Evaluation of State-Resolved Reaction Probabilities and Their Application in Population Models for He, H, and H2
Abstract
:1. Introduction
2. Population Models
2.1. Theory of Population Modeling
2.2. The Flexible Sover Yacora
3. Atomic Population Models
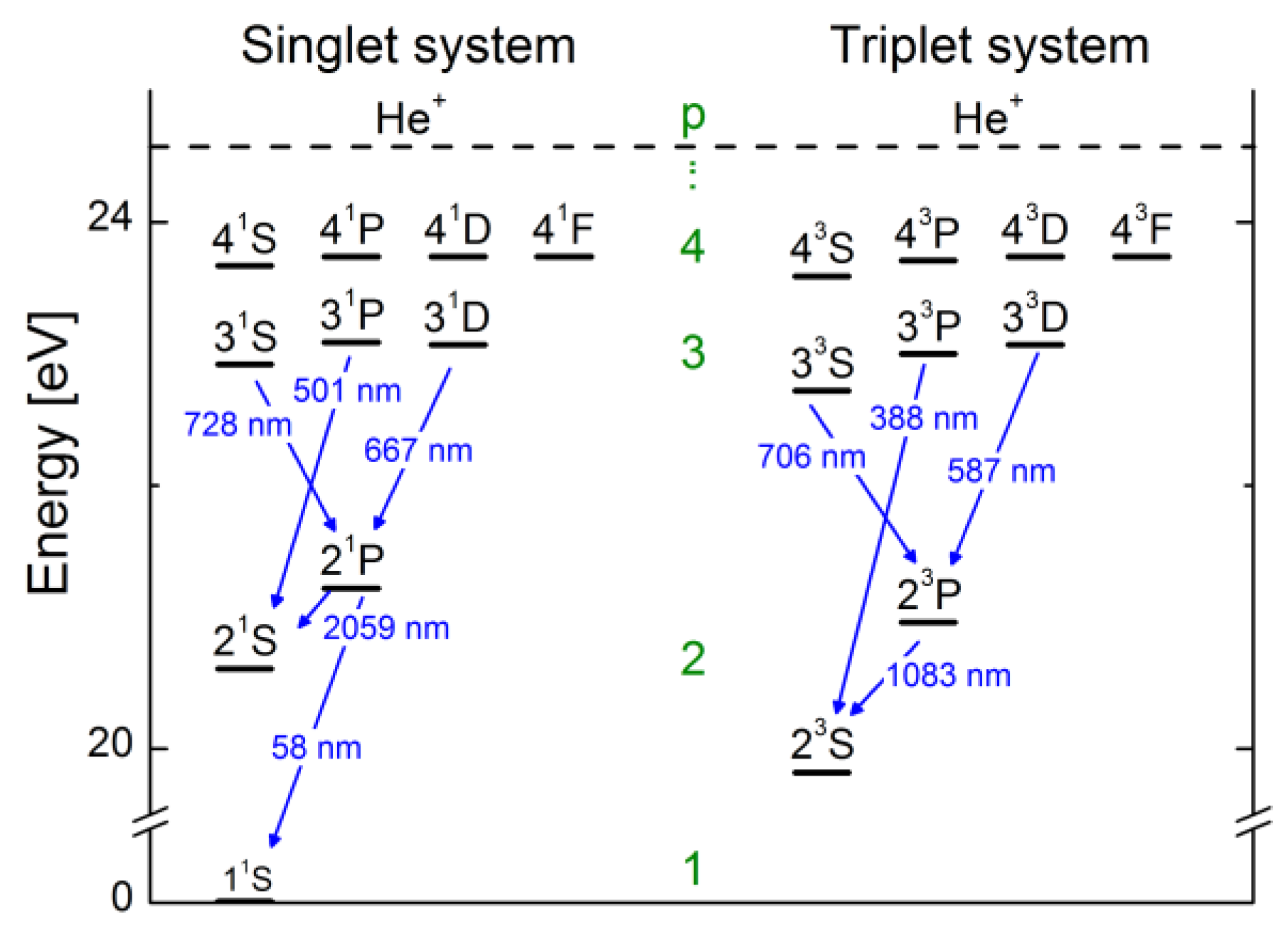
3.1. Helium
3.1.1. Properties of the Helium Atom
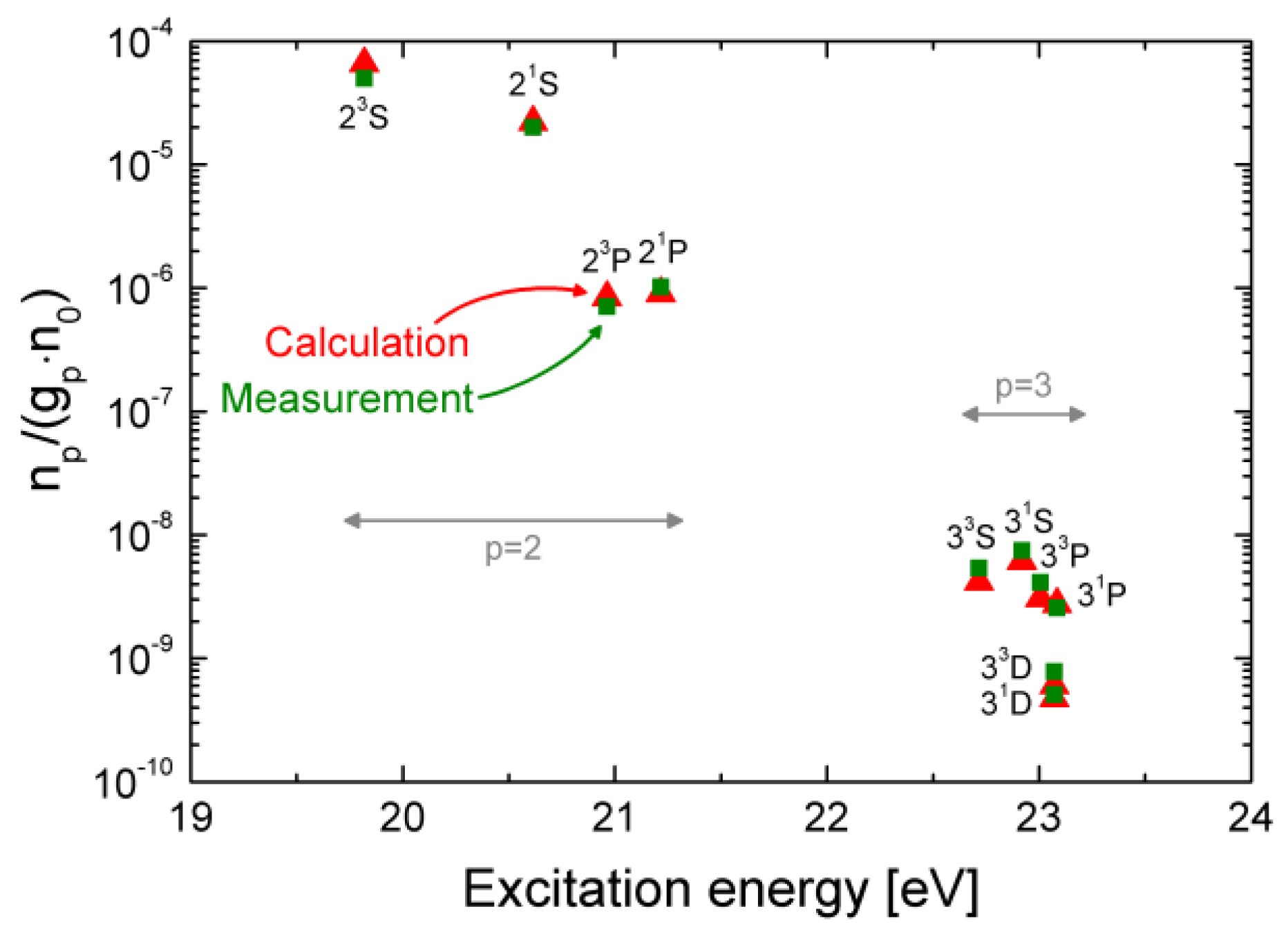
3.1.2. The CR Model for Helium and Results
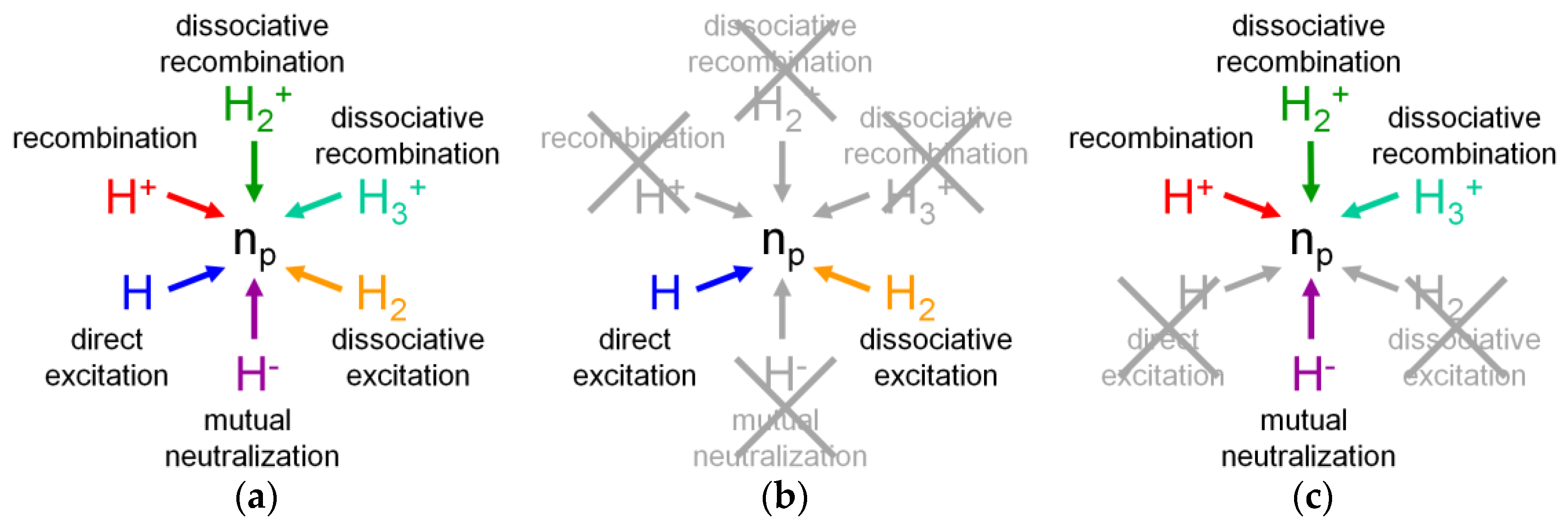
3.2. Atomic Hydrogen
3.2.1. Properties of the Hydrogen Atom
3.2.2. The CR Model for Atomic Hydrogen
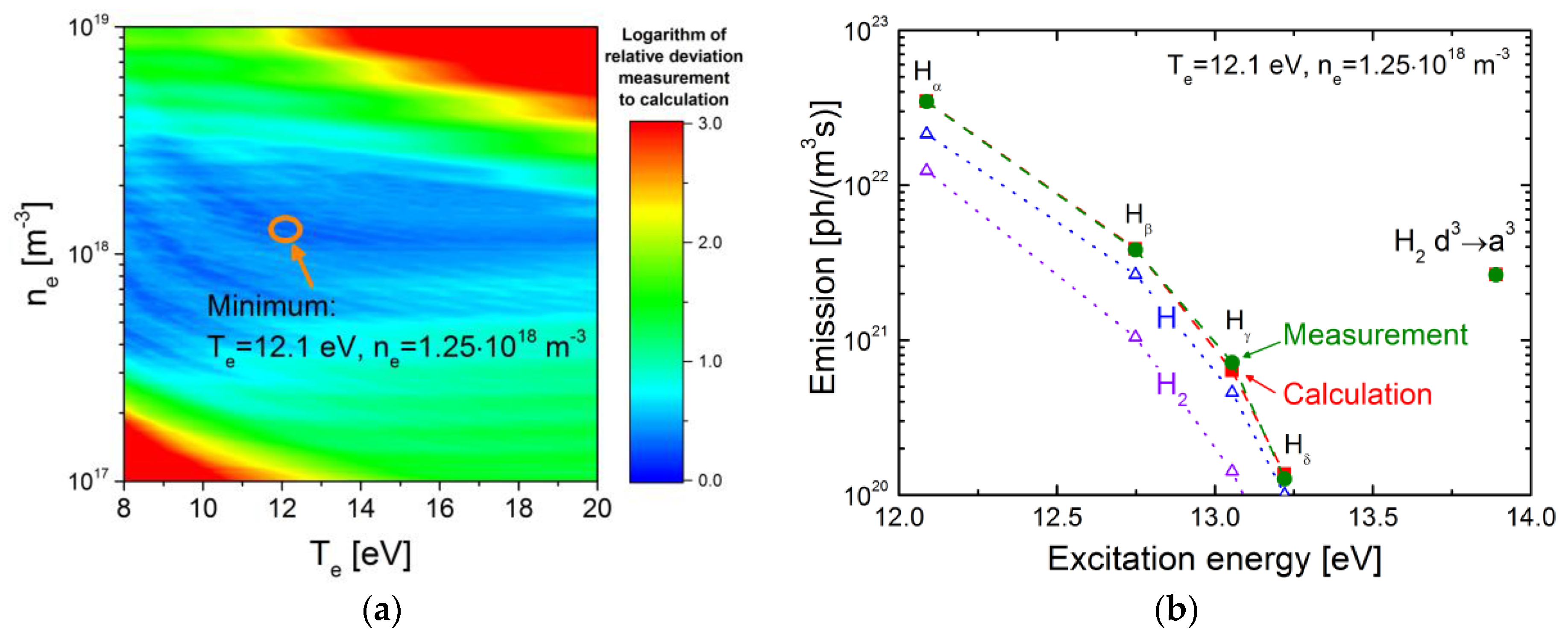
3.2.3. Application of the CR Model for H to an Ionizing Plasma
3.2.4. Application of the CR Model for H to a Recombining Plasma
4. Population Models for Molecular Hydrogen and Deuterium
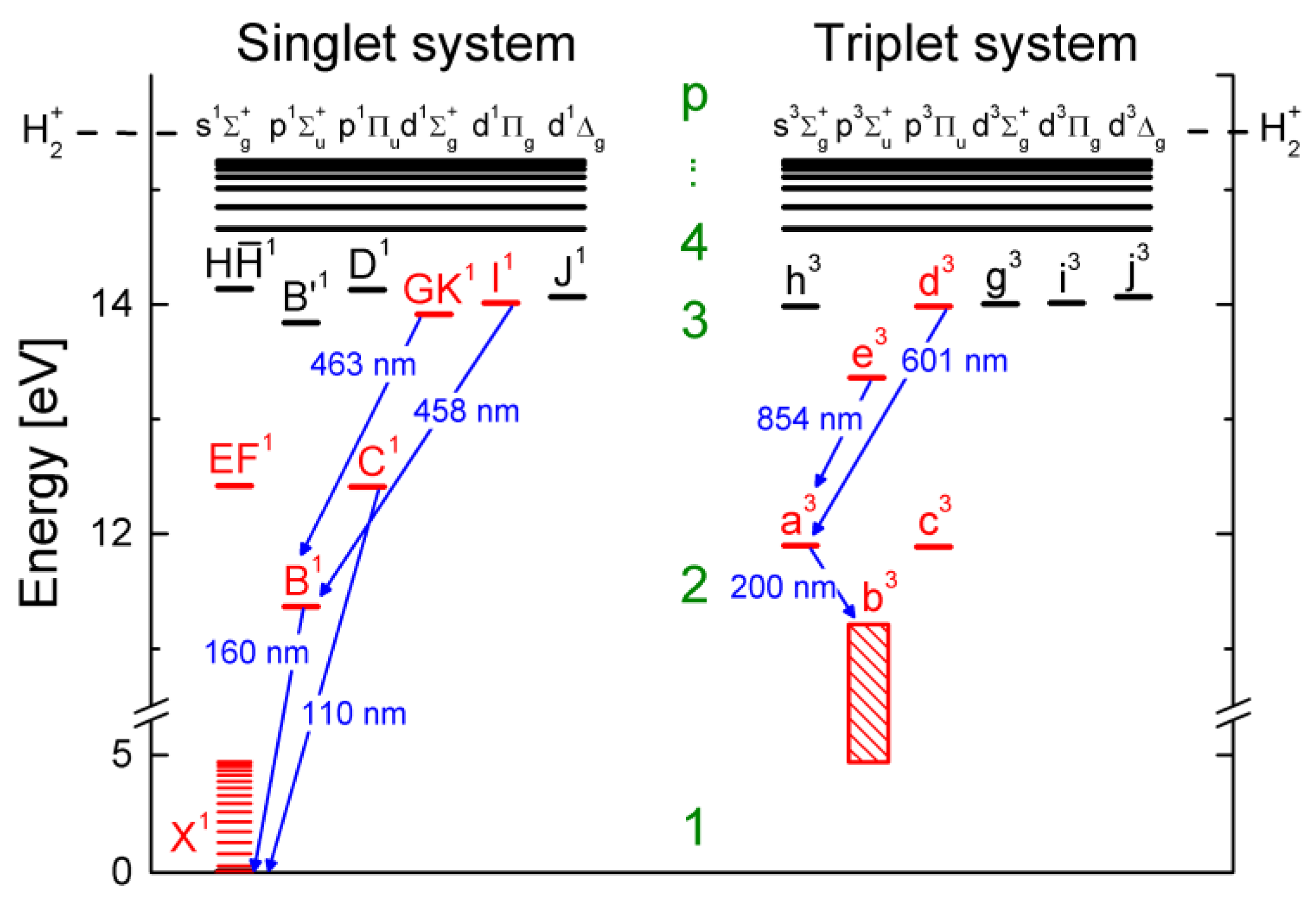
