Metallothioneins, Unconventional Proteins from Unconventional Animals: A Long Journey from Nematodes to Mammals †
Abstract
:1. Introduction
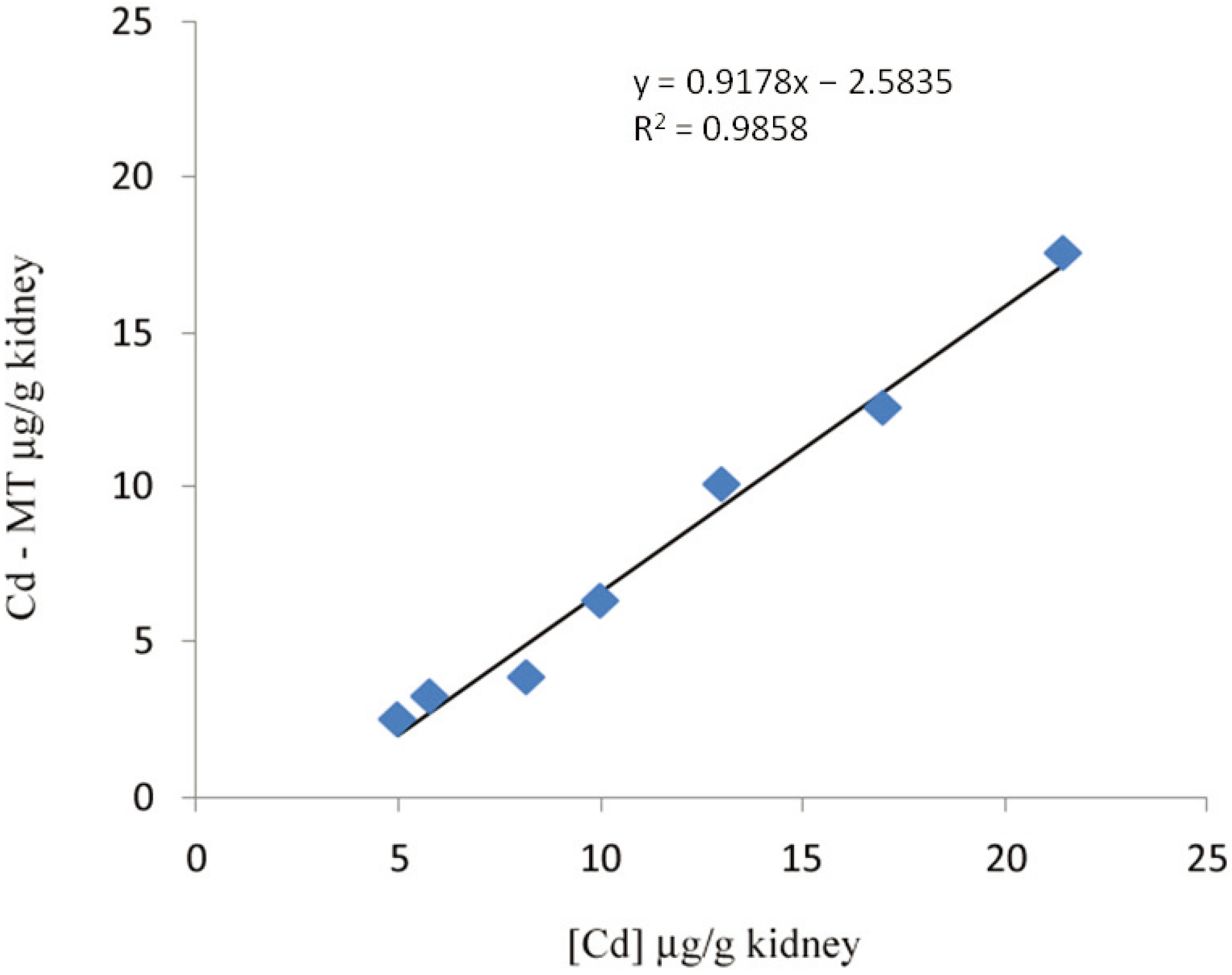
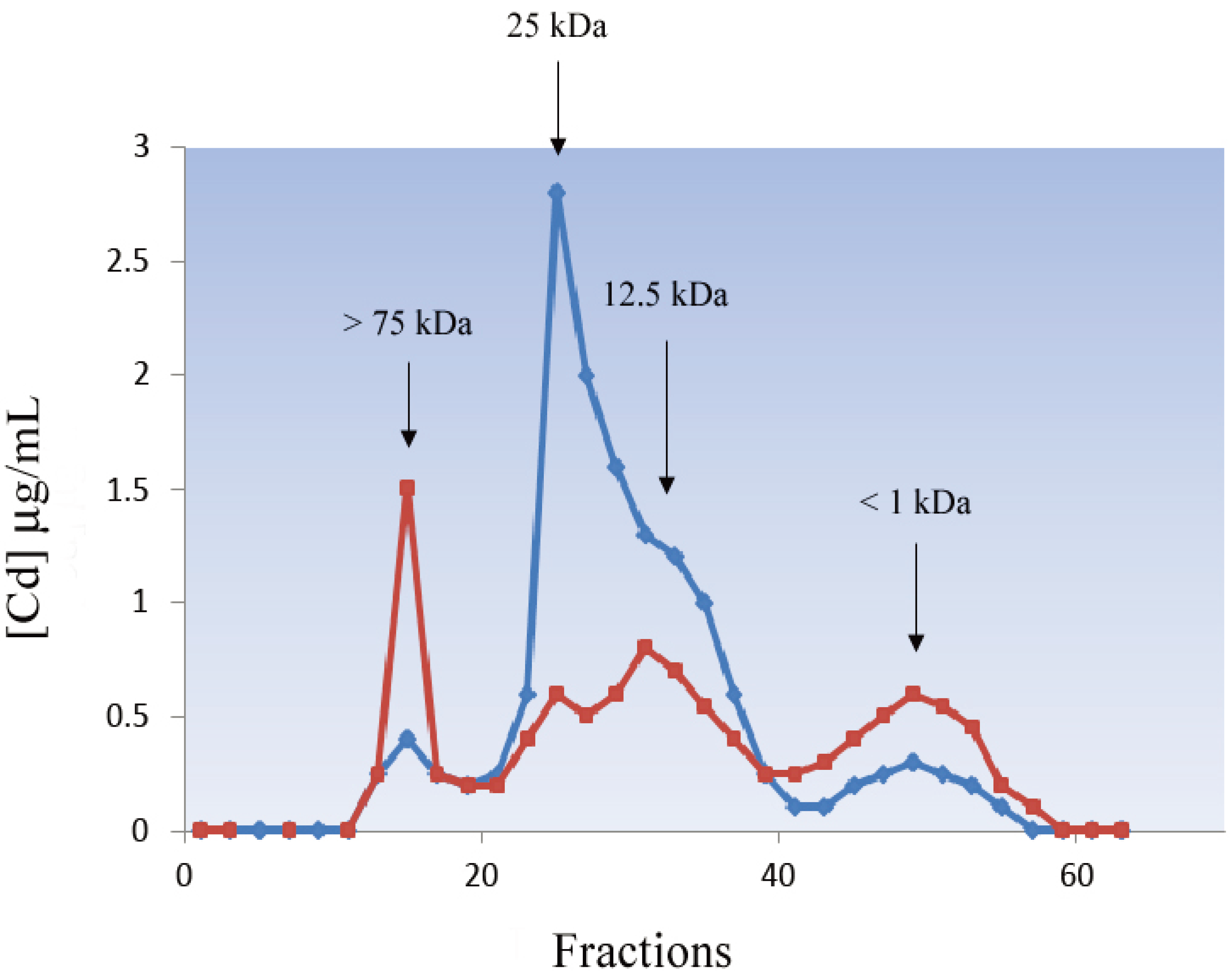
2. Methods of MT Quantification
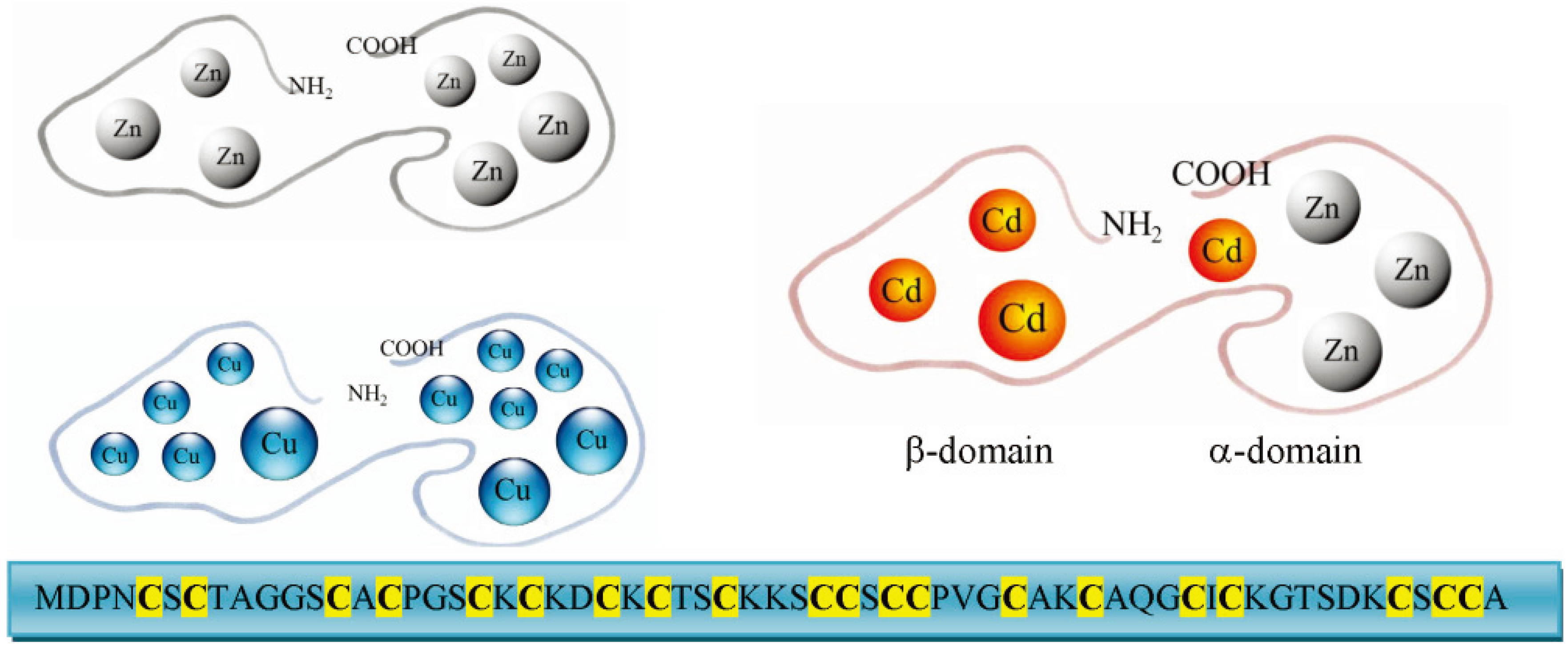
3. Molecular Characteristics and Isoforms
4. Functions
5. Unconventional Animals


5.1. Protostomia
5.1.1. Nematoda
5.1.2. Annelida
| Species | Name | Sequence | Cys | AA |
|---|---|---|---|---|
| H. sapiens | MT-SA |  | 20 | 61 |
| Vertebrates | ||||
| T. truncates | MT-2A |  | 20 | 61 |
| P. carbo | MT-2 |  | 20 | 63 |
| P. sicula | MT |  | 20 | 63 |
| T. carnifex | MT |  | 20 | 63 |
| C. auratus | MT |  | 20 | 60 |
| Invertebrates | ||||
| S. purpuratus | SpMTA | 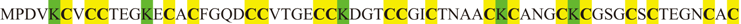 | 20 | 64 |
| M. edulis | MT10Ib |  | 21 | 73 |
| S. inaequivalvis | MT |  | 18 | 62 |
| H. pomatia | CuMT |  | 18 | 65 |
| C. sapidus | CuMT-2 |  | 21 | 64 |
| D. melanogaster | MtnE |  | 10 | 41 |
| P. nuntia | MT |  | 17 | 68 |
| L. rubellus | MT-1 |  | 20 | 79 |
| C. elegans | CeMT-1 |  | 19 | 75 |
5.1.3. Arthropoda
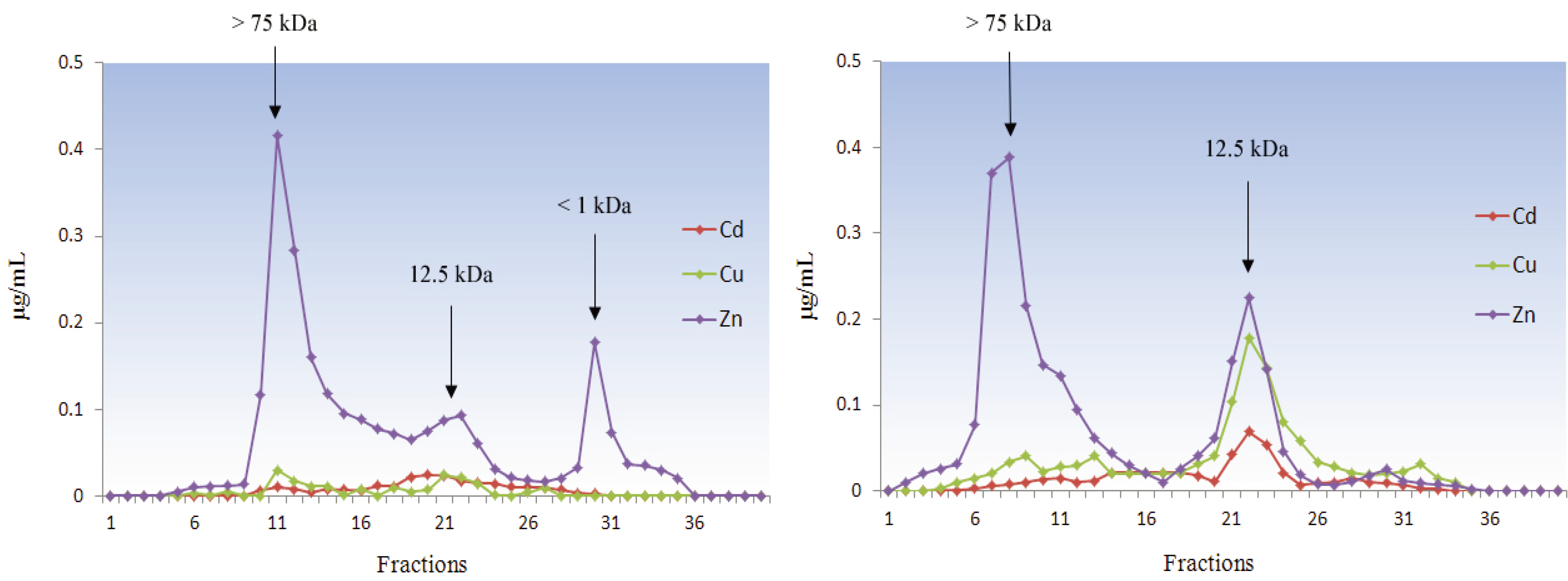
5.1.4. Mollusca
5.2. Deuterostomia

5.2.1. Echinodermata
5.2.2. Chordata
5.2.2.1. Fish
5.2.2.2. Amphibians
5.2.2.3. Reptiles
5.2.2.4. Birds
5.2.2.5. Mammals
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Margoshes, M.; Vallee, B.L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1987, 79, 4813–4814. [Google Scholar] [CrossRef]
- Kägi, J.H.; Vallee, B.L. Metallothionein: A cadmium and zinc-containing protein from equine renal cortex. II. Physico-chemical properties. J. Biol. Chem. 1961, 236, 2435–2442. [Google Scholar]
- Carginale, V.; Scudiero, R.; Capasso, C.; Capasso, A.; Kille, P.; di Prisco, G.; Parisi, E. Cadmium-induced differential accumulation of metallothionein isoforms in the antarctic icefish, which exhibits no basal metallothionein protein but high endogenous mRNA levels. Biochem. J. 1998, 332, 475–481. [Google Scholar]
