Excitation of the 2S State of Atomic Hydrogen by Electron Impact
Abstract
:1. Introduction
2. Calculations
3. Cross Sections
4. Spin-Flip Cross Section
5. Differential Cross Sections
6. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A
References
- Burke, P.G.; Schey, H.M.; Smith, K. Collisions of Slow Electrons and Positrons with Atomic Hydrogen. Phys. Rev. 1963, 129, 1258. [Google Scholar] [CrossRef]
- Callaway, J. Scattering of electrons by atomic hydrogen at intermediate energies: Elastic scattering and n = 2 excitation from 12 to 54 eV. Phys. Rev. A 1985, 32, 775–783. [Google Scholar] [CrossRef]
- Callaway, J.; Unnikrishnan, K.; Oza, D.H. Optical potential of electron-hydrogen scattering at intermediate energies. Phys. Rev. A 1987, 36, 2576–2584. [Google Scholar] [CrossRef]
- Scott, M.P.; Scholz, T.T.; Walters, H.R.; Burke, P.G. Electron scattering by atomic hydrogen at intermediate energies: Integrated elastic, 1s-2s and 1s-2p cross sections. J. Phys. B 1989, 22, 3055–3077. [Google Scholar] [CrossRef]
- Bartschat, K.; Hudson, E.T.; Scott, M.P.; Burke, P.G.; Burke, V.M. Electron-atom scattering at low and intermediate energies using a pseudo-state/R-matrix basis. J. Phys. B 1996, 29, 115–123. [Google Scholar] [CrossRef]
- Lloyd, M.D.; McDowell, M.R.C. An application of the polarized-orbital approximation to electron impact excitation of atomic hydrogen. J. Phys. B 1969, 2, 1313–1322. [Google Scholar] [CrossRef]
- Temkin, A. A Note on the Scattering of Electrons from Atomic Hydrogen. Phys. Rev. 1959, 116, 358. [Google Scholar] [CrossRef]
