Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity?
Abstract
:1. Scope and Limitations
2. Introduction
3. In Vivo Microalgae VOCs
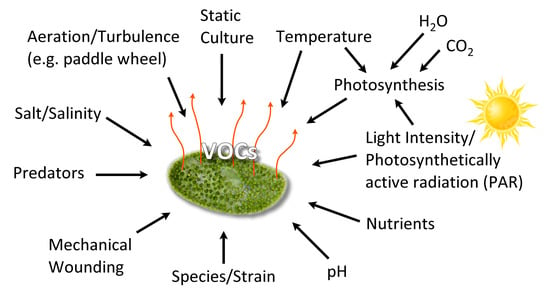
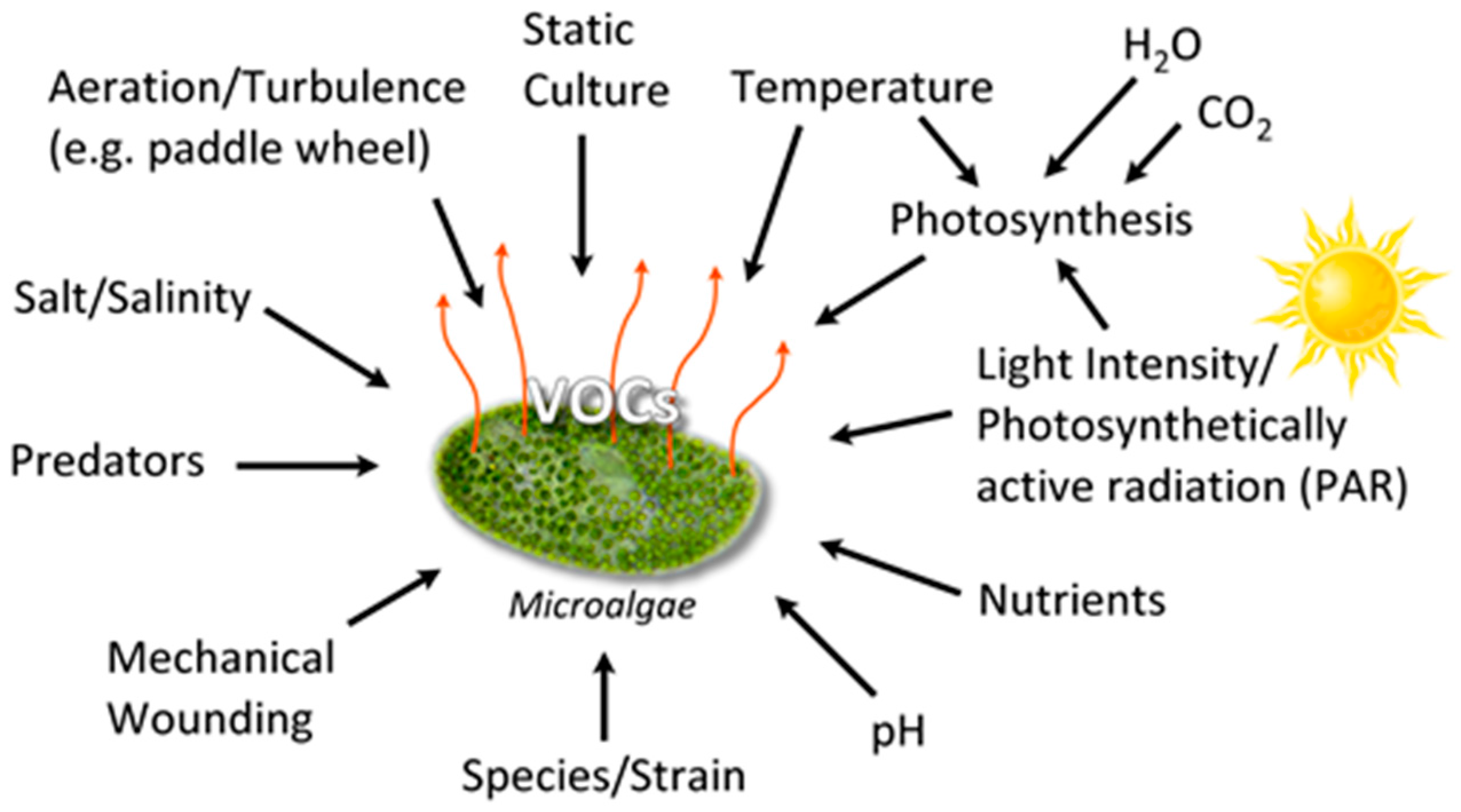
3.1. VOC Production Conditions
3.2. Inorganic Volatiles
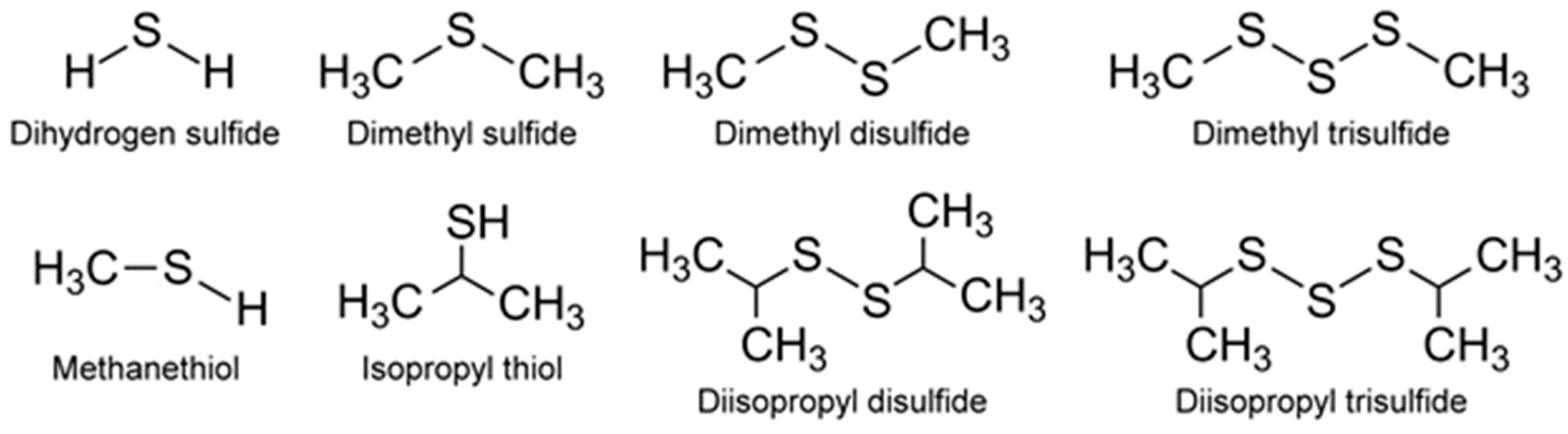
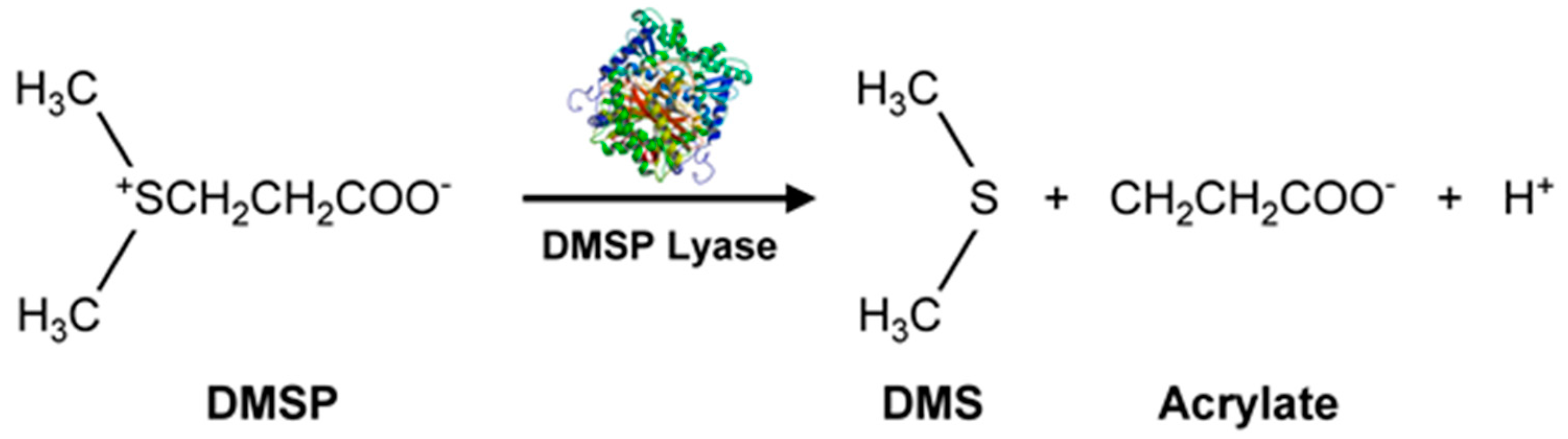
3.3. VOCs-Containing Sulfur
3.4. VOCs-Containing Halogens
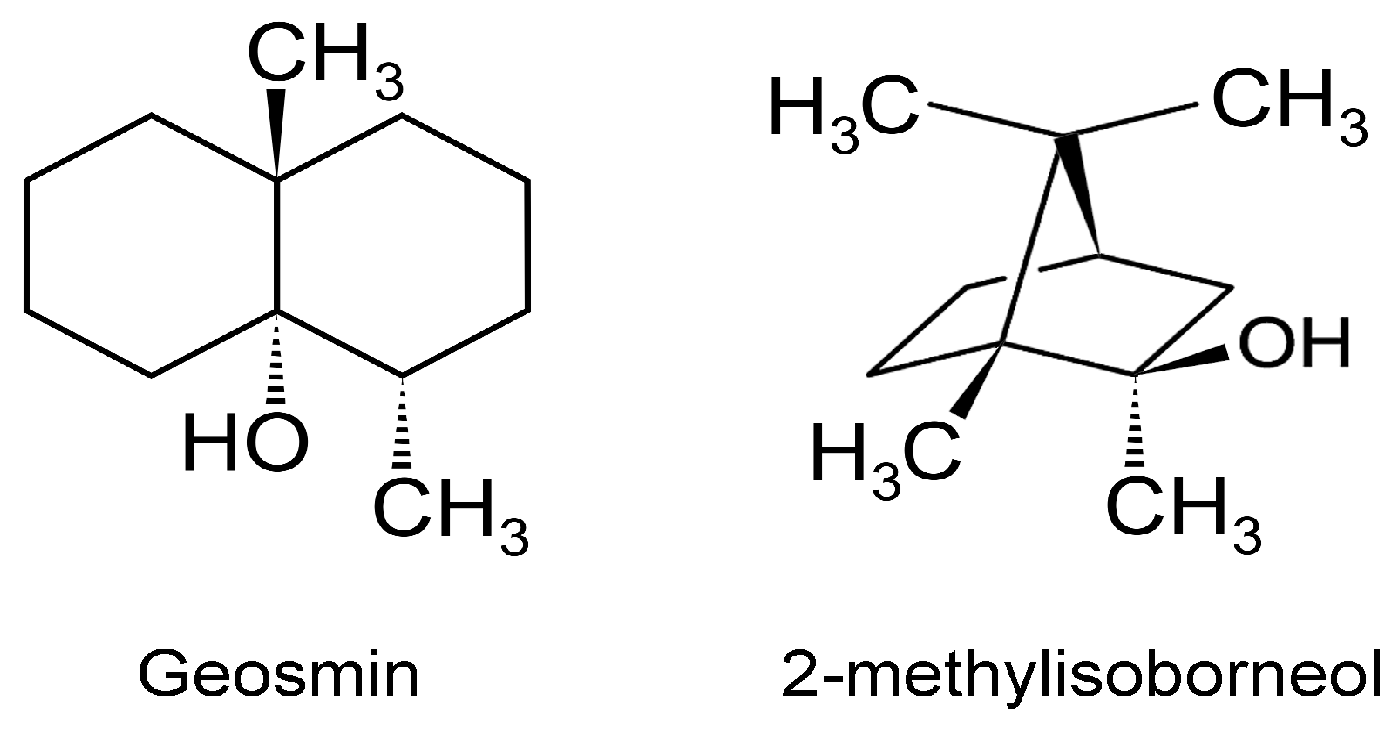
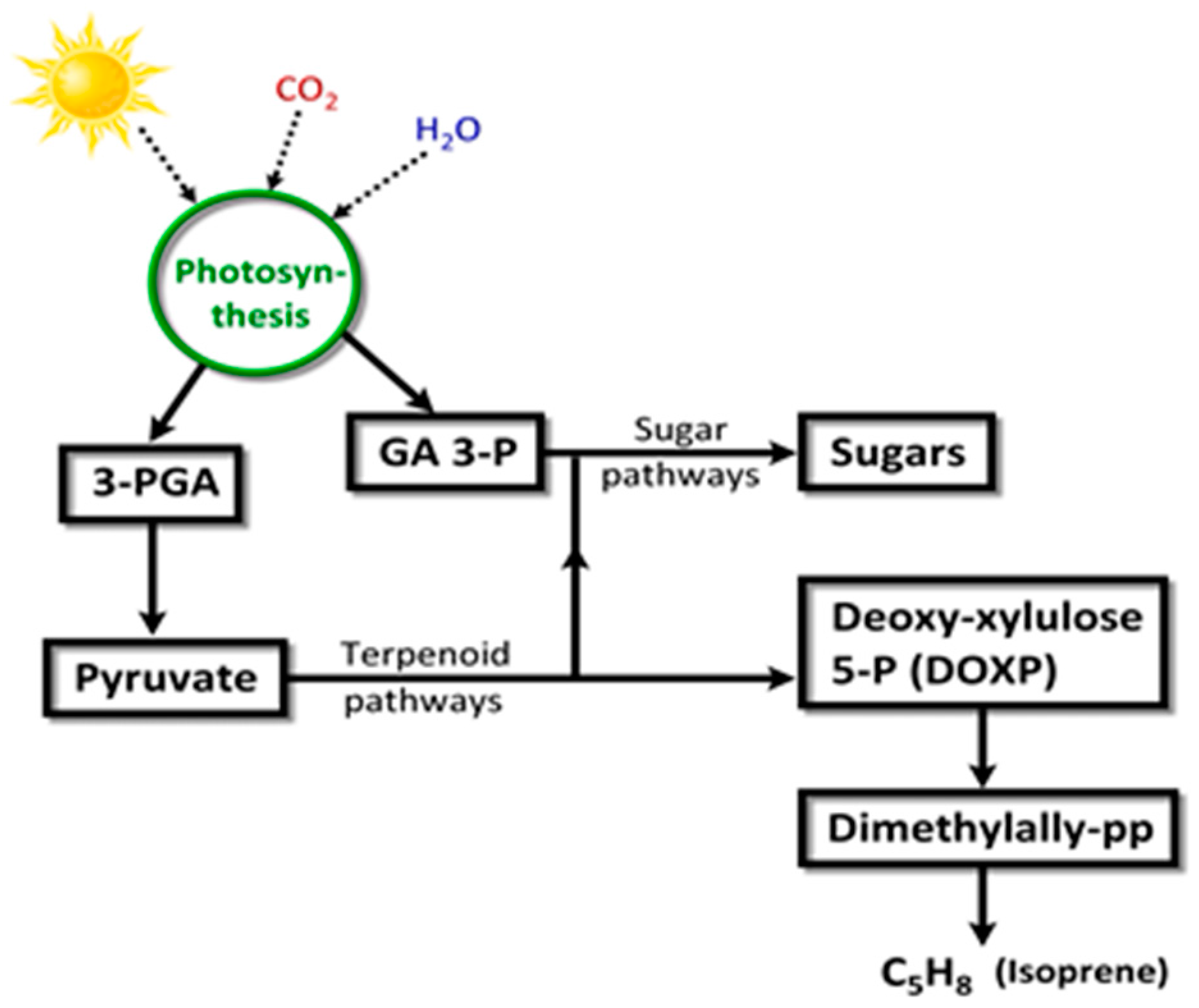
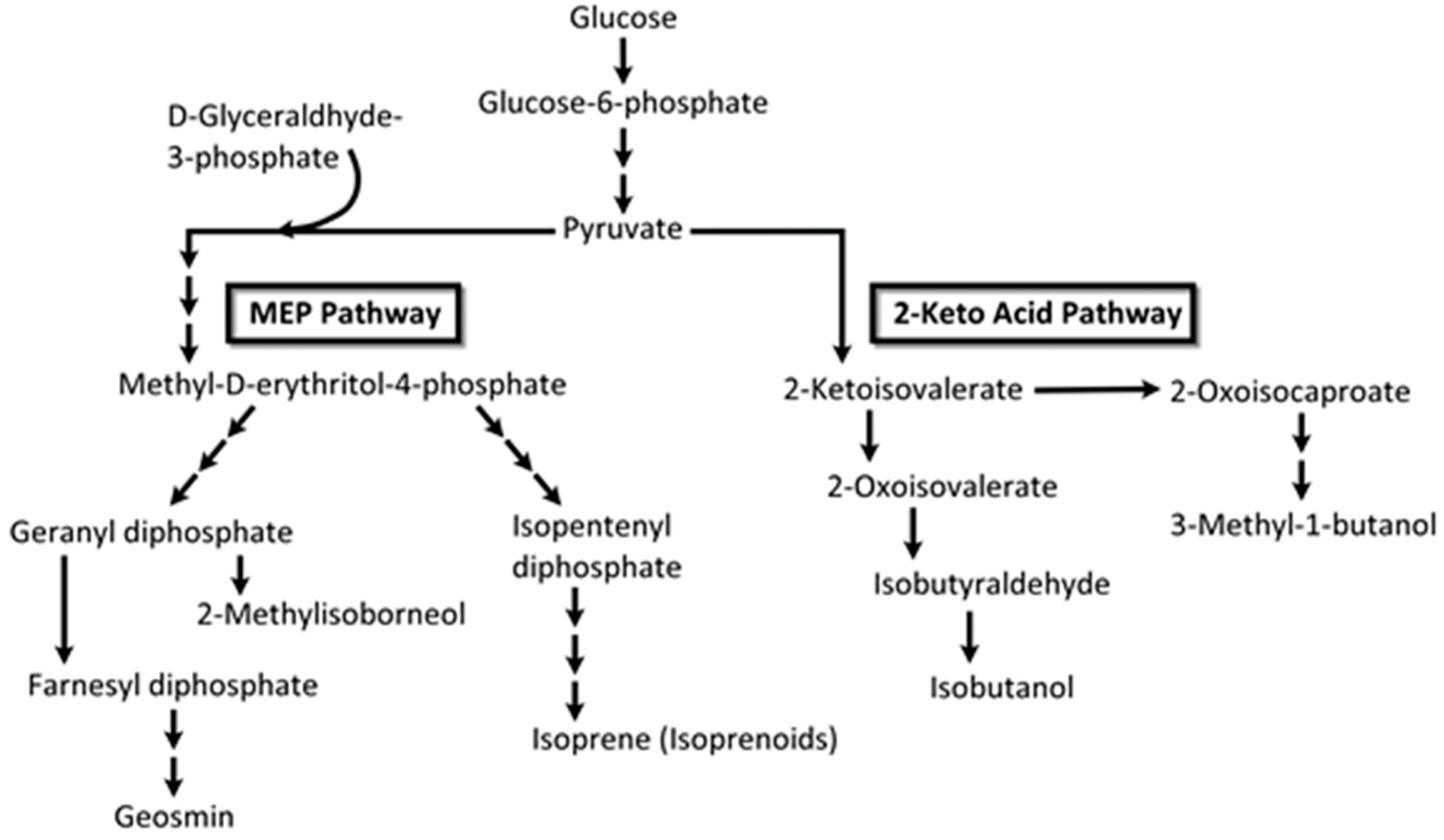
3.5. Other VOCs
4. Functions of Microalgal VOCs
Do Microalgae Communicate?
5. Presymptomatic Diagnostics—A Potential VOC Application?
5.1. Presymptomatic Diagnostics
5.2. VOCs for Presymptomatic Diagnostics
5.3. VOC Metabolomics (Volatilomics)
5.4. VOCs during Growth/Senescence
5.5. VOCs in Predator–Prey Interactions
5.6. Caveats to VOC-Based Presymptomatic Diagnostics
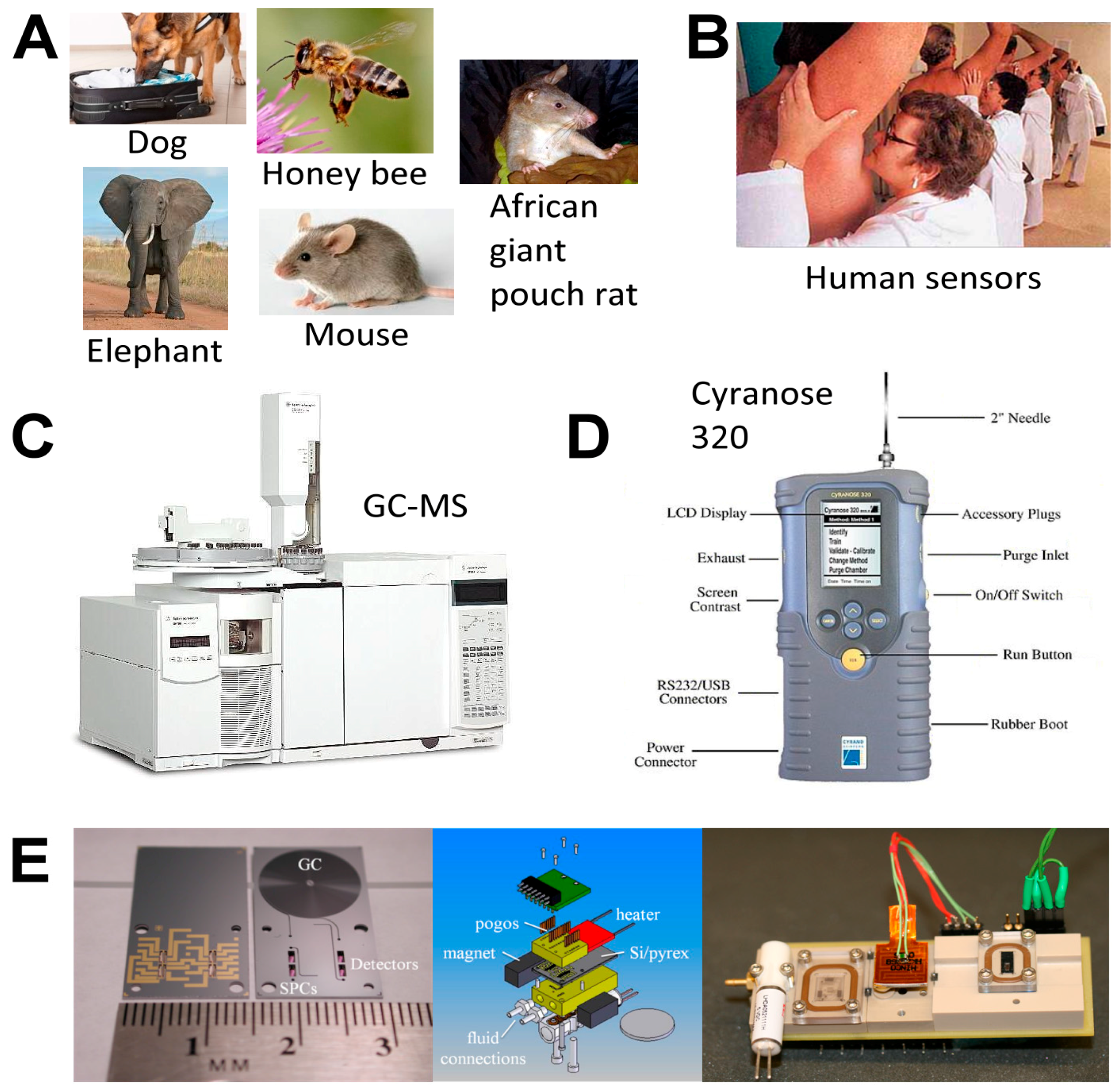
6. Tools and Techniques for VOCs Collection and Detection
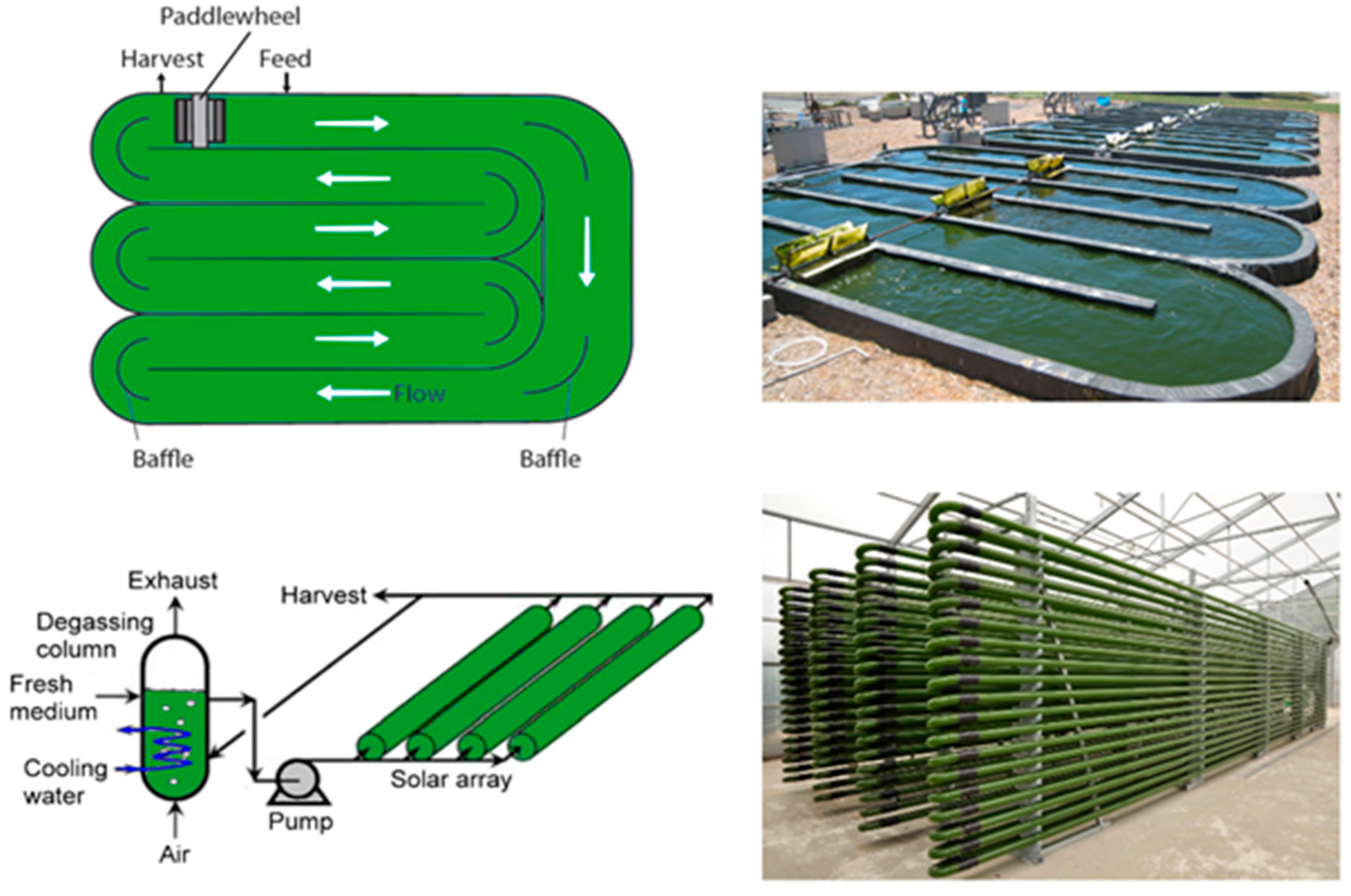
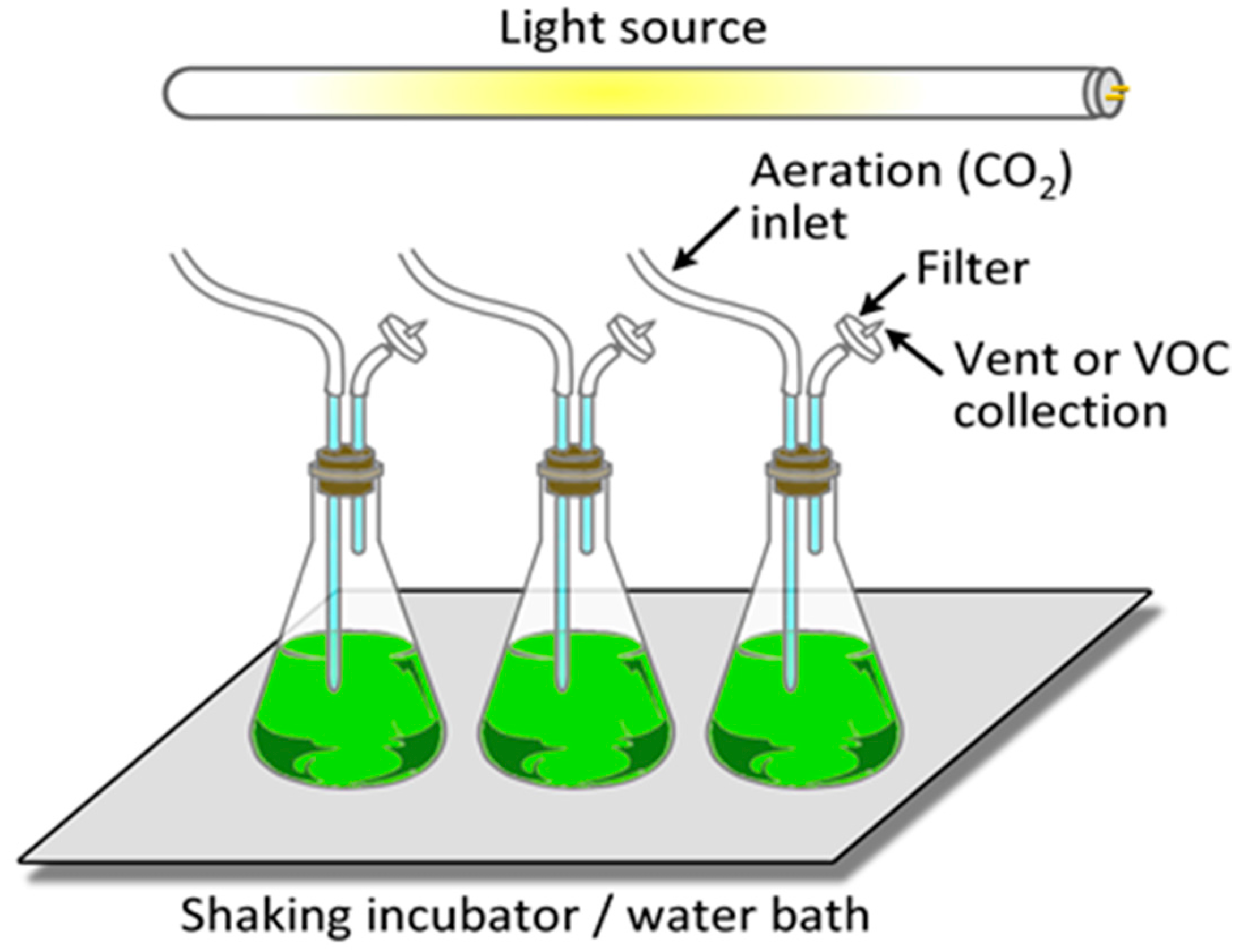
6.1. Microalgal Cultivation
6.2. Microalgal VOCs Analysis
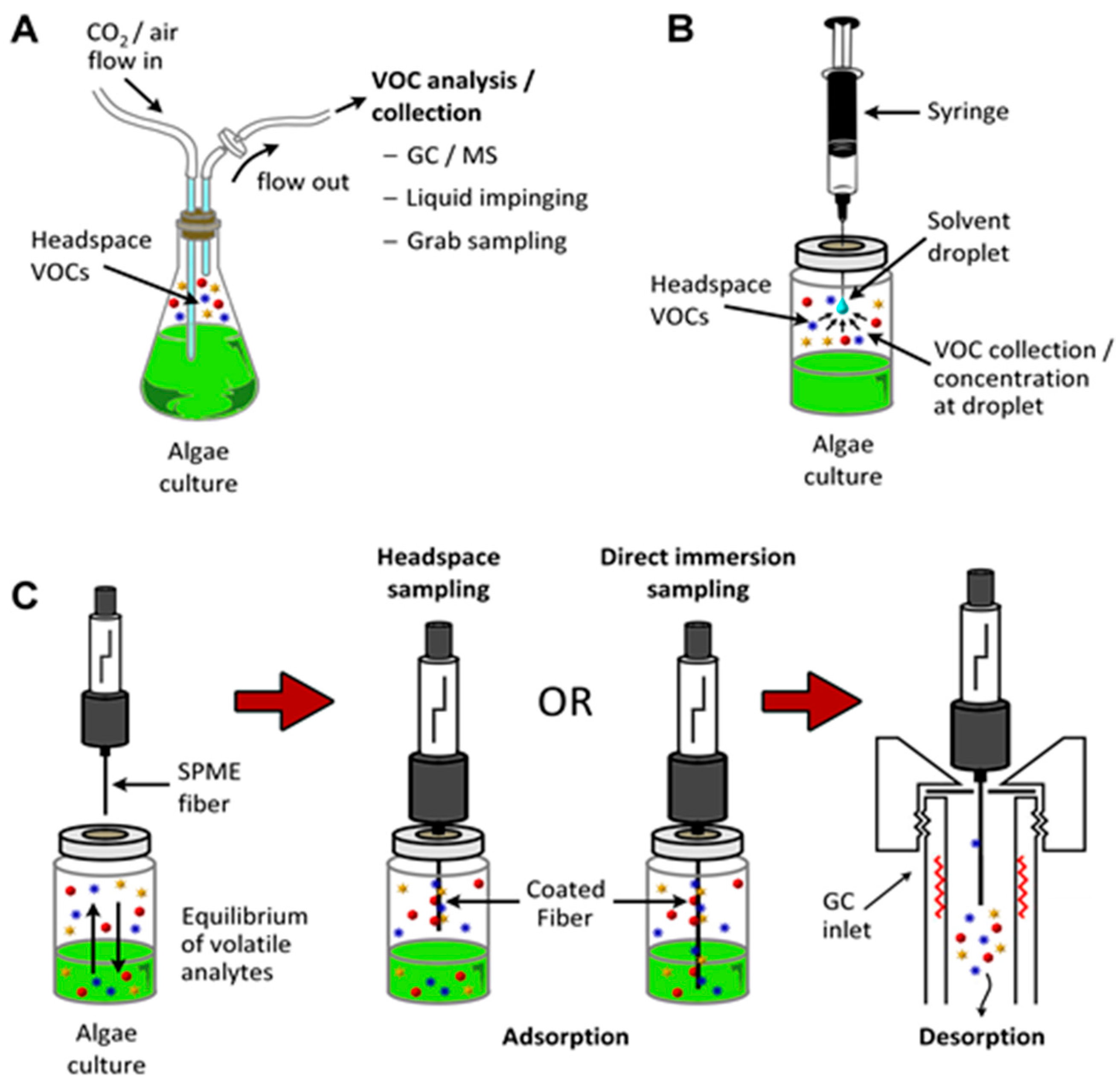
6.3. VOC Sample Collection
6.4. VOC Analysis
7. Future Perspectives
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fink, P.; von Elert, E.; Juttner, F. Volatile foraging kairomones in the littoral zone: Attraction of an herbivorous freshwater gastropod to algal odors. J. Chem. Ecol. 2006, 32, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Shkrob, I.; Dor, I. Separation and identification of hydrocarbons and other volatile compounds from cultured blue-green alga Nostoc sp. by gas chromatography-mass spectrometry using serially coupled capillary columns with consecutive nonpolar and semipolar stationary phases. J. Chromatogr. 1999, 682, 221–229. [Google Scholar] [CrossRef]
- Sun, S.-M.; Chung, G.-H.; Shin, T.-S. Volatile compounds of the green alga, Capsosiphon fulvescens. J. Appl. Phycol. 2012, 24, 1003–1013. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Herrmann, A. The Chemistry and Biology of Volatiles; John Wiley: Sussex, UK, 2010. [Google Scholar]
- Moore, J.P. Volatile Organic Compounds: Occurrence, Behavior and Ecological Implications; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- Nicolay, X. Odors in the Food Industry; Springer: New York, NY, USA, 2006. [Google Scholar]
