571–574. [Google Scholar] [CrossRef]
- Neufeld, D.S.; Wright, S.H. Basolateral transport of taurine in the epithelial cells of isolated, perfused Mytilus californianus gills. J. Exp. Biol. 1995, 198, 465–473. [Google Scholar] [PubMed]
- Manahan, D.T. The uptake and metabolism of dissolved amino acids by bivalve larvae. Biol. Bull. 1983, 164, 236–250. [Google Scholar] [CrossRef]
- Welborn, J.R.; Manahan, D.T. Taurine metabolism in larvae of marine molluscs (Bivalvia, Gastropoda). J. Exp. Biol. 1995, 198, 1791–1799. Available online: http://jeb.biologists.org/content/198/8/1791.long (accessed on 4 July 2017). [PubMed]
- Tikunov, A.P.; Johnson, C.B.; Lee, H.; Stoskopf, M.K.; Macdonald, J.M. Metabolomic investigations of American oysters using 1H-NMR spectroscopy. Mar. Drugs 2010, 8, 2578–2596. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Mauceri, A.; Corsaro, C.; Maisano, M.; Parrino, V.; Lo Paro, G.; Messina, G.; Fasulo, S. Impact of environmental pollution on cages mussels Mytilus galloprovincialis using NMR-based metabolomics. Mar. Pollut. Bull. 2013, 77, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Maisano, M.; Giannetto, A.; Parrino, V.; Mauceri, A.; Fasulo, S. Neurotoxicological effects on marine mussel Mytilus galloprovincialis caged at petrochemical contaminated areas (eastern Sicily, Italy): 1H NMR and immunohistochemical assays. Comp. Biochem. Phsyiol. C Toxicol. Pharmacol. 2015, 169, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Maisano, M.; Mauceri, A.; Fasulo, S. 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2017, 142, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.R. The free amino-acid of invertebrate nerve. Biochem. J. 1952, 52, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bricteux-Grégoire, S.; Duchâteau-Bosson, G.; Jeuniaux, C.; Florkin, M. Constituants osmotiquement actifs des muscles adducteurs de Mytilus edulis adaptée a l’eau de mer ou a l’eau saumâtre. Arch. Int. Physiol. Biochim. 1964, 72, 116–123. (In French) [Google Scholar] [PubMed]
- Rice, M.A.; Stephens, G.C. Influx and transepithelial flux of amino acids in the mussel Mytilus edulis. J. Exp. Biol. 1988, 135, 275–287. Available online: http://jeb.biologists.org/content/135/1/275 (accessed on 4 July 2017).
- Wright, S.H.; Secomb, T.W.; Bradley, T.J. Apical membrane permeability of Mytilus gill: Influence of ultrastructure, salinity, and competitive inhibitors on amino acid fluxes. J. Exp. Biol. 1987, 129, 205–230. Available online: http://jeb.biologists.org/content/129/1/205 (accessed on 4 July 2017).
- Wright, S.H.; Secomb, T.W. Epithelial amino acid transport in marine mussels: Role in net exchange of taurine between gills and seawater. J. Exp. Biol. 1986, 121, 251–270. Available online: http://jeb.biologists.org/content/121/1/251 (accessed on 4 July 2017).