- Poet, R. The exact solution for a simplified model of electrons scattering by hydrogen atoms. J. Phys. B 1978, 11, 3081–3094. [Google Scholar] [CrossRef]
- Bhatia, A.K. Hybrid theory of electron-hydrogen elastic scattering. Phys. Rev. A 2007, 75, 032713. [Google Scholar] [CrossRef]
- Bhatia, A.K. Application of P-wave hybrid theory to the scattering of electrons from He+ and resonances in He and H−. Phys. Rev. A 2012, 86, 032709. [Google Scholar] [CrossRef]
- Bhatia, A.K. Hybrid theory of P-wave electron-Li+ elastic scattering and photoabsorption in two-electron systems. Phys. Rev. A 2013, 87, 042705. [Google Scholar] [CrossRef]
- Temkin, A.; Lamkin, J.C. Application of the Method of Polarized Orbitals to the Scattering of Electrons from Hydrogen. Phys. Rev. 1961, 121, 788–794. [Google Scholar] [CrossRef]
- Shertzer, J.; Temkin, A. Direct calculation of the scattering amplitude without partial-wave analysis. III. Inclusion of correlation effects. Phys. Rev. A 2006, 74, 052701. [Google Scholar] [CrossRef]
- McCarthy, I.E.; Saha, B.C.; Stelbovics, A.T. The polarization potential in electron-atom scattering. J. Phys. B 1981, 14, 2871–2893. [Google Scholar] [CrossRef]
- Kauppila, W.E.; Ott, W.R.; Fite, W.L. Excitation of Atomic Hydrogen to the Metastable 22S1/2 State by Electron-Impact. Phys. Rev. A 1970, 1, 1099. [Google Scholar] [CrossRef]
- Fite, W.L.; Brachman, R.T. Collisions of Electrons with Hydrogen Atoms. II. Excitation of Lyman-Alpha Radiation. Phys. Rev. 1958, 112, 1141–1151. [Google Scholar] [CrossRef]