- Carpenè, E.; Andreani, G.; Isani, G. Metallothionein functions and structural characteristics. J. Trace Elem. Med. Biol. 2007, 21, 35–39. [Google Scholar] [CrossRef]
- Dieter, H.H.; Müller, L.; Abel, J.; Summer, K.H. Metallothionein-determination in biological materials: Interlaboratory comparison of 5 current methods. Experientia Suppl. 1987, 52, 351–358. [Google Scholar] [CrossRef]
- Isani, G.; Andreani, G.; Kindt, M.; Carpenè, E. Metallothioneins (MTs) in marine molluscs. Cell. Mol. Biol. 2000, 46, 311–330. [Google Scholar]
- Ryvolova, M.; Krizkova, S.; Adam, V.; Beklova, M.; Trnkova, L.; Hubalek, J.; Kizek, R. Analytical methods for metallothionein detection. Curr. Anal. Chem. 2011, 7, 243–261. [Google Scholar]
- Torreggiani, A.; Tinti, A. Raman spectroscopy a promising technique for investigations of metallothioneins. Metallomics 2010, 2, 246–260. [Google Scholar] [CrossRef]
- Carpenè, E.; Andreani, G.; Monari, M.; Castellani, G.; Isani, G. Distribution of Cd, Zn, Cu and Fe among selected tissues of the earthworm (Allolobophora caliginosa) and Eurasian woodcock (Scolopax rusticola). Sci. Total Environ. 2006, 363, 126–135. [Google Scholar] [CrossRef]
- Brouwer, M.; Whaling, P.; Engel, D.W. Copper-metallothioneins in the American lobster, Homarus americanus: Potential role as Cu(I) donors to apohemocyanin. Environ. Health Perspect. 1986, 65, 93–100. [Google Scholar]
- Kägi, J.H.; Kojima, Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987, 52, 25–61. [Google Scholar] [CrossRef]
- Vašák, M. Dynamic metal-thiolate cluster structure of metallothioneins. Environ. Health Perspect. 1986, 65, 193–197. [Google Scholar]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 95, 8428–8430. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Leszczyszyn, O.I. Metallothioneins: Unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 2010, 27, 720–741. [Google Scholar]