- Beebe, S.C.; Howell, T.J.; Bennett, P.C. Using scent detection dogs in conservation settings: A review of scientific literature regarding their selection. Front. Vet. Sci. 2016, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Camara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2014, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Bishwal, S.C.; Das, A.; Dabral, D.; Varshney, A.; Badireddy, V.K.; Nanda, R. Investigation of gender-specific exhaled breath volatome in humans by GCxGC-TOF-MS. Anal. Chem. 2014, 86, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, C.; Calvo, A.M.; Latge, J.P. The volatome of Aspergillus fumigatus. Eukaryot. Cell 2017, 13, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid volatolomics and disease detection. Angew. Chem. Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef] [PubMed]
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 46, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Zuri, L.; Haick, H. Combined volatolomics for monitoring of human body chemistry. Sci. Rep. 2014, 4, 4611. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-L. Biotechnological applications of microalgae. IJSME 2012, 6 (Suppl. 1), S24–S37. [Google Scholar]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Odjadjare, E.C.; Mutanda, T.; Olaniran, A.O. Potential biotechnological application of microalgae: A critical review. Crit. Rev. Biotechnol. 2017, 37, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Majumdar, A. Monitoring and reporting VOCs in ambient air. In Air Quality Monitoring, Assessment and Management; Nicolas, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 137–148. [Google Scholar]
- Barsanti, L.; Paolo, G. Algae Anatomy, Biochemistry and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- AlgaeBase. Available online: www.algaebase.org (accessed on 15 March 2017).
- Tomasellli, L. The microalgal cell. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2004; p. 3. [Google Scholar]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E. Basic characteristics of the algae. In Phycology, 4th ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 3–29. [Google Scholar]
- Abdullah, A.A.; Altaf-Ul-Amin, Md.; Ono, N.; Sato, T.; Sugiura, T.; Morita, A.H.; Katsuragi, T.; Muto, A.; Nishioka, T.; Kanaya, S. Development and mining of a volatile organic compound database. BioMed Res. Int. 2015, 139254, 139254. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Hinojosa, F.; Gaxiola-Castro, G.; Segovia-Zavala, J.A.; Munoz-Barbosa, A.; Orozco-Borbon, M.V. The effect of vertical mixing on primary production in a Bay of the Gul of California. Estuar. Coast. Shelf Sci. 1997, 45, 135–148. [Google Scholar] [CrossRef]