- Deaton, L.E. Hyperosmotic volume regulation in the gills of the ribbed mussel, Geukensia demissa: Rapid accumulation of betaine and alanine. J. Exp. Mar. Biol. Ecol. 2001, 260, 185–197. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic, and counteracting cryoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Bishop, S.H.; Ellis, L.L.; Burcham, J.M. 6. Amino acid metabolism in molluscs. In The Mollusca; Hochachka, P.W., Ed.; Academic Press: New York, NY, USA, 1983; Volume 1, pp. 275–279. [Google Scholar]
- Stephens, G.C.; Schinske, R.A. Uptake of amino acids by marine invertebrates. Limnol. Oceanograp. 1961, 6, 175–181. [Google Scholar] [CrossRef]
- Crawford, C.C.; Webb, K.L. Amino acid flux in an estuary. Science 1968, 159, 1463–1464. [Google Scholar] [CrossRef]
- Péquignat, E. A kinetic and autoradiographic study of the direct assimilation of amino acids and glucose by organs of the mussel Mytilus edulis. Mar. Biol. 1973, 19, 227–244. [Google Scholar] [CrossRef]
- Zandee, D.I.; Kluytmans, J.H.; Zurburg, W.; Pieters, H. Seasonal variations in biochemical composition of Mytilus edulis with reference to energy metabolism and gametogenesis. Neth. J. Sea Res. 1980, 14, 1–29. [Google Scholar] [CrossRef]
- Gasteiger, E.L.; Haake, P.C.; Gergen, J.A. An investigation of the distribution and function of homarine (N-methyl picolinic acid). Ann. N. Y. Acad. Sci. 1960, 90, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Netherton, J.C.; Gurin, S. Biosynthesis and physiological role of homarine in marine shrimp. J. Biol. Chem. 1982, 257, 11971–11975. Available online: http://www.jbc.org/content/257/20/11971.long (accessed on 4 July 2017). [PubMed]
- Nishitani, H.; Kikuchi, S.; Okumura, K.; Taguchi, H. Finding of a homarine-synthesizing enzyme in turban shell and some properties of the enzyme. Arch. Biochem. Biophys. 1995, 322, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Wright, S.H. Integumental taurine transport in Mytilus gill: Short-term adaptation to reduced salinity. J. Exp. Biol. 1992, 162, 265–279. Available online: http://jeb.biologists.org/content/162/1/265.long (accessed on 4 July 2017). [PubMed]
- Manahan, D.T. Amino acid fluxes to and from seawater in axenic veliger larvae of a bivalve (Crassostrea gigas). Mar. Ecol. Prog. Ser. 1989, 53, 247–255. Available online: http://www.jstor.org/stable/24833690 (accessed on 4 July 2017). [CrossRef]
- Widdows, J. Physiological ecology of mussel larvae. Aquaculture 1991, 94, 147–163. [Google Scholar] [CrossRef]
- Kasschau, M.R.; Skisak, C.M.; Cook, J.P.; Mills, W.R. β-Alanine metabolism and high salinity stress in the sea anemone, Bundosoma cavernata. J. Comp. Physiol. B 1984, 154, 181–186. [Google Scholar] [CrossRef]
- Kluytmans, J.H.; Zandee, D.I.; Zurburg, W.; Pieters, H. The influence of seasonal changes on energy metabolism in Mytilus edulis (L.)—III. Anaerobic energy metabolism. Comp. Biochem. Physiol. 1980, 67B, 307–315. [Google Scholar] [CrossRef]
- Gilles, R. Osmoregulation in three molluscs: Acanthochitona discrepans (Brown), Glycymeris glycymeris (L.), and Mytilus edulis (L.). Biol. Bull. 1972, 142, 25–35. [Google Scholar] [CrossRef]
- Hoyaux, J.; Gilles, R.; Jeuniaux, C. Osmoregulation in molluscs of the intertidal zone. Comp. Biochem. Physiol. 1976, 53A, 361–365. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [PubMed]
- Meng, J.; Zhu, Q.; Zhang, L.; Li, C.; Li, L.; She, Z.; Huang, B.; Zhang, G. Genome and transcriptome analyses provide insight into the euryhaline adaptation mechanism of Crassostrea gigas. PLoS ONE 2013, 8, e58563. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Awapara, J. Metabolism of sulfur amino acids in Mytilus edulis and Rangia cuneata. Biol. Bull. 1960, 118, 173–182. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves: A Practical Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; Volume 471, p. 177. [Google Scholar]
- Newell, R.I.E. Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (North and Mid-Atlantic)—Blue Mussel; U.S. Fish and Wildlife service Biological Report; TR EL-82-4; U.S. Fish and Wildlife Service: Fort Belvoir, VA, USA, 1989; Volume 82.