- Fite, W.L.; Stebbings, R.F.; Brachman, R.T. Collisions of Electrons with Hydrogen Atoms. IV. Excitation of Lyman-Alpha Radiation near Threshold. Phys. Rev. 1959, 116, 356–357. [Google Scholar] [CrossRef]
- Williams, J.F.; Willis, B.A. Electron scattering from atomic hydrogen I. Differential cross sections for excitation of n = 2 states. J. Phys. B 1975, 8, 1641–1669. [Google Scholar] [CrossRef]
- Scott, B.L. Angular Distributions for e− -H Scattering near the First Inelastic Threshold. Phys. Rev. 1965, 140, A699. [Google Scholar] [CrossRef]
- Fon, W.C.; Aggarwal, K.M.; Ratnavelu, K. Electron impact excitation of n = 2 states of atomic hydrogen at energies just above the ionization threshold. J. Phys. B 1992, 25, 2625. [Google Scholar] [CrossRef]
- Omidvar, K. 2s and 2p Electron-impact Excitation in Atomic Hydrogen. Phys. Rev. 1964, 133, A970. [Google Scholar] [CrossRef]
- Bhatia, A.K. Electron-hydrogen P-wave elastic scattering. Phys. Rev. A 2004, 69, 032714. [Google Scholar] [CrossRef]
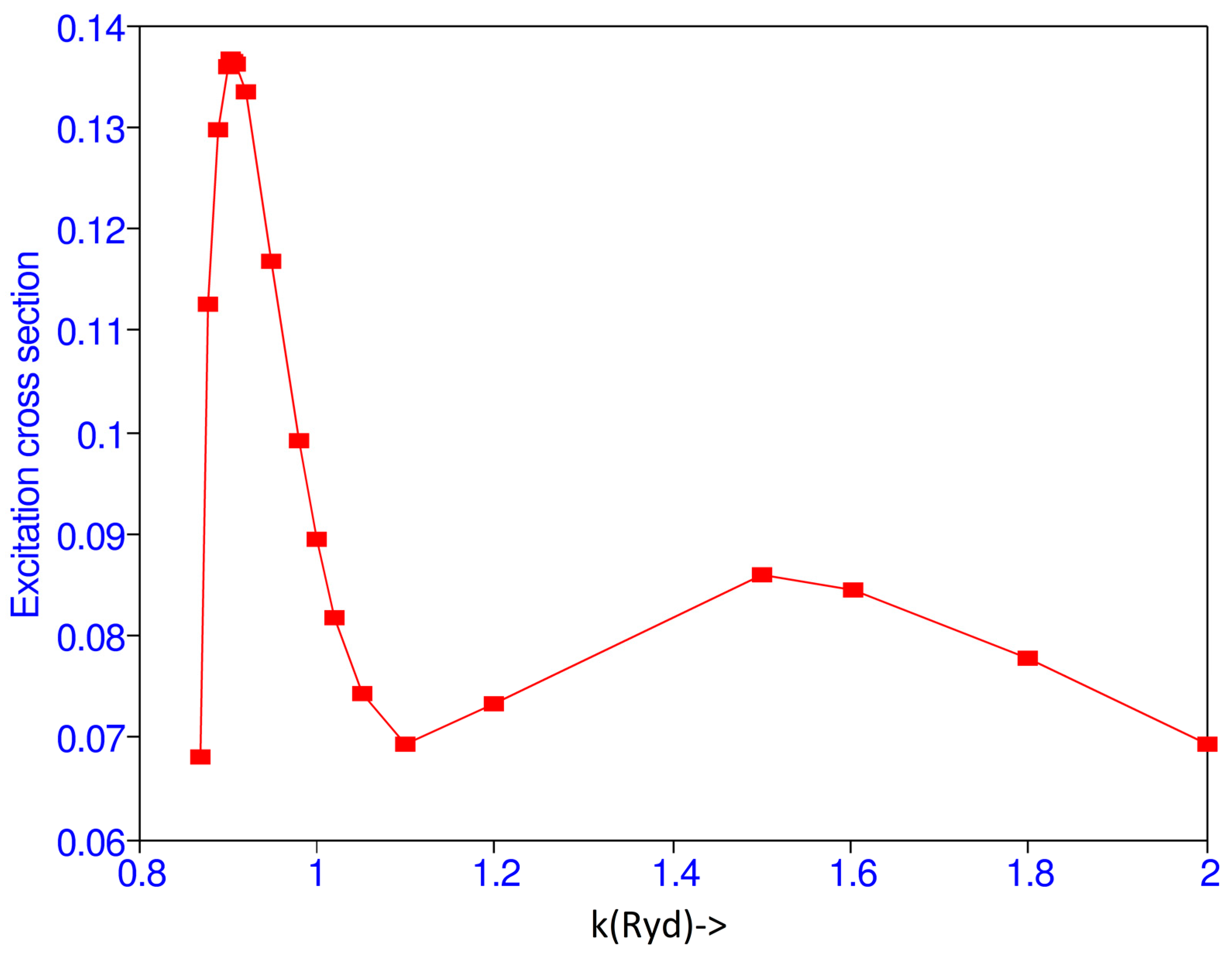
| k | Present | Lloyd and McDowell [6] | Poet [8] | Burke et al. [1] | Callaway [2] | Scott et al. [4] |
|---|---|---|---|---|---|---|
| 0.87 | 6.8208(-2) | |||||
| 0.88 | 1.1267(-1) | |||||
| 0.89 | 1.2971(-1) | |||||
| 0.90 | 1.3589(-1) | 0.1789 | 0.09918 | |||
| 0.904 | 1.3667(-1) | |||||
| 0.905 | 1.3673(-1) | |||||
| 0.906 | 1.3662(-1) | |||||
| 0.907 | 1.3660(-1) | |||||
| 0.91 | 1.3630(-1) | |||||
| 0.92 | 1.3345(-1) | |||||
| 0.95 | 1.1684(-1) | |||||
| 0.98 | 9.9106(-2) | 0.161 a | ||||