4.1. The Hydrogen Molecule
4.2. Characteristics of the Models for H2 and D2
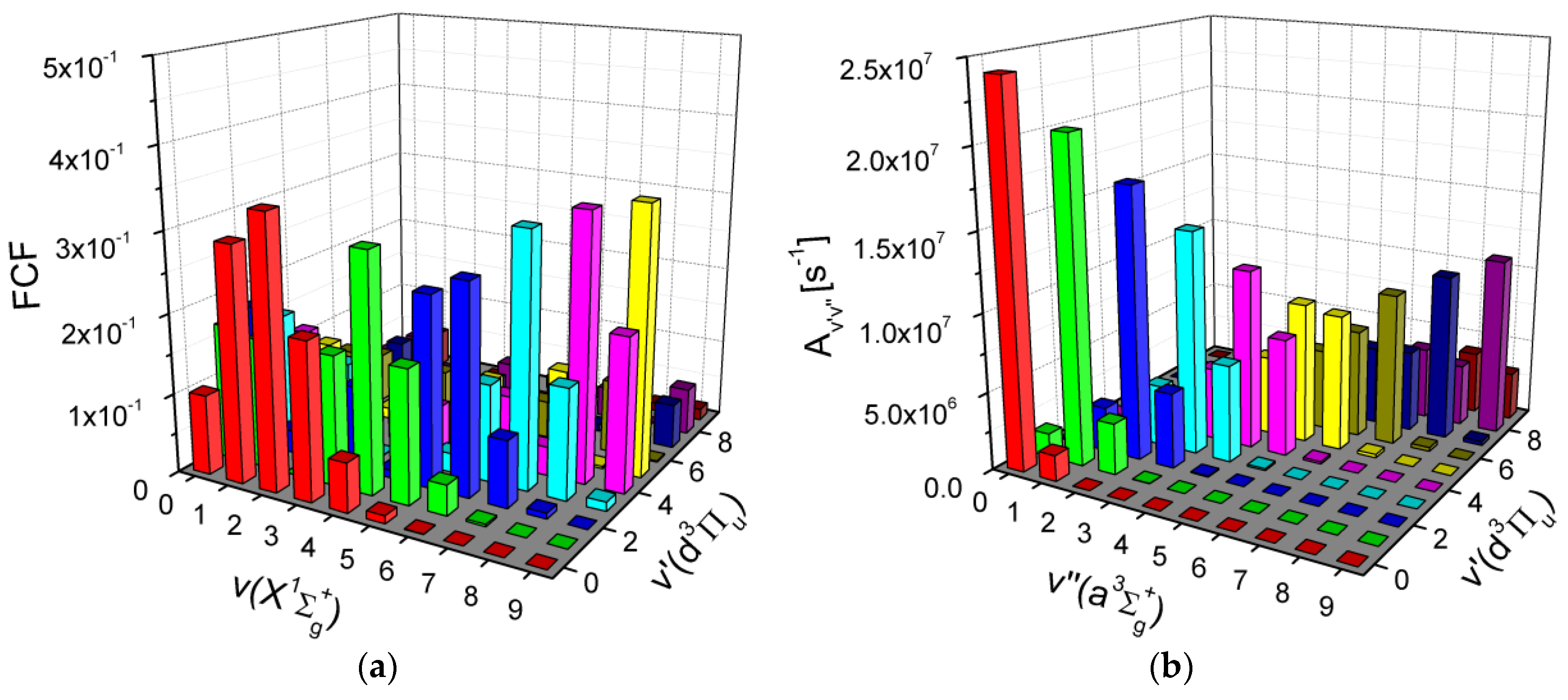
4.3. Franck–Condon Factors and Einstein Coefficients
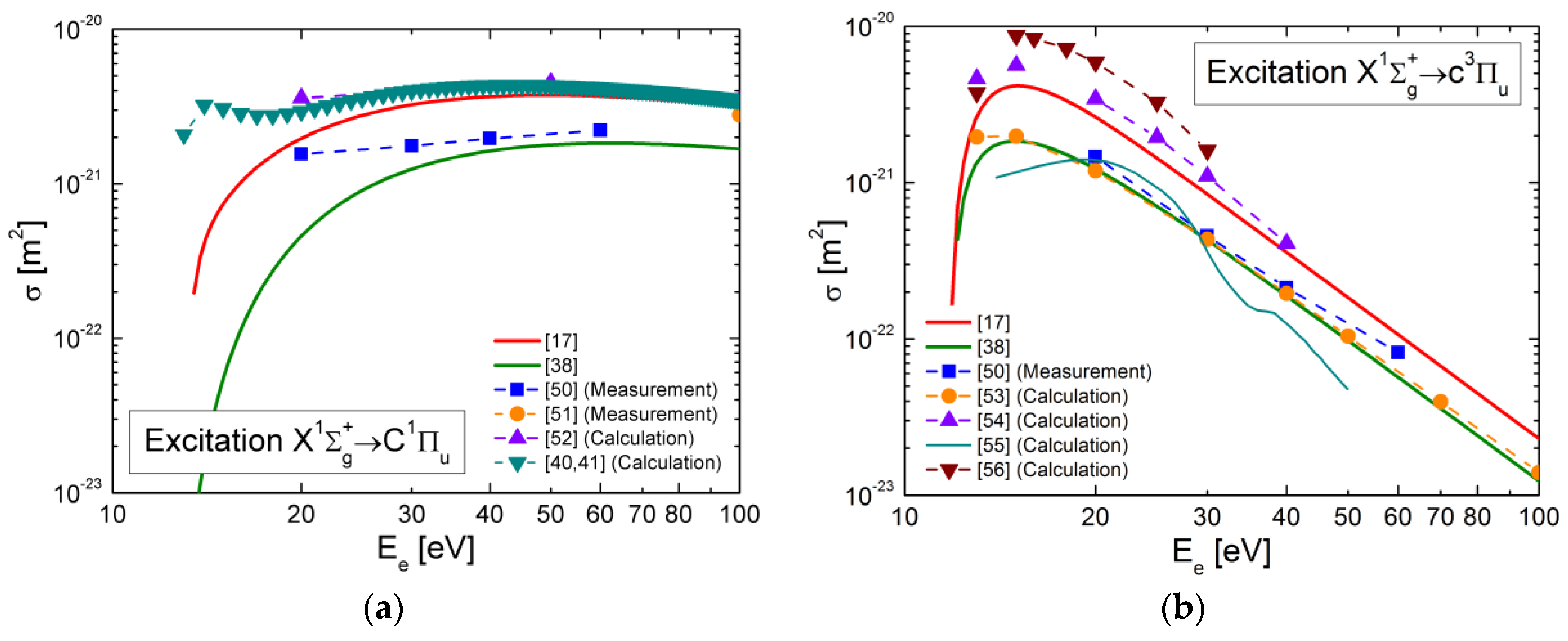
4.4. Electron Collision Excitation Cross-Sections
4.5. Electron Collision Ionization Cross-Sections
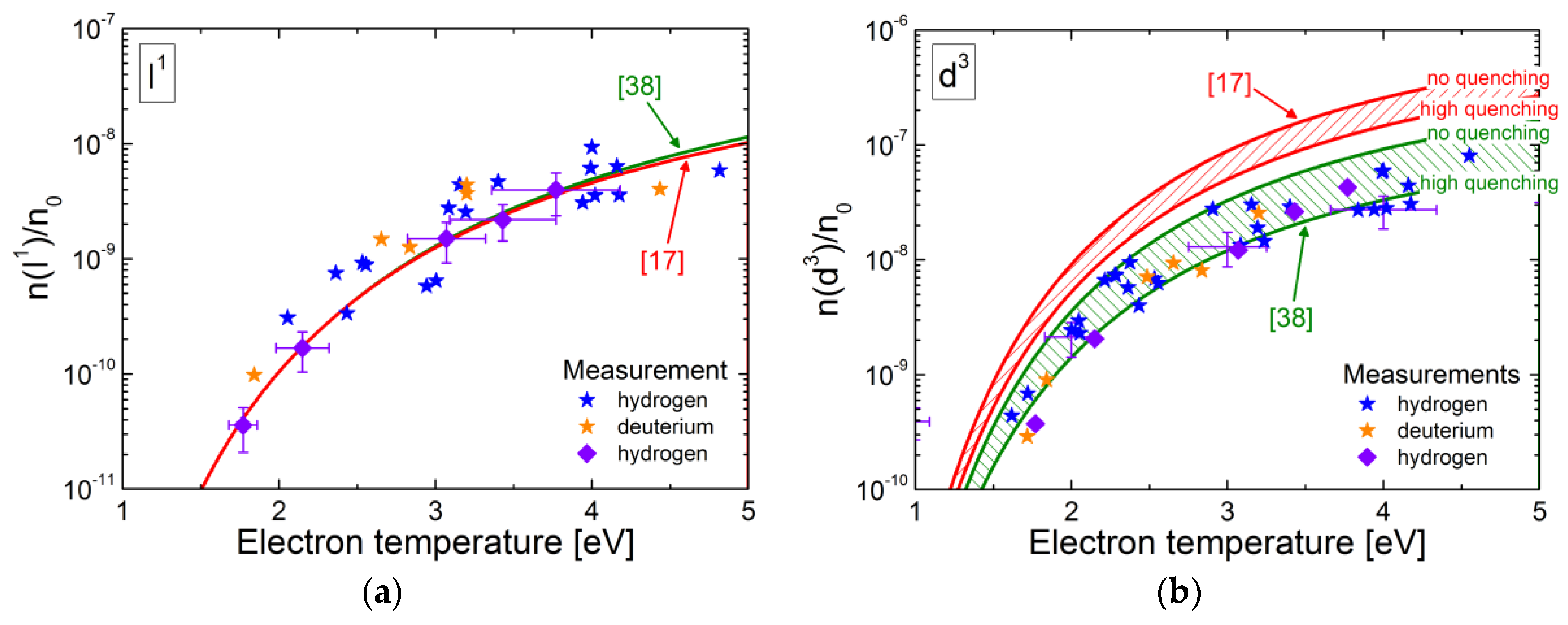
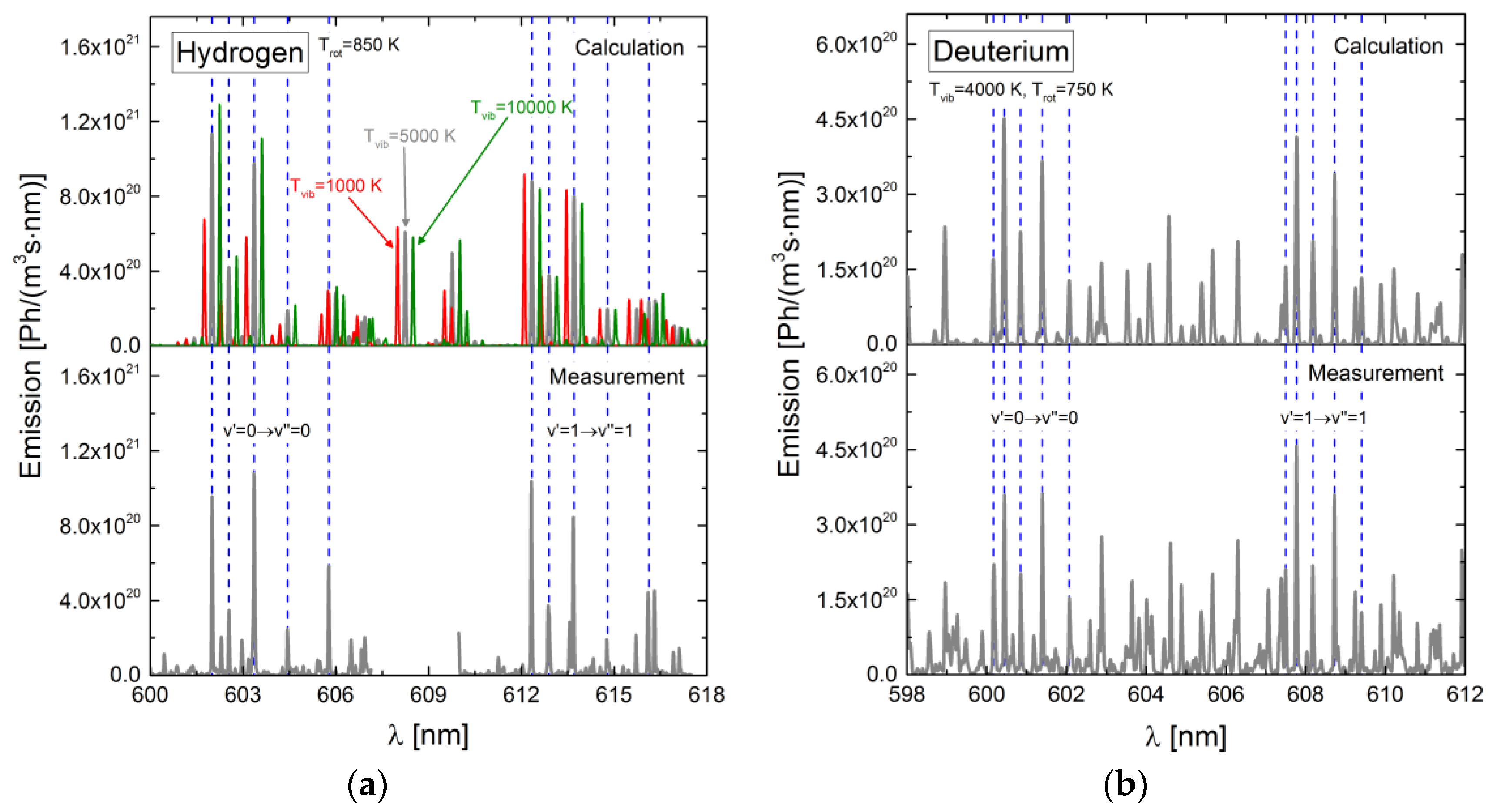
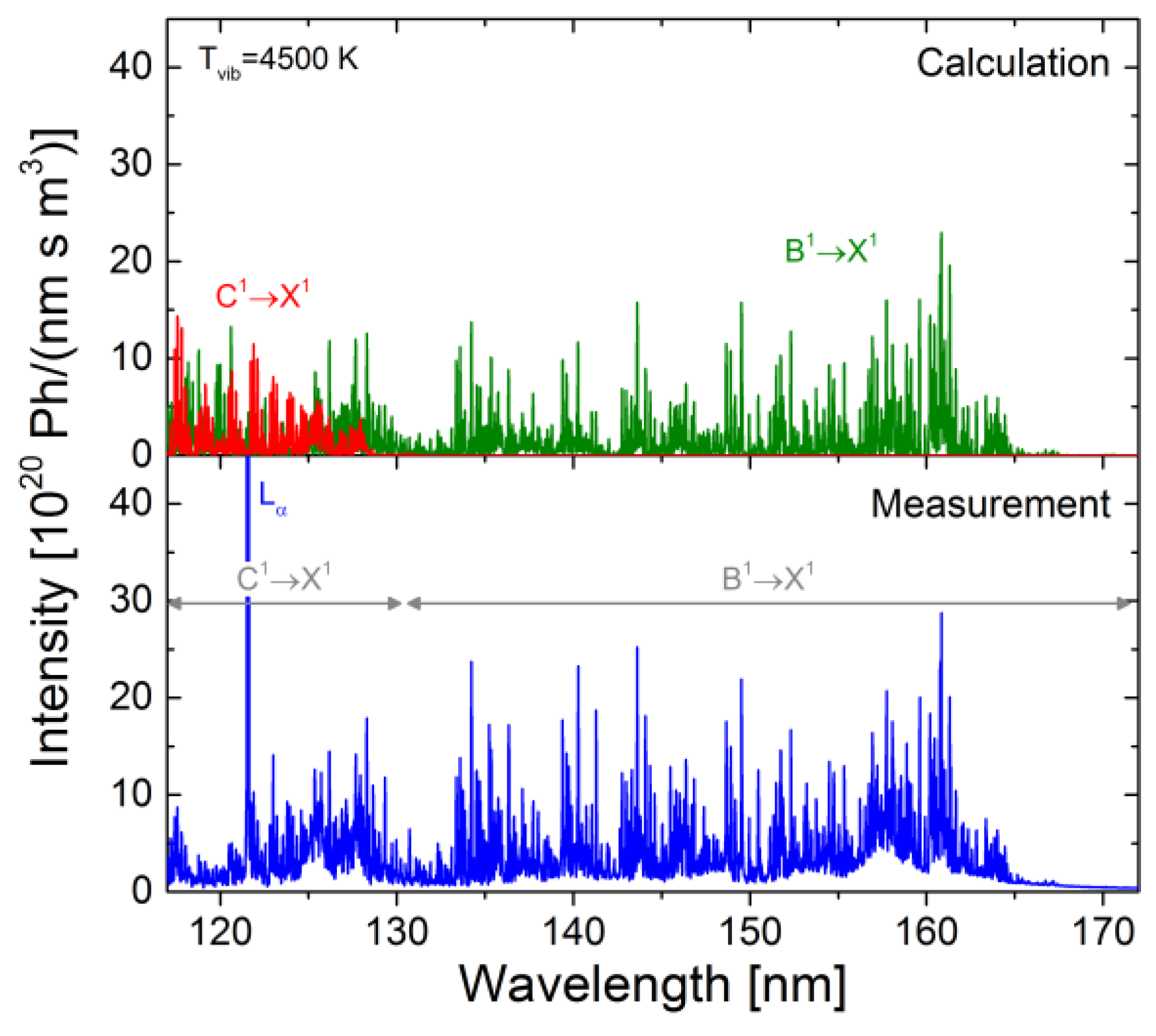
4.6. Application of the Models
5. Conclusions
Abbreviations
| OES | Optical emission spectroscopy |
| TALIF | Two-Photon Excited laser Induced Fluorescence |
| TDLAS | Tunable Diode Laser Absorption Spectroscopy |
| CR model | Collisional radiative model |
| EEDF | Electron energy distribution function |
| ECR | Electron cyclotron resonance |
| RF | Radio frequency |