- Vašák, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef]
- Yang, Y.; Maret, W.; Vallee, B.L. Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc. Natl. Acad. Sci. USA 2001, 98, 5556–5559. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Biophys. Acta 2012, 1823, 1416–1425. [Google Scholar]
- Klug, A.; Rhodes, D. Zinc fingers: A novel protein fold for nucleic acid recognition. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 473–482. [Google Scholar] [CrossRef]
- Won, E.J.; Rhee, J.S.; Ra, K.; Kim, K.T.; Au, D.W.; Shin, K.H.; Lee, J.S. Molecular cloning and expression of novel metallothionein (MT) gene in the polychaete Perinereis nuntia exposed to metals. Environ. Sci. Pollut. Res. Int. 2011, 19, 2606–2618. [Google Scholar]
- Russo, R.; Zito, F.; Matranga, V. Tissue-specificity and phylogenetics of Pl-MT mRNA during Paracentrotus lividus embryogenesis. Gene 2013, 519, 305–310. [Google Scholar] [CrossRef]
- Höckner, M.; Dallinger, R.; Stürzenbaum, S.R. Nematode and snail metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1057–1065. [Google Scholar]
- Maruyama, K.; Hori, R.; Nishihara, T.; Kondo, M. Isolation and characterization of metallothionein from nematode (Caenorhabditis elegans). Eisei Kagaku 1986, 32, 22–27. [Google Scholar] [CrossRef]
- Bofill, R.; Orihuela, R.; Romagosa, M.; Domenech, J.; Atrian, S.; Capdevila, M. Caenorhabditis elegans metallothionein isoform specificity-metal binding abilities and the role of histidine in CeMT1 and CeMT2. FEBS J. 2009, 276, 7040–7056. [Google Scholar] [CrossRef]
- Imagawa, M.; Onozawa, T.; Okumura, K.; Osada, S.; Nishihara, T.; Kondo, M. Characterization of metallothionein cDNAs induced by cadmium in the nematode Caenorhabditis elegans. Biochem. J. 1990, 268, 237–240. [Google Scholar]