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Manal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. Available online: http://www.hmdb.ca/ (accessed on 4 July 2017). [CrossRef] [PubMed]
- Qiu, J.-W.; Tremblay, R.; Bourget, E. Ontogenetic changes in hyposaline tolerance in the mussels Mytilus edulis and M. trossulus: Implications for distributions. Mar. Ecol. Prog. Ser. 2002, 228, 143–152. [Google Scholar] [CrossRef]
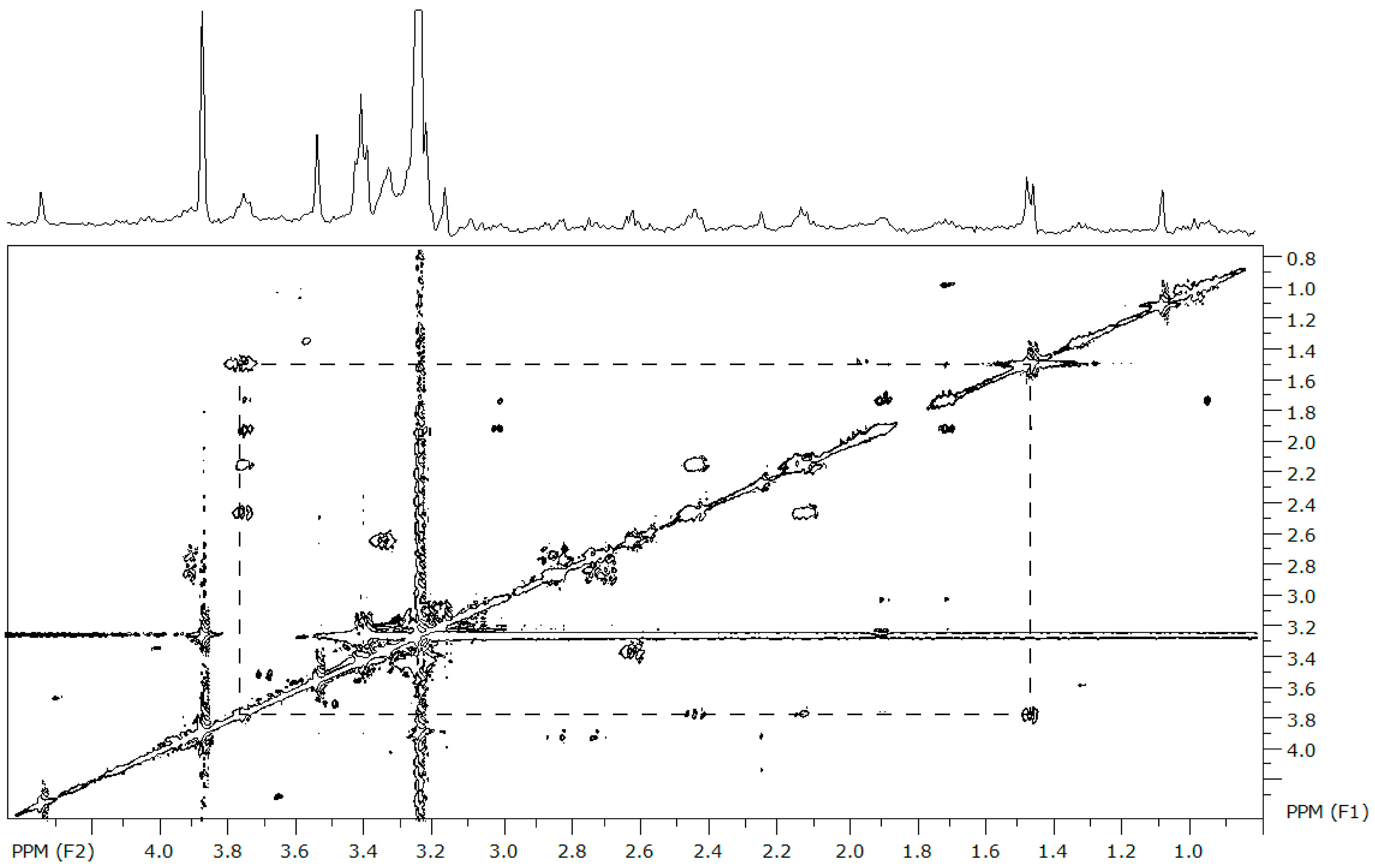

| No | Metabolite | Chemical Shift (Multiplicity) 1 |
|---|---|---|
| Common to all samples | ||
| 1 | Alanine | 1.46 (d), 3.77 (t) |
| 2 | Aspartate | 2.68 (dd), 2.82 (dd), 3.87 (dd) |
| 3 | Betaine | 3.26 (s), 3.91 (s) |
| 4 | Glycine | 3.55 (s) |
| 5 | Homarine | 4.36 (s), 7.96 (m), 8.03 (d), 8.53 (m), 8.69 (d) |
| 6 | Hypotaurine | 2.63 (t), 3.36 (t) |
| 7 | Isoleucine | 0.98 (m), 1.03 (m) |
| 8 | Taurine | 3.25 (t), 3.43 (t) |
| 9 | Threonine | 1.32 (d), 3.58 (d), 4.25 (t) |
| Common to larvae and juveniles | ||
| 10 | Arginine | 1.73 (m), 1.93 (m), 3.23 (m), 3.76 (m) |
| 11 | β-Alanine | 2.56 (t), 3.18 (t) |
| 12 | Glutamate | 2.05 (m), 2.14 (m), 2.38 (m), 3.76 (m) |
| 13 | Glutamine | 2.14 (m), 2.42 (m), 3.76 |
| 14 | Leucine | 0.96 (t), 1.72 (m) |
| 15 | Lysine | 1.73 (m), 1.88 (m), 3.02 (t) |
| 16 | Unknown #2 2 | 1.24 (s) |
| 17 | Unknown Metabolite | 0.87 (m) |
| 18 | Unknown Metabolite | 3.66 (m), 4.28 (d) |
| 19 | Unknown Metabolite | 3.75 (d), 4.27 (t) |
| Larvae-specific | ||
| 20 | Unknown Metabolite | 2.92 (s) |
| 21 | Unknown Metabolite | 2.96 (s) |
| 22 | Lactic acid | 1.32 (d), 4.12 (m) |
| Juvenile-specific | ||
| 23 | Unknown #1 2 | 1.09 (s) |
| 24 | Unknown Metabolite | 2.25 (s) |
| 25 | Unknown Metabolite | 2.56 (s) |
| 26 | Unknown Metabolite | 3.11 (s), 3.28 (s) |
| Gill | Exposure at 20 ppt 2 | |||||
|---|---|---|---|---|---|---|
| Control | 24 h | 48 h | 72 h | F 3 | p | |
| Taurine | 357.3 ± 239.7 | 345.3 ± 36.6 (−3%) | 343.2 ± 46.9 (−4%) | 359.6 ± 45.0 (0%) | 0.02 | 0.995 |