| ITER | The internuclear thermonuclear experimental reactor (or latin for “the way”) |
| NBI | Neutral beam injection |
| FCF | Franck-Condon factor |
| ICP | Inductively coupled plasma |
References
- Fantz, U.; Falter, H.D.; Franzen, P.; Wünderlich, D.; Berger, M.; Lorenz, A.; Kraus, W.; McNeely, P.; Riedl, R.; Speth, E. Spectroscopy—A powerful diagnostic tool in source development. Nucl. Fusion 2006, 46, S297–S306. [Google Scholar] [CrossRef]
- Otorbaev, D.K.; Buuron, A.J.M.; Guerassimov, N.T.; van de Sanden, M.C.M.; Schram, D.C. Spectroscopic measurement of atomic hydrogen level populations and hydrogen dissociation degree in expanding cascaded arc plasmas. J. Appl. Phys. 1994, 76, 4499–4510. [Google Scholar] [CrossRef]
- Amorim, J.; Baravian, G.; Jolly, J. Laser-induced resonance fluorescence as a diagnostic technique in non-thermal equilibrium plasmas. J. Phys. D 2000, 33, R51–R65. [Google Scholar] [CrossRef]
- Hinkley, E.D. High-resolution infrared spectroscopy with a tunable diode. Appl. Phys. Lett. 1970, 16, 351–354. [Google Scholar] [CrossRef]
- Wünderlich, D.; Dietrich, S.; Fantz, U. Application of a collisional radiative model to atomic hydrogen for diagnostic purposes. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 62–71. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, D.; Rauner, D.; Wünderlich, D. Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Behringer, K.; Fantz, U. The influence of opacity on hydrogen excited-state population and applications to low-temperature plasmas. New J. Phys. 2000, 2, 23. [Google Scholar] [CrossRef]
- Cohen, S.D.; Hindmarsh, A.C.; Dubois, P.F. CVODE, A Stiff/Nonstiff ODE Solver in C. Comput. Phys. 1996, 10, 138–143. [Google Scholar] [CrossRef]