- Hughes, S.L.; Bundy, J.G.; Want, E.J.; Kille, P.; Stürzenbaum, S.R. The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins. J. Proteome Res. 2009, 8, 3512–3519. [Google Scholar] [CrossRef]
- OECD. Method 207: Earthworm acute toxicity tests. In Guidelines for Testing Chemicals. Section 2: Effects on Biotic Systems; Organization for Economic Cooperation and Development (OECD): Paris, France, 1984. [Google Scholar]
- Suzuki, K.; Yamamura, M.; Mori, T. Cadmium-binding proteins induced in the earthworm. Arch. Environ. Contam. Toxicol. 1980, 9, 415–424. [Google Scholar] [CrossRef]
- Gruber, C.; Stürzenbaum, S.; Gehrig, P.; Sack, R.; Hunziker, P.; Berger, B.; Dallinger, R. Isolation and characterization of a self-sufficient one-domain protein-(Cd)-metallothionein from Eisenia foetida. Eur. J. Biochem. 2000, 267, 573–582. [Google Scholar]
- Brulle, F.; Cocquerelle, C.; Wamalah, A.N.; Morgan, A.J.; Kille, P.; Lepretre, A.; Vandenbulcke, F. c-DNA cloning and expression analysis of Eisenia fetida (Annelida: Oligochaeta) phytochelatin synthase under cadmium exposure. Ecotoxicol. Environ. Saf. 2008, 71, 47–55. [Google Scholar] [CrossRef]
- Stürzenbaum, S.R.; Winters, C.; Galay, M.; Morgan, A.J.; Kille, P. Metal ion trafficking in earthworms. Identification of a cadmium-specific metallothionein. J. Biol. Chem. 2001, 276, 34013–34018. [Google Scholar] [CrossRef]
- Berthet, B.; Mouneyrac, C.; Amiard, J.C.; Amiard-Triquet, C.; Berthelot, Y.; le Hen, A.; Mastain, O.; Rainbow, P.S.; Smith, B.D. Accumulation and soluble binding of cadmium, copper, and zinc in the polychaete Hediste diversicolor from coastal sites with different trace metal bioavailabilities. Arch. Environ. Contam. Toxicol. 2003, 45, 468–478. [Google Scholar] [CrossRef]
- Overnell, J.; Trewhella, E. Evidence for the natural occurrence of (cadmium, copper) metallothionein in the crab Cancer pagurus. Comp. Biochem. Physiol. 1979, 64, 69–76. [Google Scholar]