- Mendes, L.B.B.; Vermellio, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.T.; Lane, T.W. Parasites in algae mass culture. Front. Microbiol. 2014, 5, 278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.T.; Wilkenfeld, J.S.; Lane, P.D.; Solberg, O.D.; Fuqua, Z.B.; Cornelius, N.G.; Gillespie, S.; Williams, K.P.; Samocha, T.M.; Lane, T.W. Pond crash forensics: Presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass culture of Nannochloropsis salina. Algal Res. 2016, 17, 341–347. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and eukaryotic algal odor compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A review: Microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Efroymson, R.A.; Dale, V.H. Environmental indicators for sustainable production of algal biofuels. Ecol. Indicat. 2014, 49, 1–13. [Google Scholar] [CrossRef]
- Weathers, P.J. N2O evolution by green algae. Appl. Environ. Microbiol. 1984, 48, 1251–1253. [Google Scholar] [PubMed]
- Weathers, P.J.; Niedzielski, J.J. Nitrous oxide production by cyanobacteria. Arch. Microbiol. 1986, 146, 204–206. [Google Scholar] [CrossRef]
- Florez-Leiva, L.; Tarifeno, E.; Cornejo, M.; Kiene, R.; Farias, L. High production of nitrous oxide (N2O), methane (CH4) and dimethylsulphoniopropionate (DMSP) in a massive marine phytoplankton culture. Biogeosci. Discuss. 2010, 7, 6705–6723. [Google Scholar] [CrossRef]
- Fagerstone, K.D. Measurement of Direct Nitrous Oxide Emissions from Microalgae Cultivation under Oxic and Anoxic Conditions. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2011; pp. 1–91. [Google Scholar]
- Fagerstone, K.D.; Quinn, J.C.; Bradley, T.H.; DeLong, S.K.; Marchese, A.J. Quantitative measurement of direct nitrous oxide emissions from microalgae cultivation. Environ. Sci. Technol. 2011, 45, 9449–9456. [Google Scholar] [CrossRef] [PubMed]
- Guieysse, B.; Plouviez, M.; Coilhac, M.; Cazali, L. Nitrous oxide (N2O) production in axenic Chlorella vulgaris microalgae cultures: Evidence, putative pathways, and potential environmental impacts. Biogeosciences 2013, 10, 6737–6746. [Google Scholar] [CrossRef]
- Alcantara, C.; Munoz, R.; Norvill, Z.; Plouviez, M.; Guieysse, B. Nitrous oxide emissions from high rate algal ponds treating domestic wastewater. Bioresour. Technol. 2015, 177, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Boucher, O.; Haigh, J.; Haughustaine, D.; Haywood, J.; Myhre, G.; Nakajima, T.; Shi, G.Y.; Solomon, S.; Betts, R.; et al. Climate Change 2001: The Scientific Basis; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK; pp. 349–416.