| Betaine | 266.2 ± 163.2 | 277.0 ± 50.3 (+4%) | 275.3 ± 61.3 (+3%) | 306.1 ± 45.2 (+15%) | 0.171 | 0.914 |
| Glycine | 62.8 ± 45.4 | 17.6 ± 6.0 (−72%) | 14.8 ± 5.5 (−76%) | 22.7 ± 11.3 (−63%) | 4.48 | 0.018 |
| Homarine | 17.4 ± 8.3 | 13.3 ± 3.9 (−23%) | 7.7 ± 4.1 (−56%) | 16.3 ± 4.0 (−6%) | 3.21 | 0.051 |
| Alanine | 34.2 ± 30.3 | 22.2 ± 7.7 (−35%) | 23.5 ± 7.8 (−31%) | 24.0 ± 10.0 (−30%) | 0.54 | 0.665 |
| Mantle | ||||||
| Taurine | 238.2 ± 54.9 | 224.6 ± 27.7 (−6%) | 200.3 ± 61.8 (−15%) | 220.3 ± 45.1 (−8%) | 0.51 | 0.680 |
| Betaine | 243.3 ± 30.2 | 243.0 ± 50.9 (0%) | 266.2 ± 65.2 (+9%) | 255.3 ± 72.9 (+5) | 0.19 | 0.904 |
| Glycine | 81.5 ± 10.3 | 52.9 ± 13.5 (−35%) | 37.5 ± 38.1 (−53%) | 37.8 ± 23.9 (−54%) | 3.70 | 0.034 |
| Homarine | 34.2 ± 7.0 | 29.6 ± 7.6 (−13%) | 27.3 ± 13.7 (−20%) | 39.0 ± 15.8 (+14%) | 0.99 | 0.422 |
| Alanine | 80.4 ± 25.5 | 73.1 ± 23.6 (−9%) | 58.8 ± 65.0 (−27%) | 53.9 ± 53.0 (−33%) | 0.37 | 0.778 |
| Adductor | ||||||
| Taurine | 195.6 ± 38.1 | 216.3 ± 54.1 (+11%) | 263.8 ± 36.3 (+35%) | 198.1 ± 33.6 (+1%) | 2.93 | 0.065 |
| Betaine | 151.7 ± 20.3 | 177.7 ± 77.7 (+17%) | 213.7 ± 61.4 (+41%) | 158.6 ± 29.3 (+5%) | 1.39 | 0.281 |
| Glycine | 141.6 ± 51.7 | 137.4 ± 16.9 (−3%) | 110.3 ± 36.7 (−22%) | 100.7 ± 53.0 (−29%) | 1.13 | 0.365 |
| Homarine | 53.4 ± 19.7 | 57.8 ± 22.5 (+8%) | 48.3 ± 14.7 (−10%) | 65.4 ± 40.6 (+22%) | 0.38 | 0.768 |
| Alanine | 80.8 ± 24.3 | 66.1 ± 16.4 (−18%) | 58.6 ± 24.9 (−27%) | 63.3 ± 13.1 (−22%) | 1.11 | 0.373 |
| Veliger | Exposure at 20 ppt 2 | |||||
|---|---|---|---|---|---|---|
| Control | 24 h | 48 h | 72 h | F 3 | p | |
| Taurine | 349.5 ± 86.2 | 309.9 ± 27.4 (−11%) | 244.1 ± 60.6 (−30%) | 272.0 ± 65.1 (−22%) | 2.10 | 0.146 |
| Betaine | 251.9 ± 60.6 | 207.8 ± 9.7 (−18%) | 168.3 ± 47.0 (−33%) | 205.0 ± 55.9 (−19%) | 1.94 | 0.170 |
| Glycine | 248.4 ± 72.9 | 82.4 ± 11.3 (−67%) | 61.6 ± 24.1 (−75%) | 59.6 ± 19.1 (−76%) | 25.97 | <0.001 |