- Otvos, J.D.; Olafson, R.W.; Armitage, I.M. Structure of an invertebrate metallothionein from Scylla serrata. J. Biol. Chem. 1982, 257, 2427–2431. [Google Scholar]
- Zhu, Z.; DeRose, E.F.; Mullen, G.P.; Petering, D.H.; Shaw, C.F., 3rd. Sequential proton resonance assignments and metal cluster topology of lobster metallothionein-1. Biochemistry 1994, 33, 8858–8865. [Google Scholar] [CrossRef]
- Serra-Batiste, M.; Cols, N.; Alcaraz, L.A.; Donaire, A.; Gonzalez-Duarte, P.; Vašák, M. The metal-binding properties of the blue crab copper specific CuMT-2: A crustacean metallothionein with two cysteine triplets. J. Biol. Inorg. Chem. 2010, 15, 759–776. [Google Scholar] [CrossRef]
- Asselman, J.; Shaw, J.R.; Glaholt, S.P.; Colbourne, J.K.; de Schamphelaere, K.A. Transcription patterns of genes encoding four metallothionein homologs in Daphnia pulex exposed to copper and cadmium are time- and homolog-dependent. Aquat. Toxicol. 2013, 142–143, 422–430. [Google Scholar]
- Atanesyan, L.; Gunther, V.; Celniker, S.E.; Georgiev, O.; Schaffner, W. Characterization of MtnE, the fifth metallothionein member in Drosophila. J. Biol. Inorg. Chem. 2011, 16, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Tio, L.; Pagani, M.A.; Atrian, S. Drosophila proteins interacting with metallothioneins: A metal-dependent recognition. Proteomics 2009, 9, 2568–2577. [Google Scholar] [CrossRef]
- Goldberg, E.D. The mussel watch concept. Environ. Monit. Assess. 1986, 7, 91–103. [Google Scholar] [CrossRef]
- Isani, G.; Serra, R.; Cattani, O.; Cortesi, P.; Carpenè, E. Adenylate energy charge and metallothionein as stress indices in Mytilus galloprovincialis exposed to cadmium and anoxia. J. Mar. Biol. Assoc. UK 1997, 77, 1187–1197. [Google Scholar] [CrossRef]