- Mathis, M. Method Development and In-Situ Measurements in an Algal Culture of Chlamydomonas reinhardtii for Volatile, Methylated Selenium and Sulfur Using Solid-Phase Micro-Extraction. Master’s Thesis, IBP-ETH, Zurich, Switzerland, 2014; pp. 1–72. [Google Scholar]
- Wolfe, G.V.; Steinke, M. Grazing-activated production of dimethylsulfide (DMS) by two clones of Emiliania huxleyi. Limnol. Oceanogr. 1996, 41, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.M.; De Souza, M.P.; Pickering, I.J.; Terry, N. Rapid microalgal metabolism of selenite to volatile dimethylselenide. Plant Cell Environ. 2003, 26, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, N.; Takahashi, G.; Ishikazi, M. Methylation of inorganic selenium compounds by freshwater green algae, Ankistrodesmus sp., Chlorella vulgaris and Selenastrum sp. Jpn. J. Toxicol. Environ. Health 1991, 37, 83–86. [Google Scholar] [CrossRef]
- Haas, P. The liberation of methyl sulfide by seaweed. Biochem. J. 1935, 29, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.E. Odorous algal cultures in culture collections. Water Sci. Technol. 1988, 20, 211–213. [Google Scholar]
- Bentley, R.; Chasteen, T.G. Environmental VOSCs—Formation and degradation of dimethyl sulfide, methane thiol and related materials. Chemosphere 2004, 55, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Juttner, F. Malodorous volatile organic sulfur compounds: Sources, sinks and significance in inland waters. Crit. Rev. Microbiol. 2017, 43, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; Houser, L.T. Dimethylsulfide release during macroinvertebrate grazing and its role as an activated chemical defense. Mar. Ecol. Prog. Ser. 2003, 250, 175–181. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Myburgh, J.G.; Govender, D.; Bengis, R.; Botha, A.-M. Identification of Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotoxicol. Environ. Saf. 2009, 72, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Simo, R. Production of atmospheric sulfur by oceanic plankton: Biogeochemical, ecological and evolutionary links. Trends Ecol. Evol. 2001, 16, 287–294. [Google Scholar] [CrossRef]
- Keller, M.D.; Bellows, W.K.; Guillard, R.R.L. Dimethylsulfide production in marine phytoplankton. Biogenic Sulfur in the Environment. ACS Symp. Ser. 1989, 393, 167–182. [Google Scholar]
- Gebser, B.; Pohnert, G. Synchronized regulation of different zwitterionic metabolites in the osmoadaptation of phytoplankton. Mar. Drugs 2013, 11, 2168–2182. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmessa, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the algal dimethyl sulfide-releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cell to global climate. FEMS Microbiol. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, K.A.; Strom, S.L. The algal osmolyte DMSP as a microzooplankton grazing deterrent in laboratory and field studies. J. Plankton Res. 2009, 31, 135–152. [Google Scholar] [CrossRef]
- Wolfe, G.W.; Steinke, M.; Kirst, G.O. Grazing-activated chemical defence in a unicellular marine alga. Nature 1997, 387, 894–897. [Google Scholar] [CrossRef]
- Ishida, Y. Physiological studies on evolution of dimethyl sulfide from unicellular marine algae. Mem. Coll. Agric. Kyoto Univ. 1968, 94, 47–82. [Google Scholar]
- Kadota, H.; Ishida, Y. Evolution of volatile sulfur compounds from unicellular marine algae. Bull. Misaki Mar. Biol. Inst. Kyoto Univ. 1968, 12, 35–48. [Google Scholar]
- Ettre, L.S. The early development and rapid growth of gas chromatographic instrumentation in the United States. J. Chromatogr. Sci. 2002, 40, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H.; Doemel, W.N.; Brock, T.D. Production of volatile sulfur compounds during the decomposition of algal mats. Appl. Environ. Microbiol. 1977, 34, 859–860. [Google Scholar] [PubMed]
- Matrai, P.; Keller, M.D. Dimethylsulfide in a large-scale coccolithophore bloom in the Gulf of Maine. Cont. Shelf Res. 1993, 13, 831–843. [Google Scholar] [CrossRef]
- Molina, M.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalyzed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Keng, F.S.L.; Phang, S.M.; Rahman, N.A.; Leedham, E.C.; Hughes, C.; Robinson, A.D.; Harris, N.R.P.; Pyle, J.A.; Sturges, W.T. Volatile halocarbon emissions by three tropical brown seaweeds under different irradiances. J. Appl. Phycol. 2013, 25, 1377–1386. [Google Scholar] [CrossRef]
- Carpenter, L.J. Iodine in the marine boundary layer. Chem. Rev. 2003, 103, 4953–4962. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.G.; Braesicke, P.; Harris, N.R.P.; Pyle, J.A. Seasonal and inter-annual variations in troposphere-to-stratosphere transport from the tropical tropopause layer. Atmos. Chem. Phys. 2008, 8, 3689–3703. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; Jimenez, J.L.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H.; Hameri, K.; Pirjola, L.; Kulmala, M.; Jennings, S.G.; Hoffmann, T. Marine aerosol formation from biogenic iodine emissions. Nature 2002, 417, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Sturges, W.T.; Cota, G.F.; Buckley, P.T. Bromoform emission from arctic ice algae. Nature 1992, 358, 660–662. [Google Scholar] [CrossRef]
- Thorenz, U.R.; Carpenter, L.J.; Huang, R.J.; Kundel, M.; Bosle, J.; Hoffmann, T. Emission of iodine-containing volatiles by selected microalgae species. Atmos. Chem. Phys. 2014, 14, 13327–13335. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Liss, P.S. On temperate sources of bromoform and other reactive organic bromine gases. J. Geophys. Res. Atmos. 2000, 105, 20539–20547. [Google Scholar] [CrossRef]
- Manley, S.L.; de la Cuesta, J.L. Methyl iodide production from marine phytoplankton cultures. Limnol. Oceanogr. 1997, 42, 142–147. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mithoo-Singh, P.K.; Keng, F.S.L.; Phang, S.M.; Elvidge, E.C.L.; Sturges, W.T.; Malin, G.; Rahman, N.A. Halocarbon emissions by selected seaweeds: Species-specific and compound-specific responses under changing pH. PeerJ 2017, 5, e2918. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.E.; Lukas, R.; Sadler, D.W.; Church, M.J.; Karl, D.M. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc. Natl. Acad. Sci. USA 2009, 106, 12235–12240. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.E.; Kimmance, S.A.; Stepehns, J.A.; Bellerby, R.G.J.; Brussaard, C.P.D.; Czerny, J.; Schulz, K.G.; Archer, S.D. Response of halocarbons to ocean acidification in the Arctic. Biogeoscience 2013, 10, 2331–2345. [Google Scholar] [CrossRef] [Green Version]
- Malinksky-Rushansky, N.A.; Legrand, C. Excretion of dissolved organic carbon by phytoplankton of different sizes and subsequent bacterial uptake. Mar. Ecol. Prog. Ser. 1996, 132, 249–255. [Google Scholar] [CrossRef]
- Mopper, K.; Zhou, X.; Kieber, R.J.; Kieber, D.J.; Sikorski, R.J.; Jones, R.D. Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle. Nature 1991, 353, 60–62. [Google Scholar] [CrossRef]
- Abrahamsson, K.; Ekdahl, A.; Collen, J.; Pedersen, M. Formation and distribution of halogenated volatile organics in sea water. In Naturally-Produced Organohalogens; Grimvall, A., de Leer, E.W.B., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 317–326. [Google Scholar]
- Han, W.; Clarke, W.; Pratt, S. Stabilisation of microalgae: Iodine mobilization under aerobic and anaerobic conditions. Bioresour. Technol. 2015, 193, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, N.; Tsujita, M.; Morikawa, S.; Itoh, N. Purification and characterization of a monohalomethane-producing enzyme S-adenosyl-l-methionine: Halide ion methyltransferase from a marine microalga, Pavlova pinguis. Biosci. Biotechnol. Biochem. 2001, 65, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, Y.; Ooki, A.; Hashimoto, S.; Itoh, N. A study on the production and emission of marine-derived volatile halocarbons. In Western Pacific Air-Sea Interaction Study; Uematsu, M., Yokouchi, Y., Watanabe, Y.W., Takeda, S., Yamanaka, Y., Eds.; Terrapub: Tokyo, Japan, 2014; pp. 1–25. [Google Scholar]
- Scarratt, M.G.; Moore, R.M. Production of chlorinated hydrocarbons and methyl iodide by the red microalga Porphyridium purpurum. Limnol. Oceanogr. 1999, 44, 703–707. [Google Scholar] [CrossRef]
- Chance, R.; Malin, G.; Jickells, T.; Baker, A.R. Reduction of iodate to iodide by cold water diatom cultures. Mar. Chem. 2007, 105, 169–180. [Google Scholar] [CrossRef]
- Scarratt, M.G.; Moore, R.M. Production of methyl bromide and methyl chloride in laboratory cultures of marine phytoplankton. Mar. Chem. 1998, 59, 311–320. [Google Scholar] [CrossRef]
- Vanelslander, B.; Paul, C.; Grueneberg, J.; Prince, E.K.; Gillard, J.; Sabbe, K.; Pohnert, G.; Vyverman, W. Daily bursts of biogenic cyanogen bromide (BrCN) control biofilm formation around a marine benthic diatom. Proc. Natl. Acad. Sci. USA 2012, 109, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.P.; Yang, B.; Lu, X.L.; Ding, H.B.; He, Z. Spatio-temporal variations of sea surface halocarbon concentrations and fluxes from southern Yellow sea. Biogeochemistry 2014, 121, 369–388. [Google Scholar] [CrossRef]
- Singh, H.; Chen, Y.; Standt, A.; Jacob, D.; Blake, D.; Heikes, B.; Snow, J. Evidence for the Pacific troposphere for large global sources of oxygenated organic compounds. Nature 2001, 410, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, C.D.; de Leeuw, G. Marine aerosol production: A review of the current knowledge. Philos. Trans. A Math. Phys. Eng. Sci. 2007, 365, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Fink, P. Ecological functions of volatile organic compounds in aquatic systems. Mar. Freshw. Behav. Physiol. 2007, 40, 155–168. [Google Scholar] [CrossRef]
- Watson, S.B.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste- and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Proteau, P.J. Biosynthesis of phytol in the cyanobacterium Synechocystis sp. UTEX 2470: Utilization of the non-mevalonate pathway. J. Nat. Prod. 1998, 61, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci. Biotechnol. Biochem. 2002, 66, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G.; Taylor, W.D. A guide to geosmin- and MIB-producing cyanobacteria in the United States. Water Sci. Technol. 2004, 49, 19–24. [Google Scholar] [PubMed]
- Chen, Y.-M.; Hobson, P.; Burch, M.D.; Lin, T.-F. In situ measurement of odor compound production by cyanobacteria. J. Environ. Monit. 2010, 12, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Satchwill, T.; Watson, S.B.; Dixon, E. Odorous algal-derived alkenes: Differences in stability and treatment responses in drinking water. Water Sci. Technol. 2007, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Koc, H.; Unterkofler, K.; Mochalski, P.; Kupferthaler, A.; Tesch, G.; Tesch, S.; Hinterhuber, H.; Amann, A. Physiological modeling of isoprene dynamics in exhaled breath. J. Theor. Biol. 2010, 267, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.T.; Gouveia, L.; Morais, A.R.C.; Reis, A.; Bogel-Lukasik, R. Green metrics evaluation of isoprene production by microalgae and bacteria. Green Chem. 2013, 15, 2854–2864. [Google Scholar] [CrossRef]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Lipid Res. 2005, 44, 357–429. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metabol. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Laothawornkitkul, J.; Paul, N.D.; Vickers, C.E.; Possell, M.; Mullineaux, P.M.; Hewitt, C.N.; Taylor, J.E. The role of isoprene in insect herbivory. Plant Signal. Behav. 2008, 3, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Milne, P.J.; Riemer, D.D.; Zika, R.G.; Brand, L.E. Measurement of vertical distribution of isoprene in surface seawater, its chemical fate, and its emission from several phytoplankton monocultures. Mar. Chem. 1995, 48, 237–244. [Google Scholar] [CrossRef]
- Exton, D.A.; Suggett, D.J.; McGenity, T.J.; Steinke, M. Chlorophyll-normalized isoprene production in laboratory cultures of marine microalgae and implications for global models. Limnol. Oceanogr. 2013, 58, 1301–1311. [Google Scholar] [CrossRef]
- Bonsang, B.; Polle, C.; Lambert, G. Evidence for marine production of isoprene. Geophys. Res. Lett. 1992, 19, 1129–1132. [Google Scholar] [CrossRef]
- Broadgate, W.G.; Liss, P.S.; Penkett, S.A. Seasonal emissions of isoprene and other reactive hydrocarbon gases from the ocean. Geophys. Res. Lett. 1997, 24, 2675–2678. [Google Scholar] [CrossRef]
- Moore, R.M.; Oram, D.E.; Penkett, S.A. Production of isoprene by marine phytoplankton cultures. Geophys. Res. Lett. 1994, 21, 2507–2510. [Google Scholar] [CrossRef]
- Meskhidze, N.; Nenes, A. Phytoplankton and cloudiness in the Southern Ocean. Science 2006, 314, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.L.; Chisholm, S.W.; Prinn, R.G. Isoprene production by Prochlorococcus, a marine cyanobacterium, and other phytoplankton. Mar. Chem. 2003, 80, 227–245. [Google Scholar] [CrossRef]
- Shaw, S.L.; Gantt, B.; Meskhidze, N. Production and emission of marine isoprene and monoterpenes: A review. Adv. Meterol. 2010, 408696, 1–24. [Google Scholar] [CrossRef]
- Gantt, B.; Meskhidze, N.; Kamykowski, D. A new physically-based quantification of marine isoprene and primary organic aerosol emissions. Atmos. Chem. Phys. 2009, 9, 4915–4927. [Google Scholar] [CrossRef]
- Meskhidze, N.; Sabolis, A.; Reed, R.; Kamykowski, D. Quantifying environmental stress-induced emissions of algal isoprene and monoterpenes using laboratory measurements. Biogeoscience 2015, 12, 637–651. [Google Scholar] [CrossRef]
- Srikanta Dani, K.G.; Benavides, A.M.S.; Michelozzi, M.; Peluso, G.; Torzillo, G.; Loreto, F. Relationship between isoprene emission and photosynthesis in diatoms, and its implications for global marine isoprene estimates. Mar. Chem. 2017, 189, 17–24. [Google Scholar] [CrossRef]
- Srikanta Dani, K.G.; Loreto, F. Trade-off between dimethyl sulfide and isoprene emissions from marine phytoplankton. Trend Plant Sci. 2017, 1521, 1360–1385. [Google Scholar]
- Santos, A.B.; Vieira, K.R.; Nogara, G.P.; Wagner, R.; Jacob-Lopes, E.; Zepka, L.Q. Biogeneration of volatile organic compounds by microalgae: Occurrence, behavior, ecological implications and industrial applications. In Volatile Organic Compounds; Moore, J.P., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 1–24. [Google Scholar]
- National Research Council: Committee on the sustainable development of algal biofuels. In Sustainable Development of Algal Biofuels in the United States; The National Academies Press: Washington, DC, USA, 2012; p. 246.