- Godyak, V.A. Nonequilibrium EEDF in gas discharge plasmas. IEEE Trans. Plasma Sci. 2006, 34, 755–766. [Google Scholar] [CrossRef]
- De Heer, F.J. Critically Assessed Electron-Impact Excitation Cross Sections for He (11S). In IAEA Nuclear Data Section Report; INDC(NDS)-385; IAEA: Vienna, Austria, 1998. [Google Scholar]
- Ralchenko, Y.V.; Janev, R.K.; Kato, T.; Fursa, D.V.; Bray, I.; de Heer, F.J. Cross Section Database for Collision Processes of Helium Atom with Charged Particles. I. Electron Impact Processes. In Research Reports NIFS DATA; NIFS-DATA-59; NIFS: Toki, Japan, 2000. [Google Scholar]
- Drake Gordon, W.F. Springer Handbook of Atomic, Molecular, and Optical Physics; Springer Science+Business Media, Inc.: New York, NY, USA, 2006; pp. 199–219. [Google Scholar]
- Fowler, R.H. Statistical equilibrium with special reference to the mechanism of ionization by electronic impacts. Philos. Mag. 1926, 47, 257–277. [Google Scholar] [CrossRef]
- Möller, W. Plasma and surface modeling of the deposition of hydrogenated carbon films from low-pressure methane plasmas. Appl. Phys. A 1993, 56, 527–546. [Google Scholar] [CrossRef]
- Fantz, U. Spectroscopic diagnostics and modelling of silane microwave plasmas. Plasma Phys. Control. Fusion 1998, 40, 1035–1056. [Google Scholar] [CrossRef]
- Janev, R.K. Atomic and Plasma-Material Interaction Data for Fusion. Suppl. Nucl. Fusion 1993, 4, 192. [Google Scholar]
- Janev, R.K.; Reiter, D.; Samm, U. Report Jül–4105. Forschungszentrum Jülich: Jülich, Germany, 2003; Available online: www.eirene.de/report_4105.pdf (accessed on 23 September 2016).