| 1.00 | 8.9474(-2) | 0.1443 | 0.0407 | 0.1783 | 0.154 | |
| 1.02 | 8.1852(-2) | 0.150 a | ||||
| 1.05 | 8.4057(-2) | 0.142 | ||||
| 1.10 | 6.9444(-2) | 0.1654 | 0.135 | 0.1087 | ||
| 1.20 | 7.3317(-2) | 0.1349 | 0.111 | 0.0986 | ||
| 1.50 | 8.5797(-2) | 0.0891 | 0.087 | 0.0867 | ||
| 1.60 | 8.4611(-2) | 0.081 a | ||||
| 1.80 | 7.1804(-2) | 0.074 a | ||||
| 2.00 | 6.9460(-2) | 0.1198 | 0.0168 | 0.06355 | 0.066 | 0.0661 |
| k | Present | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lmax = 0 | Lmax = 1 | Lmax = 2 | Lmax = 3 | Lmax = 4 | Lmax = 5 | Lmax = 6 | Lmax = 7 | Lmax = 8 | |
| 0.87 | 6.7949(-2) | 6.8204(-2) | 6.8209(-2) | 6.8209(-2) | |||||
| 0.88 | 1.1107(-1) | 1.1246(-1) | 1.1255(-1) | 1.1267(-1) | 1.1267(-1) | ||||
| 0.89 | 1.2678(-1) | 1.2938(-1) | 1.2970(-1) | 1.2971(-1) | 1.2971(-1) | ||||
| 0.90 | 1.3148(-1) | 1.3517(-1) | 1.3586(-1) | 1.3589(-1) | 1.3589(-1) | ||||
| 0.904 | 1.3166(-1) | 1.3574(-1) | 1.3663(-1) | 1.3667(-1) | 1.3667(-1) | ||||
| 0.905 | 1.3158(-1) | 1.3575(-1) | 1.3670(-1) | 1.3673(-1) | 1.3673(-1) | ||||
| 0.906 | 1.3131(-1) | 1.3558(-1) | 1.3657(-1) | 1.3662(-1) | 1.3662(-1) | ||||
| 0.907 | 1.3115(-1) | 1.3550(-1) | 1.3655(-1) | 1.3660(-1) | 1.3660(-1) | ||||
| 0.91 | 1.3039(-1) | 1.3501(-1) | 1.3624(-1) | 1.3630(-1) | 1.3630(-1) | ||||
| 0.92 | 1.2602(-1) | 1.3144(-1) | 1.3333(-1) | 1.3344(-1) | 1.3345(-1) | 1.3345(-1) | |||
| 0.95 | 1.0447(-1) | 1.1187(-1) | 1.1638(-1) | 1.1681(-1) | 1.1684(-1) | 1.1684(-1) | 1.1684(-1) | ||
| 0.98 | 8.0886(-2) | 9.0296(-2) | 9.8005(-2) | 9.898(-2) | 9.9093(-2) | 9.9105(-2) | 9.9105(-2) | 9.9105(-2) | |