- Walsh, K.; Jones, G.J.; Dunstan, R.H. Effect of high irradiance and iron on volatile odour compounds in the cyanobacterium Microcystis aeruginosa. Phytochemistry 1998, 49, 1227–1239. [Google Scholar] [CrossRef]
- Bravo-Linares, C.M.; Mudge, S.M. Temporal trends and identification of the sources of volatile organic compounds in coastal seawater. J. Environ. Monit. 2009, 11, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mincer, T.J.; Alcher, A.C. Methanol production by a broad phylogenetic array of marine phytoplankton. PLoS ONE 2016, 11, e0150820. [Google Scholar] [CrossRef] [PubMed]
- Heikes, B.G.; Chang, W.; Pilson, M.E.Q.; Swift, E.; Singh, H.B.; Guenther, A.; Jacob, D.J.; Field, B.D.; Fall, R.; Riemer, D.; et al. Atmospheric methanol budget and ocean implication. Glob. Biogeochem. Cycle 2002, 16, 80:1–80:3. [Google Scholar] [CrossRef]
- Colomb, A.; Yassaa, N.; Williams, J.; Peeken, I.; Locute, K. Screening volatile organic compounds (VOCs) emissions from five marine phytoplankton species by head space gas chromatography/mass spectrometry (HS-GC-MS). J. Environ. Monit. 2008, 10, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.B.; Fernandes, A.S.; Wagner, R.; Jacob-Lopes, E.; Zepka, L.Q. Biogeneration of volatile organic compounds produced by Phormidium autumnale in heterotrophic bioreactor. J. Appl. Phycol. 2016, 28, 1561–1570. [Google Scholar] [CrossRef]
- Khalil, M.A.K. Non-CO2 greenhouse gases in the atmosphere. Ann. Rev. Energy Environ. 1999, 24, 645–661. [Google Scholar] [CrossRef]
- Schaefer, H.; Mikaloff Fletcher, S.E.; Veidt, C.; Lassey, K.R.; Brailsford, G.W.; Bromley, T.M.; Dlugokencky, E.J.; Michel, S.E.; Miller, J.B.; Levin, I.; et al. A 21st-century shift from fossil-fuel to biogenic methane emissions indicated by 13CH4. Science 2016, 352, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, K.; Klintzsch, T.; Langer, G.; Nehrke, G.; Bunge, M.; Schnell, S.; Keppler, F. Evidence for methane production by the marine algae Emiliania huxleyi. Biogeoscience 2016, 13, 3163–3174. [Google Scholar] [CrossRef]
- Durme, J.V.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the volatile composition and sensory properties of five species of microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, I.; Misan, A.; Simeunovic, J.; Kovac, D.; Jambree, D.; Mandic, A. Determination of volatile organic compounds in selected strains of cyanobacteria. J. Chem. 2015, 969542, 1–6. [Google Scholar] [CrossRef]
- Obata, T.; Araie, H.; Shiraiwa, Y. Bioconcentration mechanism of selenium by a coccolithophorid, Emiliania huxleyi. Plant Cell Physiol. 2004, 45, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Stefels, J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia 2001, 443, 19–22. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2009, 15, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. Plant volatilome resources. Curr. Metabol. 2016, 4, 148–150. [Google Scholar] [CrossRef]
- Kantsa, A.; Sotiropoulou, S.; Vaitis, M.; Petanidou, T. Plant volatilome in Greece: A review on the properties, prospects, and chemogeography. Chem. Biodivers. 2015, 12, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Simpraga, M.; Verbeeck, H.; Demarcke, M.; Joo, E.; Amelynck, C.; Schoon, N.; DeWulf, J.; Van Langenhove, H.; Heinesch, B.; Aubinet, M.; et al. Comparing monoterpenoid emissions and net photosynthesis of beech (Fagus sylvatica L.) in controlled and natural conditions. Atmos. Environ. 2011, 45, 2922–2928. [Google Scholar] [CrossRef]
- Simpraga, M.; Verbeeck, H.; Demarcke, M.; Joo, E.; Pokorska, O.; Melynck, C.A.; Schoon, N.; DeWulf, J.; Van Langenhove, H.; Heinesch, B.; et al. Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmos. Environ. 2011, 45, 5254–5259. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Effmert, U.; Kalderas, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Fernandez, A.; Ferrer-i-Cancho, R. The infochemical core. J. Quant. Linguist. 2016, 23, 133–153. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J. Volatile affairs in microbial interactions. Int. Soc. Microb. Ecol. J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.E. The consequences of volatile organic compounds mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kijuta, Y.; Matsuda, K. Plant communication. Mediated by individual or blended VOCs? Plant Signal. Behav. 2012, 7, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Blande, J.D. Molecular plant volatile communication. Adv. Exp. Med. Biol. 2012, 739, 17–31. [Google Scholar] [PubMed]
- Syed, Z.; Leal, W.S. Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. USA 2008, 105, 13598–13603. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Leal, W.S. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. USA 2009, 106, 18803–18808. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.M.; Stanczyk, N.M.; Betz, H.S.; Pulido, H.; Sim, D.G.; Read, A.F.; Mescher, M.C. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 2014, 111, 11079–11084. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, Y.; Heath, J.; Kim, M. Applications of animal biosensors: A review. IEEE Sens. J. 2015, 15, 637–645. [Google Scholar]
- Zuo, Z.-J.; Zhu, Y.-R.; Bai, Y.-L.; Wang, Y. Volatile communication between Chlamydomonas reinhardtii cells under salt stress. Biochem. Syst. Ecol. 2012, 40, 19–24. [Google Scholar] [CrossRef]
- Amavizca, E.; Bashan, Y.; Ryu, C.-M.; Farag, M.A.; Bebout, B.M.; de-Bashan, L.E. Enhanced performance of the microalga Chlorella sorokiniana remotely induced by the plant growth-promoting bacteria Azospirillum brasilense and Bacillus pumilus. Sci. Rep. 2017, 7, 41310. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, L.; Yang, W.; Bai, Y.; Hou, P.; Zhao, J.; Zhou, L.; Zuo, Z. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2017, 135, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.N.; Gilligan, C.A.; Cunniffe, N.J. Detecting presymptomatic infection is necessary to forecast major epidemics in the earliest stages of infectious disease outbreaks. PLoS Comput. Biol. 2016, 12, e1004836. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Danaher, P.J.; Devadiga, A.; Legendre, D.A.; Nail, K.L.; Schmitt, P.; Wai, J. Effect of influenza vaccination on oxidative stress products in breath. J. Breath Res. 2010, 4, 026001. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, E.M.M.; van de Kant, K.D.G.; Jobsis, Q.; van Schayck, O.C.P.; Smolinska, A.; Dallinga, J.W.; van Schooten, F.J.; den Hartog, G.J.M.; de Jongste, J.C.; Rijkers, G.T.; et al. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am. J. Resp. Crit. Care Med. 2015, 191, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R.P.; Kenny, L.C. ‘Omic’ technologies: Genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynecol. 2011, 13, 189–195. [Google Scholar] [CrossRef]