- Gueguen, M.; Amiard, J.C.; Arnich, N.; Badot, P.M.; Claisse, D.; Guerin, T.; Vernoux, J.P. Shellfish and residual chemical contaminants: Hazards, monitoring, and health risk assessment along French coasts. Rev. Environ. Contam. Toxicol. 2011, 213, 55–111. [Google Scholar]
- Regoli, F.; Pellegrini, D.; Cicero, A.M.; Nigro, M.; Benedetti, M.; Gorbi, S.; Fattorini, D.; D’Errico, G.; di Carlo, M.; Nardi, A.; et al. A multidisciplinary weight of evidence approach for environmental risk assessment at the Costa Concordia wreck: Integrative indices from Mussel Watch. Mar. Environ. Res. 2013, S0141–S1136. [Google Scholar]
- Spagnuolo, C.; D’Alessandro, R.; Chiancone, E. Dimeric and tetrameric hemoglobins from the mollusc Scapharca inaequivalvis. Reaction of the oxidized derivatives with azide and fluoride. Biochim. Biophys. Acta 1991, 1074, 270–276. [Google Scholar] [CrossRef]
- Howard, A.G.; Nickless, G. Protein binding of cadmium, zinc and copper in environmentally insulted limpets Patella vulgata. J. Chromatogr. 1975, 104, 457–459. [Google Scholar] [CrossRef]
- Noel-Lambot, F. Distribution of cadmium, zinc and copper in the mussel Mytilus edulis. Existence of cadmium-binding proteins similar to metallothioneins. Experientia 1976, 32, 324–326. [Google Scholar] [CrossRef]
- Carpenè, E.; Crisetig, G.; Cortesi, P.; Serrazanetti, G. Cadmium binding and zinc variations in Mytilus galloprovincialis. Boll. Soc. Ital. Biol. Sper. 1979, 55, 1217–1223. [Google Scholar]
- Ridlington, J.W.; Fowler, B.A. Isolation and partial characterization of a cadmium-binding protein from the American oyster (Crassostrea virginica). Chem. Biol. Interact. 1979, 25, 127–138. [Google Scholar] [CrossRef]
- Olafson, R.W.; Sim, R.G.; Kearns, A. Physiological and chemical characterization of invertebrate metallothionein-like proteins. Experientia Suppl. 1979, 34, 197–204. [Google Scholar] [CrossRef]