- Sawada, K.; Fujimoto, T. Effective ionization and dissociation rate coefficients of molecular hydrogen in plasma. J. Appl. Phys. 1995, 78, 2913–2924. [Google Scholar] [CrossRef]
- Kulander, F.C.; Guest, M.F. Excited electronic states of H3 and their role in the dissociative recombination of H3+. J. Phys. B 1979, 12, L501–L504. [Google Scholar] [CrossRef]
- Datz, S.; Sundström, G.; Biedermann, C.; Broström, L.; Danared, H.; Mannervik, S.; Mowat, J.R.; Larsson, M. Branching processes in the dissociative recombination of H3+. Phys. Rev. Lett. 1995, 74, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Eerden, M.J.J.; van de Sanden, M.C.M.; Otorbaev, D.K.; Schram, D.C. Cross section for the mutual neutralization reaction H2++H−, calculated in a multiple-crossing Landau-Zener approximation. Phys. Rev. A 1995, 51, 3362–3365. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.; Balance, C.P.; Badnell, N.R.; Summers, H.P. An R-matrix with pseudostates approach to the electron-impact excitation of HI for diagnostic applications in fusion plasmas. J. Phys. B 2000, 33, 1255–1262. [Google Scholar] [CrossRef]
- Speth, E.; Falter, H.D.; Franzen, P.; Fantz, U.; Bandyopadhyay, M.; Christ, S.; Encheva, A.; Fröschle, M.; Holtum, D.; Heinemann, B.; et al. Overview of the RF source development programme at IPP Garching. Nucl. Fusion 2006, 46, S220–S238. [Google Scholar] [CrossRef]
- Fantz, U.; Franzen, P.; Kraus, W.; Falter, H.D.; Berger, M.; Christ-Koch, S.; Fröschle, M.; Gutser, R.; Heinemann, B.; Martens, C.; et al. Low pressure and high power rf sources for negative hydrogen ions for fusion applications (ITER neutral beam injection) (invited). Rev. Sci. Instrum. 2008, 79, 02A511. [Google Scholar] [CrossRef] [PubMed]
- Fantz, U.; Wünderlich, D. A novel diagnostic technique for H−(D−) densities in negative hydrogen ion sources. New J. Phys. 2006, 8, 301. [Google Scholar] [CrossRef]
- Mazouffre, S.; Boogaarts, M.G.H.; Bakker, I.S.J.; Vankan, P.; Engeln, R.; Schram, D.C. Transport of ground-state hydrogen atoms in a plasma expansion. Phys. Rev. E 2001, 64, 16411. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, O.; Schram, D.C.; Engeln, R. Formation and relaxation of rovibrationally excited H2 molecules due to plasma-surface interaction. Phys. Rev. E 2008, 78, 016407. [Google Scholar] [CrossRef] [PubMed]
- Van Harskamp, W.E.N.; Brouwer, C.M.; Schram, D.C.; van de Sanden, M.C.M.; Engeln, R. Detailed H(n = 2) density measurements in a magnetized hydrogen plasma jet. Plasma Sources Sci. Technol. 2012, 21, 024009. [Google Scholar] [CrossRef]
- Bendinelli, O.; Ciotti, L.; Parmeggiani, G. Series inversion of Abel equation for very peaked profiles: The R1/4-law. Astrom. Astrophys. 1993, 279, 668–673. [Google Scholar]
- Lavrov, B.P.; Melnikov, A.S.; Käning, M.; Röpcke, J. UV continuum emission and diagnostics of hydrogen-containing nonequilibrium plasmas. Phys. Rev. E 1999, 59, 3526–3543. [Google Scholar] [CrossRef]
- Vankan, P.; Schram, D.C.; Engeln, R. High rotational excitation of molecular hydrogen in plasmas. Chem. Phys. Lett. 2004, 400, 196–200. [Google Scholar] [CrossRef]
- Fantz, U.; Heger, B. Spectroscopic diagnostics of the vibrational population in the ground state H2 and D2 of molecules. Plasma Phys. Control. Fusion 1998, 40, 2023–2032. [Google Scholar] [CrossRef]
- Ajello, J.M.; Srivastava, S.K.; Yung, Y.L. Laboratory studies of UV emissions of H2 by electron impact. The Werner- and Lyman-band systems. Phys. Rev. A 1982, 25, 2485–2498. [Google Scholar] [CrossRef]
- Day, R.L.; Anderson, R.J.; Sharpton, F.A. Electron excitation of the singlet-g states of H2. J. Chem. Phys. 1979, 71, 3683–3688. [Google Scholar] [CrossRef]
- Fantz, U.; Schalk, B.; Behringer, K. Calculation and interpretation of the continuum radiation of hydrogen molecules. New J. Phys. 2000, 2, 7. [Google Scholar] [CrossRef]
- Wedding, A.B.; Phelps, A.V. Quenching and excitation transfer for the c3Πu− and a3Σg+ states of H2 in collisions with H2. J. Chem. Phys. 1988, 89, 2965–2974. [Google Scholar] [CrossRef]
- Fantz, U.; Heger, B.; Wünderlich, D.; Pugno, R.; ASDEX Upgrade Team. Photon efficiency (S+D)/XB of hydrogen molecules at low electron temperatures. J. Nucl. Mater. 2003, 313, 743–747. [Google Scholar] [CrossRef]
- Miles, W.T.; Thompson, R.; Green, A.E.S. Electron-impact cross sections and energy deposition in molecular hydrogen. J. Appl. Phys. 1972, 43, 678–686. [Google Scholar] [CrossRef]
- Gryzinski, M. Classical theory of atomic collisions. I. Theory of inelastic collisions. Phys. Rev. 1965, 138, A336–A358. [Google Scholar] [CrossRef]
- Celiberto, R.; Janev, R.K.; Laricchiuta, A.; Capitelli, M.; Wadhera, J.M.; Atems, D.E. Cross section data for electron-impact inelastic processes of vibrationally excited molecules of hydrogen and its isotopes. At. Data Nucl. Data Tables 2001, 77, 161–213. [Google Scholar] [CrossRef]
- Celiberto, R.; (Bari, Italy). Private Communication, 2004.