- Shnayderman, M.; Mansfield, B.; Yip, P.; Clark, H.A.; Krebs, M.D.; Cohen, S.J.; Zeskind, J.E.; Ryan, E.T.; Dorkin, H.L.; Callahan, M.V.; et al. Species-specific bacteria identification using differential mobility spectrometry and bioinformatics pattern recognition. Anal. Chem. 2005, 77, 5930–5937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bean, H.D.; Kuo, Y.M.; Jill, J.E. Fast detection of volatile organic compounds from bacterial cultures by secondary electrospray ionization-mass spectrometry. J. Clin. Microbiol. 2010, 48, 4426–4431. [Google Scholar] [CrossRef] [PubMed]
- Thorn, R.M.S.; Reynolds, D.M.; Greenman, J. Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J. Microbiol. Methods 2011, 84, 258–264. [Google Scholar] [CrossRef] [PubMed]
- De Bok, F.A.M.; Janssen, P.W.M.; Bayjanov, J.R.; Sieuwerts, S.; Lommen, A.; van Hylckama Vlieg, J.E.T.; Molenaar, D. Volatile compound fingerprinting of mixed-culture fermentations. Appl. Environ. Microbiol. 2011, 77, 6233–6239. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Bohm, K.; Geisen, S.; Wubs, E.R.J.; Song, C.; de Boer, W.; Garbeva, P. The prey’s scent—Volatile organic compound mediated interactions between soil bacteria and their protest predators. Int. Soc. Microb. Ecol. J. 2017, 11, 817–820. [Google Scholar]
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; Ludescher, T.; King, J.; Ager, C.; Trosin, M.; Senocak, U.; Brezany, P.; Feilhauer, T.; Amann, A. ABA-Cloud: Support for collaborative breath research. J. Breath Res. 2013, 7, 026007. [Google Scholar] [CrossRef] [PubMed]
- Haug, K.; Salek, R.M.; Conesa, P.; Hastings, J.; de Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P.; et al. MataboLights—An open-access general-purpose repository of metabolomics studies and associated meta-data. J. Nucleic Acids Res. 2013, 41, D781–D786. [Google Scholar] [CrossRef] [PubMed]
- Mannschreck, K.; Bachmann, K.; Barnes, I.; Becker, K.H.; Heil, TH.; Kurtenbach, R.; Memmesheimer, M.; Mohnen, V.; Obermeier, A.; Poppe, D.; et al. A database for volatile organic compounds. J. Atmos. Chem. 2002, 42, 281–286. [Google Scholar] [CrossRef]
- Goulitquer, S.; Potin, P.; Tonon, T. Mass spectrometry-based metabolomics to elucidate functions in marine organisms and ecosystems. Mar. Drugs 2012, 10, 849–880. [Google Scholar] [CrossRef] [PubMed]
- Ijima, Y. Recent advances in the application of metabolomics to studies of biogenic volatile organic compounds (BVOC) produced by plant. Metabolites 2014, 4, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Thakur, R.S.; Baghal, R.S.; Reddy, C.R.K.; Jha, B. Seaweed metabolomics: A new facet of functional genomics. Adv. Bot. Res. 2014, 71, 31–52. [Google Scholar]
- Zuo, Z.; Zhu, Y.; Bai, Y.; Wang, Y. Acetic acid-induced programmed cell death and release of volatile organic compounds in Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2012, 51, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.P.; Frindte, K.; Dziallas, C.; Eckert, W.; Tang, K.W. Microbial methane production in oxygenated water column of an oligotrophic lake. Proc. Natl. Acad. Sci. USA 2011, 108, 19657–19661. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lv, H.; Zhen, J.; Jiang, S.; Jia, S.; Shen, S.; Gao, L.; Dai, Y. Bacterial species and biochemical characteristic investigations of Nostoc flagelliforme concentrates during its storage. J. Microbiol. Biotechnol. 2016, 26, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipid pathways in marine diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- Maibam, C.; Fink, P.; Romano, G.; Buia, M.C.; Butera, E.; Zupo, V. Centropages typicus (Crustacea, Copepoda) reacts to volatile compounds produced by planktonic algae. Mar. Ecol. 2015, 36, 819–834. [Google Scholar] [CrossRef]
- Moelzner, J.; Fink, P. The smell of good food: Volatile infochemicals as resource quality indicators. J. Anim. Ecol. 2014, 83, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Moelzner, J.; Fink, P. Gastropod grazing on a benthic alga leads to liberation of food-finding infochemicals. Oikos 2015, 124, 1603–1608. [Google Scholar] [CrossRef]
- Moelzner, J.; Fink, P. Consumer patchiness explained by volatile infochemicals in a freshwater ecosystem. Ecosphere 2015, 6, 35. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Whitten, D.G. Design considerations for high throughput screening and in vitro diagnostic assays. Comb. Chem. HTS 2007, 10, 399–412. [Google Scholar] [CrossRef]
- Pleil, J.D.; Stiegel, M.A.; Risby, T.H. Clinical breath analysis: Discriminating between human endogenous compounds and exogenous (environmental) chemical confounders. J. Breath Res. 2013, 7, 017107. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture. Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gressler, V.; Colepicolo, P.; Pinto, E. Useful strategies for algal volatile analysis. Curr. Anal. Chem. 2009, 5, 271–292. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Rose, U.S.R.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.M.C.; Wildt, J.; Kappers, I.F.; Bouwmeester, H.J.; Hofstee, J.W.; van Henten, E.J. Detection of diseased plants by analysis of volatile organic compound emission. Annu. Rev. Phytopathol. 2011, 49, 157–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicchi, C.; Maffei, M. The plant volatilome: Methods of analysis. Methods Mol. Biol. 2012, 918, 289–309. [Google Scholar] [PubMed]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef] [PubMed]
- Materic, D.; Bruhn, D.; Turner, C.; Morgan, G.; Mason, N.; Gauci, V. Methods in plant foliar volatile organic compounds research. Appl. Plant Sci. 2015, 3, 1500044. [Google Scholar] [CrossRef] [PubMed]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Wang, Y.; McCaffrey, J.; Norwood, D.L. Recent advances in headspace gas chromatography. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1823–1851. [Google Scholar] [CrossRef]
- Theis, A.L.; Waldack, A.J.; Hansen, S.M.; Jeannot, M.A. Headspace solvent microextraction. Anal. Chem. 2001, 73, 5651–5654. [Google Scholar] [CrossRef] [PubMed]
- Drozd, J. Chemical derivatization in gas chromatography. J. Chromatogr. A 1975, 113, 303–356. [Google Scholar] [CrossRef]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namiesnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.N.; Farquar, G.R.; Jones, A.D.; Frank, M. Human breath analysis: Methods for sample collection and reduction of localized background effects. Anal. Bioanal. Chem. 2010, 396, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Souza-Silva, E.A.; Pawliszyn, J. Headspace versus direct immersion solid phase microextraction in complex matrixes: Investigation of analyte behavior in multicomponent mixtures. Anal. Chem. 2015, 87, 8448–8456. [Google Scholar] [CrossRef] [PubMed]