- George, S.G.; Carpenè, E.; Coombs, T.L.; Overnell, J.; Youngson, A. Characterisation of cadmium-binding proteins from mussels, Mytilus edulis (L.), exposed to cadmium. Biochim. Biophys. Acta 1979, 580, 225–233. [Google Scholar]
- Carpenè, E.; Cortesi, P.; Crisetig, G.; Serrazanetti, G. Cadmium-binding proteins from the mantle of Mytilus edulis (L.) after exposure to cadmium. Thalassia Jugoslavica 1980, 16, 317–323. [Google Scholar]
- Mackay, E.A.; Overnell, J.; Dunbar, B.; Davidson, I.; Hunziker, P.E.; Kägi, J.H.; Fothergill, J.E. Complete amino acid sequences of five dimeric and four monomeric forms of metallothionein from the edible mussel Mytilus edulis. Eur. J. Biochem. 1993, 218, 183–194. [Google Scholar] [CrossRef]
- Aceto, S.; Formisano, G.; Carella, F.; de Vico, G.; Gaudio, L. The metallothionein genes of Mytilus galloprovincialis: Genomic organization, tissue expression and evolution. Mar. Genomics 2011, 4, 61–68. [Google Scholar] [CrossRef]
- Barsyte, D.; White, K.N.; Lovejoy, D.A. Cloning and characterization of metallothionein cDNA in the mussel Mytilus edulis L. digestive gland. Comp. Biochem. Physiol. 1999, 122, 287–296. [Google Scholar] [CrossRef]
- Leignel, V.; Hardivillier, Y.; Laulier, M. Small metallothionein MT-10 genes in coastal and hydrothermal mussels. Mar. Biotechnol. 2005, 7, 236–244. [Google Scholar] [CrossRef]
- Lemoine, S.; Bigot, Y.; Sellos, D.; Cosson, R.P.; Laulier, M. Metallothionein isoforms in Mytilus edulis (Mollusca, Bivalvia): Complementary DNA characterization and quantification of expressionin different organs after exposure to cadmium, zinc, and copper. Mar. Biotechnol. 2000, 2, 195–203. [Google Scholar]
- Serra, R.; Carpenè, E.; Marcantonio, A.C.; Isani, G. Cadmium accumulation and Cd-binding proteins in the bivalve Scapharca inaequivalvis. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1995, 111, 165–174. [Google Scholar] [CrossRef]