| 1.00 | 6.6852(-2) | 7.7800(-2) | 8.7768(-2) | 8.9260(-2) | 8.9447(-2) | 8.9471(-2) | 8.9474(-2) | 8.9474(-2) | |
| 1.02 | 5.4526(-2) | 6.7197(-2) | 7.9401(-2) | 8.1502(-2) | 8.1801(-2) | 8.1844(-2) | 8.1851(-2) | 8.1852(-2) | |
| 1.05 | 3.9620(-2) | 5.5101(-2) | 7.0508(-2) | 7.3670(-2) | 7.4202(-2) | 7.4290(-2) | 7.4305(-2) | 7.4308(-2) | |
| 1.10 | 2.2524(-2) | 4.2752(-2) | 6.2890(-2) | 6.8079(-2) | 6.9165(-2) | 6.9384(-2) | 6.3431(-2) | 6.9441(-2) | 6.9444(-2) |
| 1.20 | 7.0712(-3) | 3.4327(-2) | 6.0266(-2) | 6.9638(-2) | 7.2324-2) | 7.3047(-2) | 7.3224(-2) | 7.3300(-2) | 7.3317(-2) |
| 1.50 | 3.0064(-3) | 2.9985(-2) | 5.6548(-2) | 7.2552(-2) | 8.0324(-2) | 8.3709(-2) | 8.5124(-2) | 8.5714(-2) | 8.5967(-2) |
| 1.60 | 3.2974(-3) | 2.7537(-2) | 5.2116(-2) | 6.8368(-2) | 7.7147(-2) | 8.1383(-2) | 8.3328(-2) | 8.4208(-2) | 8.4612(-2) |
| 1.80 | 3.2385(-3) | 2.2129(-2) | 4.2387(-2) | 5.7648(-2) | 6.7307(-2) | 7.2763(-2) | 7.5671(-2) | 7.7176(-2) | 7.7951(-2) |
| 2.00 | 2.7917(-3) | 1.7333(-2) | 3.3707(-2) | 4.7147(-2) | 5.6640(-2) | 6.2647(-2) | 6.6235(-2) | 6.8297(-2) | 6.9461(-2) |
| L | F | G |
|---|---|---|
| 0 | 3.5686(-1) | 4.6042(-2) |
| 1 | −2.0022(-1) | −9.2426(-2) |
| 2 | −9.7205(-2) | −1.7338(-1) |
| 3 | −4.6353(-2) | −7.9044(-2) |
| 4 | −2.1528(-2) | −3.1113(-2) |
| 5 | −9.5763(-3) | −1.1964(-2) |
| 6 | −4.1782(-3) | −4.7157(-3) |
| 7 | −1.8169(-3) | −1.9301(-3) |
| 8 | −7.9477(-4) | −8.1743(-4) |
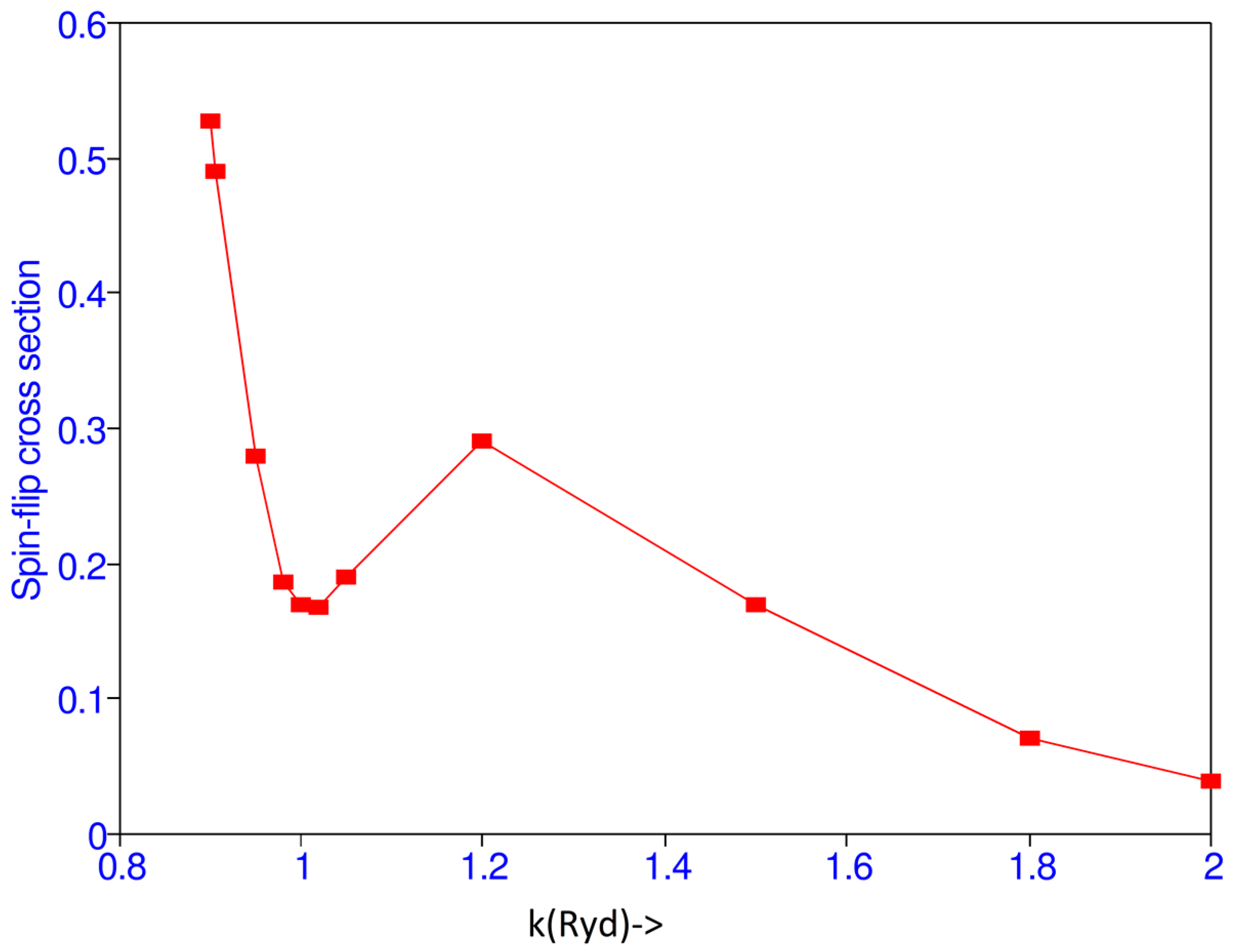
| k | Present | Burke et al. [1] |
|---|---|---|