- Yassa, N.; Colomb, A.; Lochte, K.; Peeken, I.; Williams, J. Development and application of a headspace solid-phase microextraction and gas chromatography/mass spectrometry method for the determination of dimethylsulfide emitted by eight marine phytoplankton species. Limnol. Oceanogr. 2006, 4, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Koziel, J.A.; Spinhirne, J.P.; Lloyd, J.D.; Parker, D.B. Evaluation of sample recovery of malodorous livestock gases from air sampling bags, solid-phase microextraction fibers, Tenax TA sorbent tubes, and sampling canisters. J. Air Waste Manag. Assoc. 2005, 55, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Van Harreveld, A.P. Odor concentration decay and stability in gas sampling bags. J. Air Waste Manag. Assoc. 2003, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sironi, S.; Eusebio, L.; Capellil, L.; Boiardi, E.; DeRosso, R. Ammonia diffusion through Nalophan double bags: Effect of concentration gradient reduction. Sci. World J. 2014, 2014, 214190. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, F.J.; Razavi, C.; Webb, A.K.; Jones, A.M.; Spanel, P.; Smith, D.; Lenney, W. An investigation of suitable bag materials for the collection and storage of breath samples containing hydrogen cyanide. J. Breath Res. 2012, 6, 036004. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.W.; Mani, N.; Geier, B.A.; Kwak, J.; Shepard, P.; Fan, M.; Sudberry, G.L.; Mayes, R.S.; Ott, D.K.; Martin, J.A.; et al. Storage stability of exhaled breath on Tenax TA. J. Breath Res. 2016, 10, 046008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A non-invasive method for in vivo skin volatile compounds sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lenochova, P.; Roberts, S.C.; Havlicek, J. Methods of human body odor sampling: The effect of freezing. Chem. Senses 2009, 34, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Nose, K.; Ueda, H.; Ohkuwa, T.; Kondo, T.; Araki, S.; Ohtani, H.; Tsuda, T. Identification and assessment of carbon monoxide in gas emanated from human skin. Chromatography 2006, 27, 63–65. [Google Scholar]
- Szulejko, J.E.; Kim, K.H. Re-evaluation of effective carbon number (ECN) approach to predict response factors of ‘compounds lacking authentic standards or surrogates’ (CLASS) by thermal desorption analysis with GC-MS. Anal. Chim. Acta 2014, 851, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.L.; Llobet, E.; Gardner, J.W. Electronic noses: A review of signal processing techniques. IEEE Proc. Circuits Devices Syst. 1999, 146, 297–310. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sliwinska, M.; Wisniewska, P.; Dymerski, T.; Namiesnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
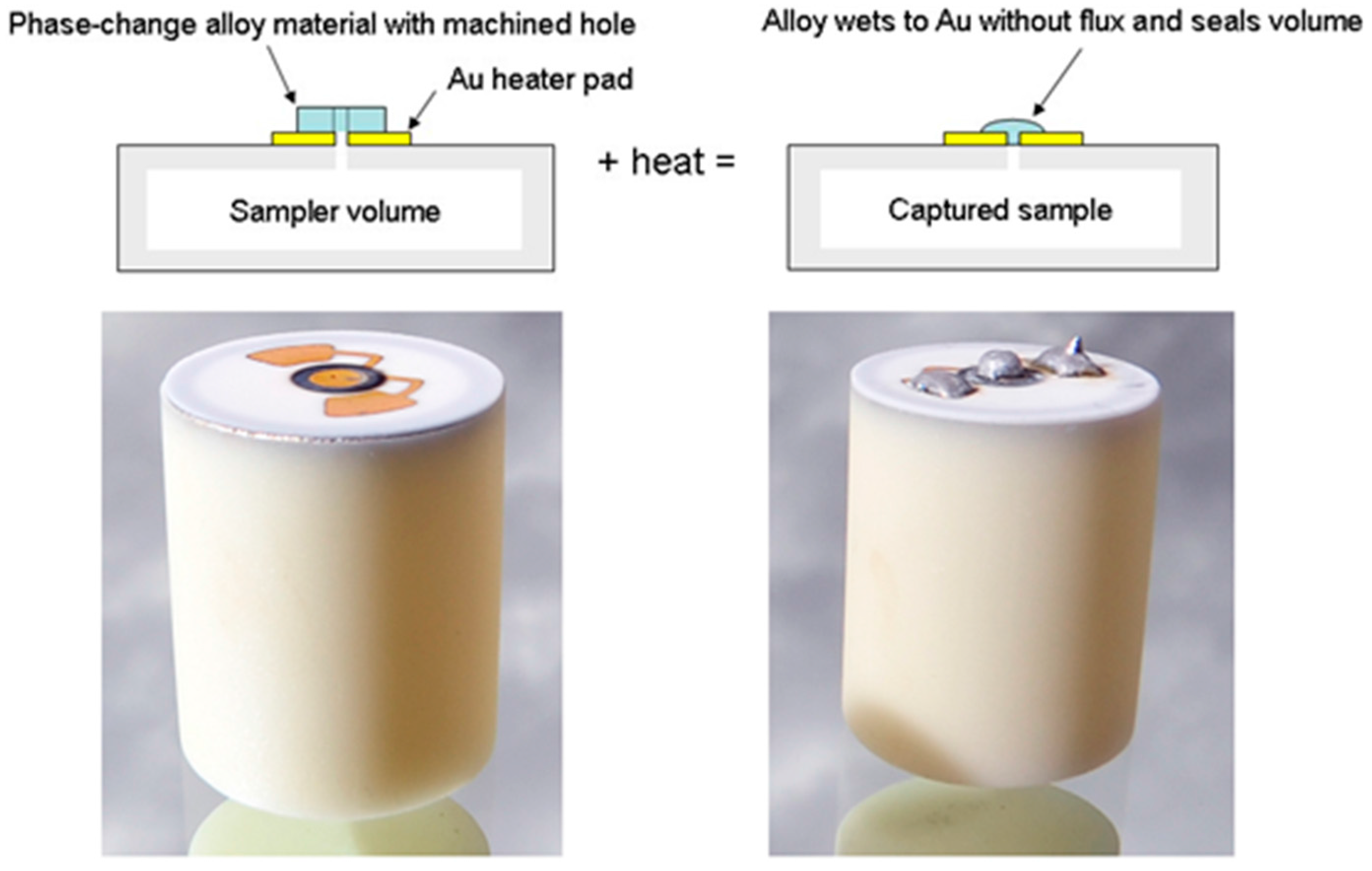
- Manginell, R.P.; Moorman, M.W.; Rejent, J.A.; Vianco, P.T.; Grazier, M.J.; Wroblewski, B.D.; Mowry, C.D.; Achyuthan, K.E. A materials investigation of a phase-change micro-valve for greenhouse gas collection and other potential applications. Rev. Sci. Instrum. 2012, 83, 031301. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.C.; Herman, J.C. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron. Dev. 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Akbar, M.; Restaino, M.; Agah, M. Chip-scale gas chromatography: From injection through detection. Microsyst. Nanoeng. 2015, 1, 15039. [Google Scholar] [CrossRef]
- Lussac, E.; Barattin, R.; Cardinael, P.; Agasse, V. Review on micro-gas analyzer systems: Feasibility, separations and applications. Crit. Rev. Anal. Chem. 2016, 46, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Manginell, R.P.; Bauer, J.M.; Moorman, M.W.; Sanchez, L.J.; Anderson, J.M.; Whiting, J.J.; Porter, D.A.; Copic, D.; Achyuthan, K.E. A monolithically-integrated μGC chemical sensor system. Sensors 2011, 11, 6517–6532. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Rice, G.; Agah, M. Characterization of a micro-helium discharge detector for gas chromatography. Sens. Actuator B 2015, 206, 190–197. [Google Scholar] [CrossRef]
- Manginell, R.P.; Mowry, C.D.; Pimentel, A.S.; Mangan, M.A.; Moorman, M.W.; Sparks, E.S.; Allen, A.; Achyuthan, K.E. Development of a mesoscale pulsed discharge helium ionization detector for portable gas chromatography. Anal. Sci. 2015, 31, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Manginell, R.P.; Pimentel, A.S.; Mowry, C.D.; Mangan, M.A.; Moorman, M.W.; Allen, A.; Schares, E.S.; Achyuthan, K.E. Diagnostic potential of the pulsed discharged helium ionization detector (PDHID) for pathogenic Mycobacterial volatile biomarkers. J. Breath Res. 2013, 7, 037107. [Google Scholar] [CrossRef] [PubMed]
- Mowry, C.D.; Pimentel, A.S.; Sparks, E.S.; Moorman, M.W.; Achyuthan, K.E.; Manginell, R.P. Pulsed discharge helium ionization detector for highly sensitive aquametry. Anal. Sci. 2016, 32, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, D.S. Pulsed discharge detector: Theory and applications. J. Chromatogr. A 2004, 1050, 63–68. [Google Scholar] [CrossRef]
- Cai, H.; Wentworth, W.E.; Stearns, S.D. Characterization of the pulsed discharge electron capture detector. Anal. Chem. 1996, 68, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, W.E.; Vasnin, S.V.; Stearns, S.D.; Meyer, C.J. Pulsed discharge helium ionization detector. Chromatographia 1992, 34, 219–225. [Google Scholar] [CrossRef]
- Syhre, M.; Chambers, S.T. The scent of Mycobacterium tuberculosis. Tuberculosis 2008, 88, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Lee, J.Y.; Ko, H.J.; Park, T.H. Bioelectronic nose: An emerging tool for odor standardization. Trends Biotechnol. 2017, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tewari, J.C.; Irudayaraj, J.M.K. Floral classification of honey using mid-infrared spectroscopy and surface acoustic wave based z-Nose sensor. J. Agric. Food Chem. 2005, 53, 6955–6966. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Capozzi, V.; Spano, G.; Biasioli, F. Proton transfer reaction-mass spectrometry: Online and rapid determination of volatile organic compounds of microbial origin. Appl. Microbiol. Biotechnol. 2015, 99, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Taiti, C.; Costa, C.; Nissim, W.G.; Bibbiani, S.; Azzarello, E.; Masi, E.; Pandolfi, C.; Pallottino, F.; Mensatti, P.; Mancuso, S. Assessing VOC emission by different wood cores using the PTR-ToF-MS technology. Wood Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Kameyama, S.; Tanimoto, H.; Inomata, S.; Tsunogai, U.; Ooki, A.; Yokouchi, Y.; Takeda, S.; Obata, H.; Uematsu, M. Equilibrator inlet-proton transfer reaction-mass spectrometry (EI-PTR-MS) for sensitive, high-resolution measurement of dimethyl sulfide dissolved in seawater. Anal. Chem. 2009, 81, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, S.; Tanimoto, H.; Inomata, S.; Suzuki, K.; Komatsu, D.D.; Hirota, A.; Konno, U.; Tsunogai, U. Application of PTR-MS to an incubation experiment of the marine diatom Thalassiosira pseudonana. Geochem. J. 2011, 45, 355–363. [Google Scholar] [CrossRef]