| β-alanine | 77.9 ± 40.7 | 38.7 ± 10.3 (−50%) | 38.6 ± 13.6 (−50%) | 44.0 ± 25.7 (−44%) | 2.37 | 0.115 |
| Homarine | 75.2 ± 21.4 | 54.6 ± 5.2 (−27%) | 48.6 ± 11.4 (−35%) | 49.5 ± 13.0 (−34%) | 3.30 | 0.052 |
| Alanine | 13.5 ± 4.5 | 12.8 ± 2.7 (−5%) | 10.9 ± 5.9 (−19%) | 11.9 ± 7.6 (−12%) | 0.17 | 0.918 |
| Pediveliger | ||||||
| Taurine | 537.5 ± 228.3 | 450.0 ± 207.0 (−16%) | 365.3 ± 158.1 (−32%) | 297.3 ± 51.0 (−45%) | 1.45 | 0.227 |
| Betaine | 408.7 ± 174.2 | 307.3 ± 136.4 (−25%) | 249.2 ± 91.1 (−39%) | 188.2 ± 15.9 (−54%) | 2.45 | 0.114 |
| Glycine | 384.6 ± 167.9 | 122.9 ± 60.8 (−68%) | 72.1 ± 22.8 (−81%) | 48.5 ± 5.3 (−87%) | 11.83 | 0.001 |
| β-alanine | 91.1 ± 46.6 | 54.1 ± 38.1 (−41%) | 29.6 ± 21.6 (−68%) | 25.0 ± 2.8 (−73%) | 3.58 | 0.047 |
| Homarine | 70.0 ± 23.6 | 57.8 ± 25.0 (−17%) | 49.4 ± 18.4 (−29%) | 32.0 ± 4.2 (−54%) | 2.64 | 0.097 |
| Alanine | 29.3 ± 18.6 | 18.7 ± 16.5 (−36%) | 16.5 ± 3.9 (−44%) | 9.5 ± 3.3 (−68%) | 1.67 | 0.227 |
| Compound | Shift (ppm) | Type 1 | H 2 |
|---|---|---|---|
| TSP | 0 | s | 9 |
| Alanine | 1.46 | d | 3 |
| β-Alanine | 2.54 | t | 2 |
| Taurine | 3.43 | t | 2 |
| Glycine | 3.55 | s | 2 |
| Betaine | 3.91 | s | 2 |
| Homarine | 4.36 | s | 2 |
| Maleic Acid | 6.32 | s | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, M.A.; Bishop, K.D.; Rawson, P.D. NMR Profiling of Metabolites in Larval and Juvenile Blue Mussels (Mytilus edulis) under Ambient and Low Salinity Conditions. Metabolites 2017, 7, 33. https://doi.org/10.3390/metabo7030033
May MA, Bishop KD, Rawson PD. NMR Profiling of Metabolites in Larval and Juvenile Blue Mussels (Mytilus edulis) under Ambient and Low Salinity Conditions. Metabolites. 2017; 7(3):33. https://doi.org/10.3390/metabo7030033
Chicago/Turabian StyleMay, Melissa A., Karl D. Bishop, and Paul D. Rawson. 2017. "NMR Profiling of Metabolites in Larval and Juvenile Blue Mussels (Mytilus edulis) under Ambient and Low Salinity Conditions" Metabolites 7, no. 3: 33. https://doi.org/10.3390/metabo7030033