| 0.90 | 5.2831(-1) | 0.157 |
| 0.905 | 4.9029(-1) | |
| 0.95 | 2.7794(-1) | |
| 0.98 | 1.8597(-1) | |
| 1.0 | 1.6885(-1) | 0.2212 |
| 1.02 | 1.6641(-1) | |
| 1.05 | 1.8922(-1) | |
| 1.1 | 2.1959(-1) | |
| 1.2 | 2.8980(-1) | 0.1146 |
| 1.5 | 1.6894(-1) | 0.0285 |
| 1.8 | 7.0457(-2) | |
| 2.0 | 3.8774(-2) | 0.0059 |
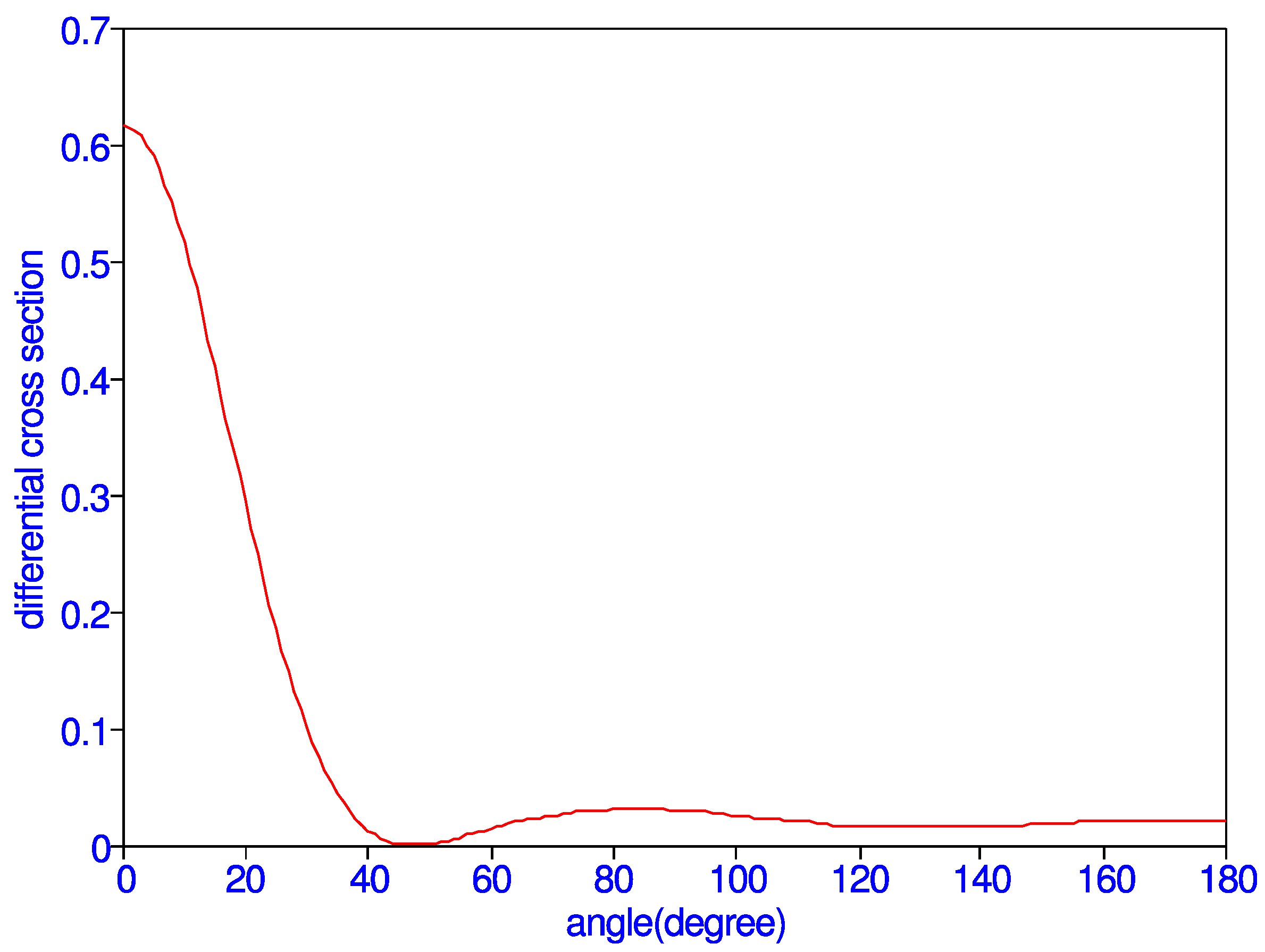
| 0 | 6.1752(-1) | 65 | 2.1103(-2) | 130 | 1.6477(-2) |
| 5 | 5.9068(-1) | 70 | 2.5993(-2) | 135 | 1.6807(-2) |
| 10 | 5.1623(-1) | 75 | 2.9160-2) | 140 | 1.7405(-2) |
| 15 | 4.1029(-1) | 80 | 3.0793(-2) | 145 | 1.8120(-2) |
| 20 | 2.9386(-1) | 85 | 3.1106(-2) | 150 | 1.8854(-2) |
| 25 | 1.8651(-1) | 90 | 3.0280(-2) | 155 | 1.9557(-2) |
| 30 | 1.0164(-1) | 95 | 2.8520(-2) | 160 | 2.0211(-2) |
| 35 | 4.4444(-2) | 100 | 2.6109(-2) | 165 | 2.0804(-2) |
| 40 | 1.3019(-2) | 105 | 2.3419(-2) | 170 | 2.1298(-2) |
| 45 | 1.1171(-3) | 110 | 2.0854(-2) | 175 | 2.1634(-2) |
| 50 | 1.3008(-3) | 115 | 1.8754(-2) | 180 | 2.1754(-2) |
| 55 | 7.2108(-3) | 120 | 1.7321(-2) | ||
| 60 | 1.4565(-2) | 125 | 1.6593(-2) |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, A.K. Excitation of the 2S State of Atomic Hydrogen by Electron Impact. Atoms 2018, 6, 7. https://doi.org/10.3390/atoms6010007
Bhatia AK. Excitation of the 2S State of Atomic Hydrogen by Electron Impact. Atoms. 2018; 6(1):7. https://doi.org/10.3390/atoms6010007
Chicago/Turabian StyleBhatia, Anand K. 2018. "Excitation of the 2S State of Atomic Hydrogen by Electron Impact" Atoms 6, no. 1: 7. https://doi.org/10.3390/atoms6010007