- De Gouw, J.; Warneke, C. Measurement of volatile compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef] [PubMed]
- Exton, D.A.; Smith, D.J.; McGenity, T.J.; Steinke, M.; Hills, A.J.; Suggett, D.J. Application of a fast isoprene sensor (FIS) for measuring isoprene production from marine samples. Limnol. Oceanogr. Methods 2010, 8, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.C.; Bartle, K.D.; Seakins, P.W.; Lewis, A.C. Direct measurement of atmospheric formaldehyde using gas chromatography-pulsed discharge ionization detector. Anal. Commun. 1999, 36, 101–104. [Google Scholar] [CrossRef]
- Dojahn, J.G.; Wentworth, W.E.; Stearns, S.D. Characterization of formaldehyde by gas chromatography using multiple pulsed-discharge photoionization detectors and a flame ionization detector. J. Chromatogr. Sci. 2001, 39, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, S.; Liu, F.; Liu, J.; Zhu, Z.; Li, G. Direct measurement of formaldehyde and methanol emissions from gasohol engine via pulsed discharge helium ionization detector. Fuel 2010, 89, 2179–2184. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Loisel, H.; Breon, F.M. A species dependent bio-optical model of case I waters for global ocean color processing. Deep Sea Res. Part I 2006, 53, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Nichol, J.; Wong, M.S. Estimation of ambient BVOC emissions using remote sensing techniques. Atmos. Environ. 2011, 45, 2937–2943. [Google Scholar] [CrossRef]
- Villa, T.F.; Gonzalez, F.; Miljievic, B.; Ristovski, Z.D.; Morawska, L. An overview of small unmanned aerial vehicles for air quality measurements: Present applications and future prospectives. Sensors 2016, 16, 1072. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.J.; Savidge, W.; Short, R.T.; Cardenas-Valencia, A.M.; Fulweiler, R.W. A review of the emerging field of underwater mass spectrometry. Front. Mar. Sci. 2016, 3, 209. [Google Scholar] [CrossRef]
- Robbins, I.C.; Kirckpatrick, G.J.; Blackwell, S.M.; Hillier, J.; Knight, C.A.; Moline, M.A. Improved monitoring of HABs using autonomous underwater vehicles (AUV). Harmful Algae 2006, 5, 749–761. [Google Scholar] [CrossRef]
- Zhang, Y.; Kieft, B.; McEwen, R.; Stanway, J.; Bellingham, J.; Ryan, J.; Hobson, B.; Porgett, D.; Birch, J.; Scholin, C. Tracking and sampling of a phytoplankton patch by an autonomous underwater vehicle in drifting mode. In Proceedings of the IEEE Ocean’s 15 MTS/IEEE, Washington, DC, USA, 19–22 October 2015. [Google Scholar]
- Tonacci, A.; Lacava, G.; Lippa, M.A.; Lupi, L.; Cocco, M.; Domenici, C. Electronic nose and AUV: A novel perspective in marine pollution monitoring. Mar. Technol. Soc. J. 2015, 49, 18–24. [Google Scholar] [CrossRef]
- Sorigue, D.; Legeret, B.; Cuine, S.; Morales, P.; Mirabella, B.; Guedeney, G.; Li-Beisson, Y.; Jetter, R.; Peltier, G.; Beisson, F. Microalgae synthesize hydrocarbons from long-chain fatty acids via a light-dependent pathway. Plant Physiol. 2016, 171, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Munoz, S.M.; Sandoval, A. Effects of ionic strength on the production of short chain volatile hydrocarbons by Dunaliella salina (Teodoresco). Chemosphere 2004, 54, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Xu, J.; Li, Y.; Zhou, C.; Yan, X. Change of volatile components in six microalgae with different growth phases. J. Sci. Food Agric. 2017, 97, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Hom, E.F.Y.; Aiyar, P.; Schaeme, D.; Mittag, M.; Sasso, S. A chemical perspective on microalgal-microbial interactions. Trends Plant Sci. 2015, 20, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Venuleo, M.; Raven, J.A.; Giordano, M. Intraspecific chemical communication in microalgae. New Phytol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xiao, R.; Li, J.; Shi, B.; Liang, Y.; Lu, W.; Chen, L. Headspace solid-phase microextraction with on-fiber derivatization for the determination of aldehydes in algae by gas chromatography-mass spectrometry. J. Sep. Sci. 2011, 34, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Wound-activated chemical defense in unicellular planktonic algae. Ang. Chem. Int. Ed. 2000, 39, 4352–4354. [Google Scholar] [CrossRef]



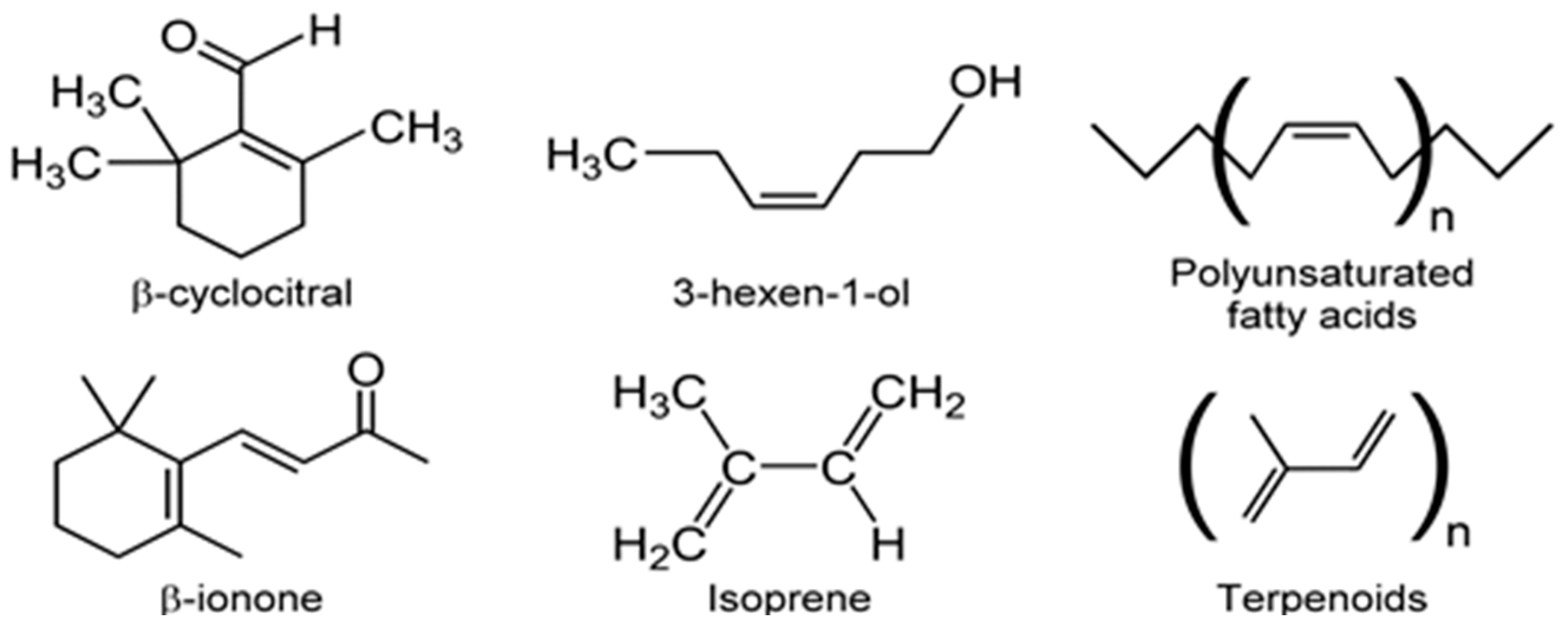
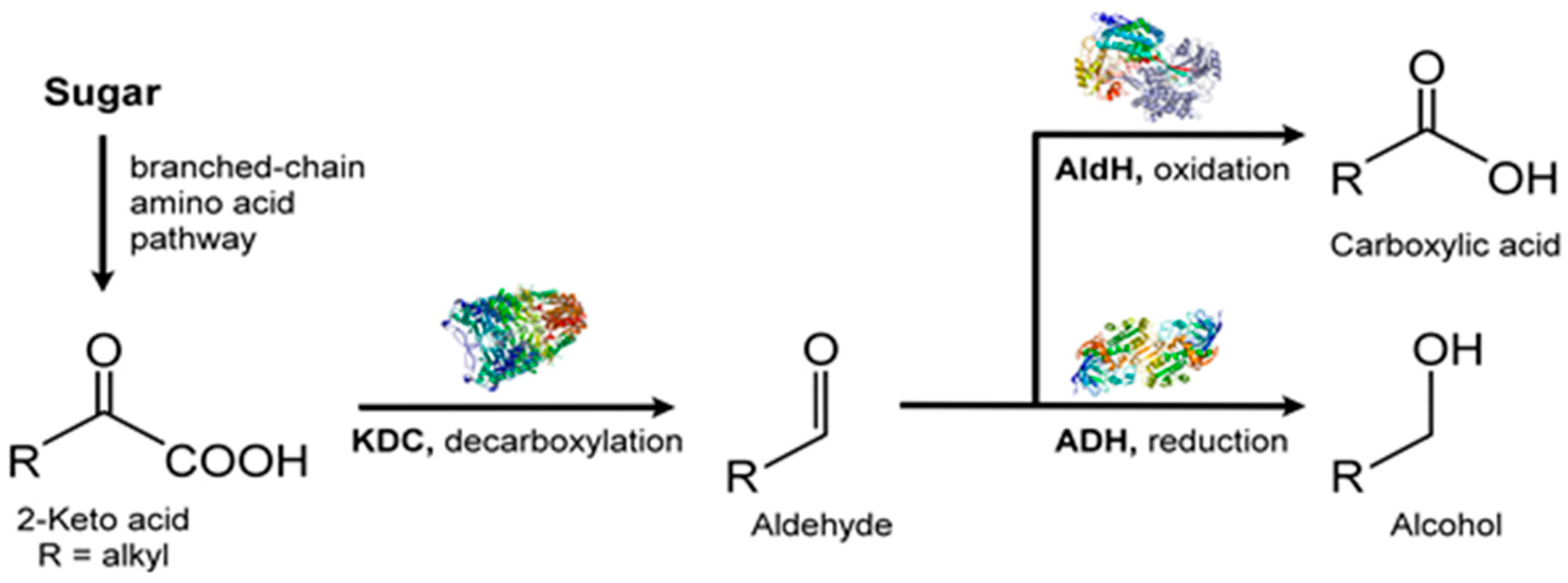
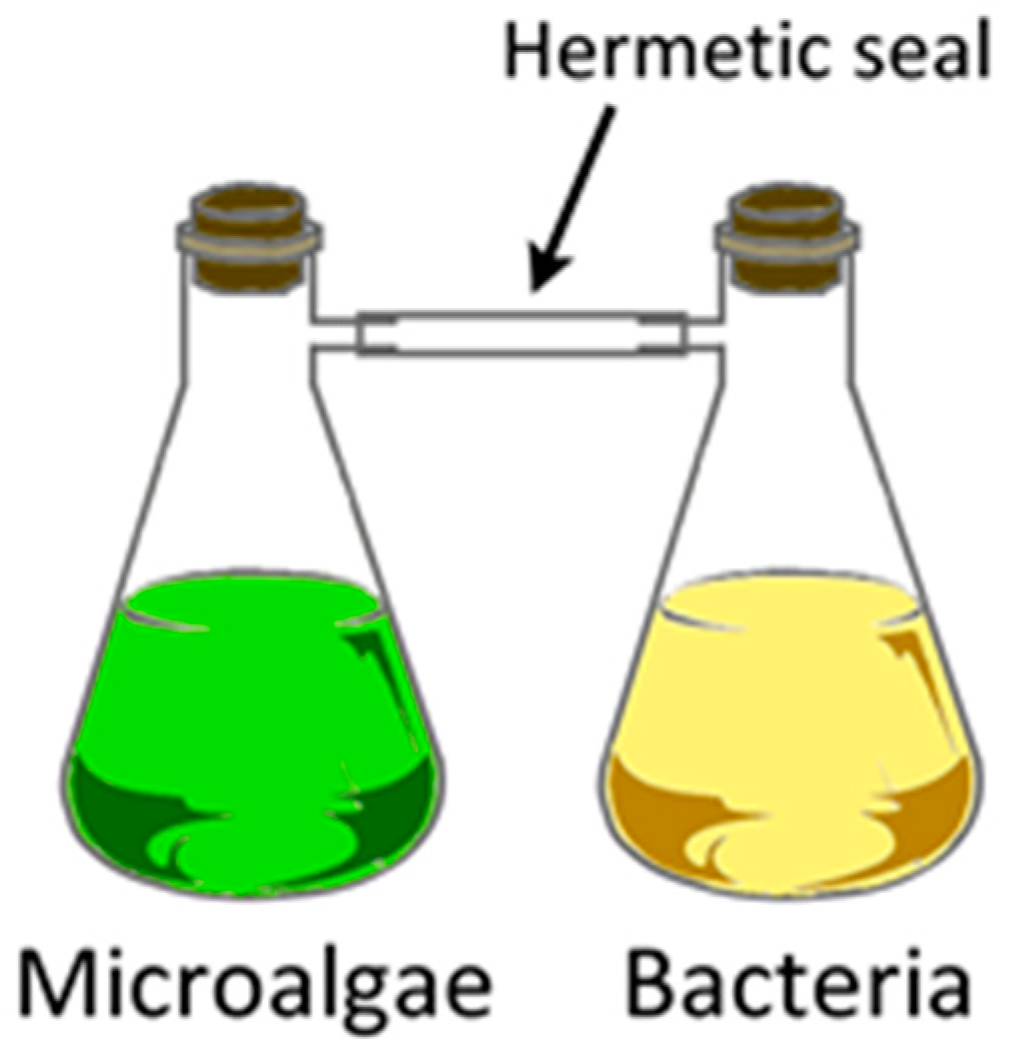
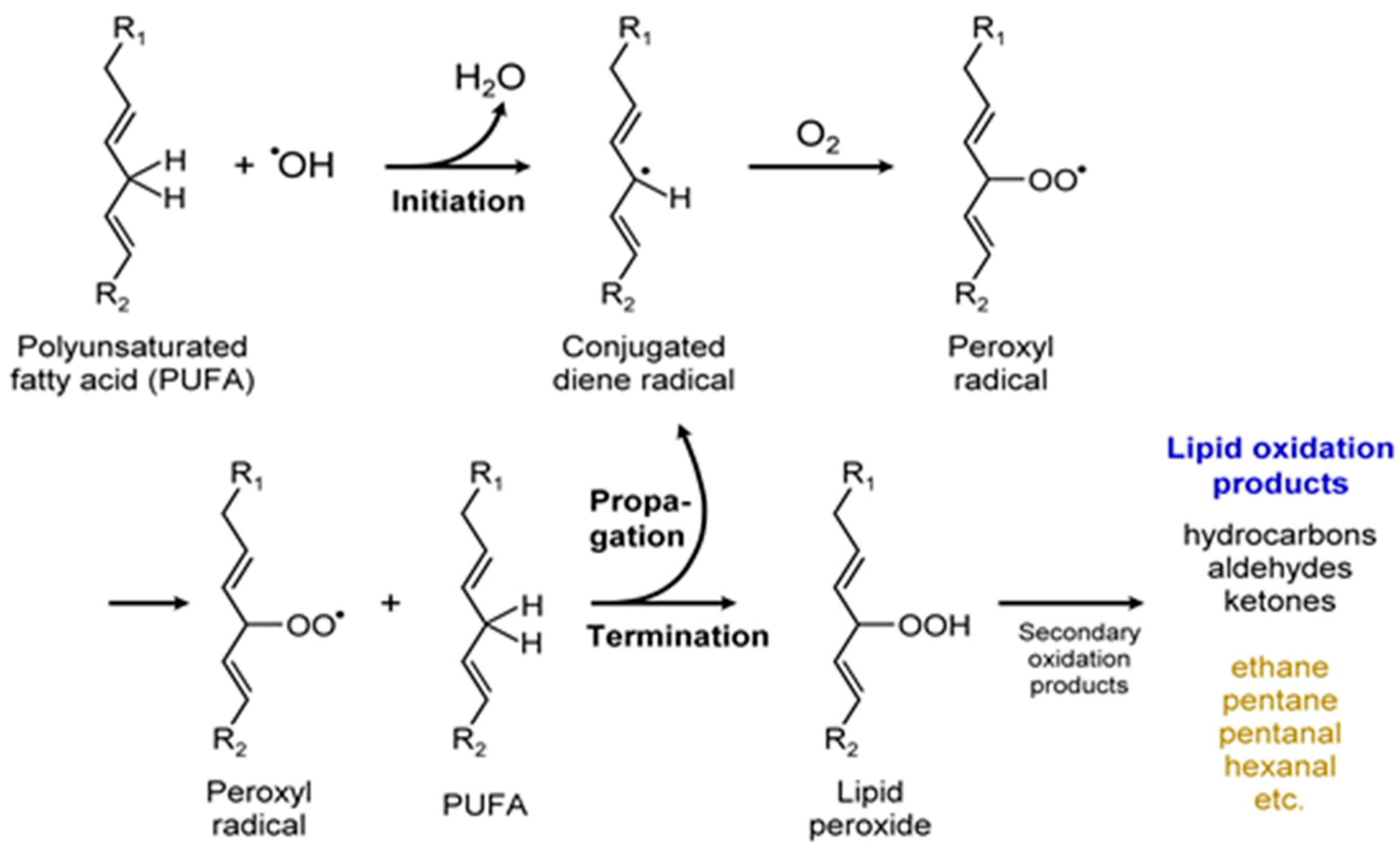

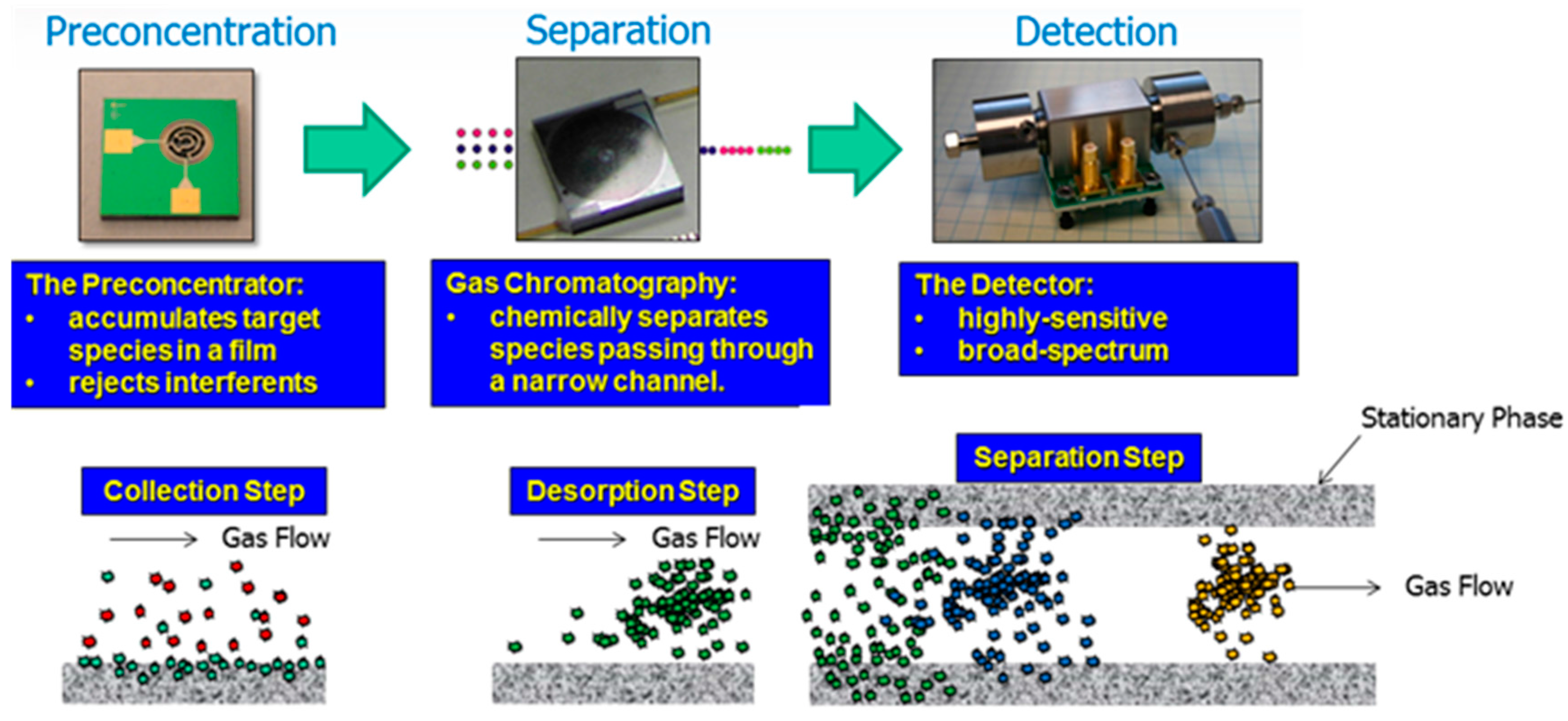
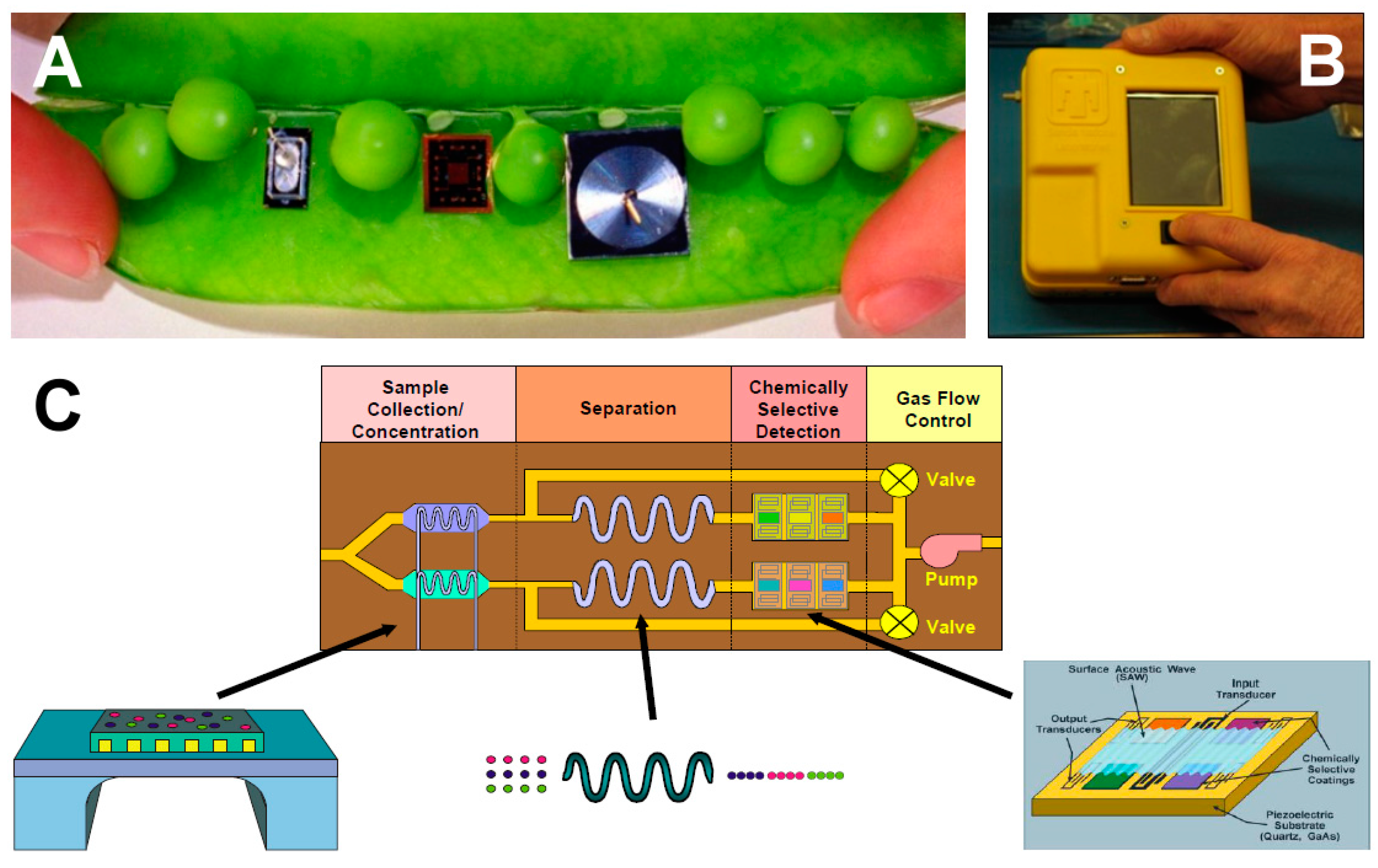

| Class | Abbreviation | Vapor Pressure (VP) (p) and Atmospheric Pressure (Patm) | VP (mm Hg) at 25 °C | Boiling Point (°C) | Example |
|---|---|---|---|---|---|
| Very Volatile Organic Compound | VVOC | p >> Patm | >380 | <0 up to 50 | Propane |
| Volatile Organic Compound | VOC | p > Patm | 0.1 to 380 | ~50 to 250 | Acetone |
| Semi-Volatile Organic Compound | SVOC | p = Patm | 10−7 to 10−1 | 250 to 400 | Plasticizers |
| Non-Volatile Organic Compound | NVOC | p << Patm | <10−7 | Not applicable | Glucose C6H12O6 |
| VOC | Metabolic Basis |
|---|---|
| H2, CH4 (Hydrogen, Methane) | Carbohydrate |
| C2H6CO (Acetone) | Acetoacetate decarboxylation |
| NH3, CH3NH2 (Ammonia, Methylamine) | Protein |
| H/Cs, C5H12, C2H6, C2H4 (Hydrocarbons) | Lipid peroxidation |
| NO (Nitric oxide) | Nitric oxide synthase |
| C2H5OH, CS2, COS (Carbonyl sulfide) | Intestinal bacteria |
| CO (Carbon monoxide) | Heme catabolism |
| CH3SH, C2H6S (Methane/Ethane thiols) | Methionine metabolism |
| CH3CHO (Acetaldehyde) | Ethanol metabolism |
| C5H10 (Cyclopentane) | Cholesterol metabolism |
| Property | Electronic Nose | μGC/μPC/μPDHID | GC-MS |
|---|---|---|---|
| Specific VOC identified? | No | Yes | Yes |
| Instruments comparable? | No | Yes | No |
| Signals comparable? | No | Yes | No |
| Onboard pattern analysis | Yes | Yes | No |
| Sensitivity limits | ppm-to-ppb | ppb-to-ppt | ppt-ppq |
| Sensor drifts? | Yes | No | No |
| Sensor poisoning? | Yes | No | No |
| Responses are variable? | Yes | No | No |
| Individual VOC quantifiable? | No | Yes | Yes |
| Power requirements | Low-to-moderate | Low-to-moderate | High |
| Skill/training of operator | Moderate | Moderate | High |
| Portability? | Yes | Yes | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achyuthan, K.E.; Harper, J.C.; Manginell, R.P.; Moorman, M.W. Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity? Metabolites 2017, 7, 39. https://doi.org/10.3390/metabo7030039
Achyuthan KE, Harper JC, Manginell RP, Moorman MW. Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity? Metabolites. 2017; 7(3):39. https://doi.org/10.3390/metabo7030039
Chicago/Turabian StyleAchyuthan, Komandoor E., Jason C. Harper, Ronald P. Manginell, and Matthew W. Moorman. 2017. "Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity?" Metabolites 7, no. 3: 39. https://doi.org/10.3390/metabo7030039