- Fantz, U.; Wünderlich, D. Franck-Condon factors, transition probabilities, and radiative lifetimes for hydrogen molecules and their isotopomeres. At. Data Nucl. Data Tables 2006, 92, 853–973. [Google Scholar] [CrossRef]
- Franck, J. Elementary processes of photochemical reactions. Trans. Faraday Soc. 1926, 21, 536–542. [Google Scholar] [CrossRef]
- Condon, E. A Theory of intensity distribution in band systems. Phys. Rev. 1926, 28, 1182–1201. [Google Scholar] [CrossRef]
- Herbelin, J.M.; Emanuel, G. Einstein coefficients for diatomic molecules. J. Chem. Phys. 1974, 60, 689–696. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra and Molecular Structure, I. Spectra of Diatomic Molecules; Van Nostrand: New York, NY, USA, 1950. [Google Scholar]
- Wünderlich, D.; Fantz, U. Franck-Condon factors for molecule-ion reactions of H2 and its isotopomers. At. Data Nucl. Data Tables. 2011, 97, 152–185. [Google Scholar] [CrossRef]
- Fantz, U.; Wünderlich, D. Franck-Condon Factors, Transition Probabilities and Radiative Lifetimes for Hydrogen Molecules and Their Isotopomeres. Available online: https://www-amdis.iaea.org/data/INDC-457/ (accessed on 20 September 2016).
- Wünderlich, D.; Fantz, U. Franck-Condon Factors for Molecule-Ion Reactions of H2 and Its Isotopomers. Available online: http://www.sciencedirect.com/science/article/pii/S0092640X10000926#m0005 (accessed on 23 September 2016).
- Khakoo, M.A.; Trajmar, S. Electron-impact excitation of the a3Σg+, B1Σu+, c3Πu, and C1Πu states of H2. Phys. Rev. A 1986, 34, 146–156. [Google Scholar] [CrossRef]
- Shemansky, D.E.; Ajello, J.M.; Hall, D.T. Electron impact excitation of H2: Rydberg band systems and the benchmark dissociative cross section for H Lyman-Alpha. Astrophys. J. 1985, 296, 765–773. [Google Scholar] [CrossRef]
- Arrighini, G.P.; Biondi, F.; Guidotti, C. A study of the inelastic scattering of fast electrons from molecular hydrogen. Mol. Phys. 1980, 41, 1501–1514. [Google Scholar] [CrossRef]
- Chung, S.; Lin, C.C.; Lee, E.T.P. Dissociation of the hydrogen molecule by electron impact. Phys. Rev. A 1975, 12, 1340–1349. [Google Scholar] [CrossRef]
- Chung, S.; Lin, C.C. Application of the close-coupling method to excitation of electronic states and dissociation of H2 by electron impact. Phys. Rev. A 1978, 17, 1874–1891. [Google Scholar] [CrossRef]
- Mu-Tao, L.; Lucchese, R.R.; McKoy, V. Electron-impact excitation and dissociation processes in H2. Phys. Rev. A 1982, 26, 3240–3248. [Google Scholar] [CrossRef]
- Lima, M.A.P.; Gibson, T.L.; McKoy, V. Cross sections for excitation of the b3Σu+, a3 Σg+, and c3 Πu states of H2 by low-energy electrons. Phys. Rev. A 1988, 38, 4527–4536. [Google Scholar] [CrossRef]
- Wünderlich, D. Vibrationally resolved ionization cross sections for the ground state and electronically excited states of the hydrogen molecule. Chem. Phys. 2011, 390, 75–82. [Google Scholar] [CrossRef]
- Rapp, D.; Englander-Golden, P. Total cross sections for ionization and attachment in gases by electron impact. I. Positive ionization. J. Chem. Phys. 1965, 43, 1464–1479. [Google Scholar] [CrossRef]
- Adamcyk, B.; Boerboom, A.J.H.; Schram, B.L.; Kistemaker, J. Partial ionization cross sections of He, Ne, H2, and CH4 for electrons from 20 to 500 eV. J. Chem. Phys. 1966, 44, 4640–4642. [Google Scholar] [CrossRef]
- Crowe, A.; McConkey, J.W. Dissociative ionization by electron impact. I. Protons from H2. J. Phys. B 1973, 6, 2088–2107. [Google Scholar] [CrossRef]
- Straub, H.C.; Renault, P.; Lindsay, B.G.; Smith, K.A. Absolute partial cross sections for electron-impact ionization of H2, N2, and O2 from threshold to 1000 eV. Phys. Rev. A 1996, 54, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, R.; Capitelli, M.; Cacciatore, M. Electron impact direct dissociative-ionization cross sections from vibrationally excited H2 molecules and translational energy distribution functions of protons. Chem. Phys. 1990, 140, 209–215. [Google Scholar] [CrossRef]
- Wünderlich, D. Vibrationally Resolved Ionization Cross Sections for the Ground State and Electronically Excited Etates of the Hydrogen Molecule. Available online: http://www.sciencedirect.com/science/article/pii/S0301010411004344#appd002 (accessed on 23 September 2016).
- Pospieszczyk, A.; Mertens, P.; Sergienko, G.; Huber, A.; Philipps, V.; Reiter, D.; Rusbüldt, D.; Schweer, B.; Vietzke, E.; Greenland, P.T. In situ measurement and modeling of hydrogen recycling and transport processes—The role of molecules. J. Nucl. Mater. 1999, 266, 138–145. [Google Scholar] [CrossRef]
- Xiao, B.; Kado, S.; Kajita, S.; Yamasaki, D. Rovibrational distribution determination of H2 in low temperature plasmas by Fulcher-a band spectroscopy. Plasma Phys. Control. Fusion 2004, 46, 653–668. [Google Scholar] [CrossRef]
- Mihaljcic, B.; Browning, P.K.; Gibson, K.J. Spatially resolved spectroscopy of detached recombining plasmas in the University of Manchester Linear System divertor simulator. Phys. Plasmas 2007, 14, 013501. [Google Scholar] [CrossRef]
- Fantz, U. Basics of plasma spectroscopy. Plasma Sources Sci. Technol. 2006, 15, S137–S147. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wünderlich, D.; Fantz, U. Evaluation of State-Resolved Reaction Probabilities and Their Application in Population Models for He, H, and H2. Atoms 2016, 4, 26. https://doi.org/10.3390/atoms4040026
Wünderlich D, Fantz U. Evaluation of State-Resolved Reaction Probabilities and Their Application in Population Models for He, H, and H2. Atoms. 2016; 4(4):26. https://doi.org/10.3390/atoms4040026
Chicago/Turabian StyleWünderlich, Dirk, and Ursel Fantz. 2016. "Evaluation of State-Resolved Reaction Probabilities and Their Application in Population Models for He, H, and H2" Atoms 4, no. 4: 26. https://doi.org/10.3390/atoms4040026