- Isani, G.; Monari, M.; Andreani, G.; Fabbri, M.; Carpenè, E. Effect of copper exposure on the antioxidant enzymes in bivalve mollusc Scapharca inaequivalvis. Vet. Res. Commun. 2003, 27, 691–693. [Google Scholar]
- Girotti, S.; Bolelli, L.; Fini, F.; Monari, M.; Andreani, G.; Isani, G.; Carpenè, E. Trace metals in arcid clam Scapharca inaequivalvis: Effects of molluscan extracts on bioluminescent bacteria. Chemosphere 2006, 65, 627–633. [Google Scholar] [CrossRef]
- Andreani, G.; Carpenè, E.; Capranico, G.; Isani, G. Metallothionein cDNA cloning, metallothionein expression and heavy metals in Scapharca inaequivalvis along the Northern Adriatic coast of Italy. Ecotoxicol. Environ. Saf. 2011, 74, 366–372. [Google Scholar] [CrossRef]
- Roesijadi, G.; Vestling, M.M.; Murphy, C.M.; Klerks, P.L.; Fenselau, C.C. Structure and time-dependent behavior of acetylated and non-acetylated forms of a molluscan metallothionein. Biochim. Biophys. Acta 1991, 1074, 230–236. [Google Scholar]
- Tanguy, A.; Moraga, D. Cloning and characterization of a gene coding for a novel metallothionein in the pacific oyster Crassostrea gigas (CgMT2): A case of adaptive response to metal-induced stress? Gene 2001, 273, 123–130. [Google Scholar] [CrossRef]
- Tanguy, A.; Mura, C.; Moraga, D. Cloning of a metallothionein gene and characterization of two other cDNA sequences in the Pacific oyster Crassostrea gigas (CgMT1). Aquat. Toxicol. 2001, 55, 35–47. [Google Scholar] [CrossRef]
- Dallinger, R.; Berger, B.; Hunziker, P.E.; Birchler, N.; Hauer, C.R.; Kägi, J.H. Purification and primary structure of snail metallothionein. Similarity of the N-terminal sequence with histones H4 and H2A. Eur. J. Biochem. 1993, 216, 739–746. [Google Scholar] [CrossRef]
- Berger, B.; Dallinger, R.; Gehrig, P.; Hunziker, P.E. Primary structure of a copper-binding metallothionein from mantle tissue of the terrestrial gastropod Helix pomatia L. Biochem. J. 1997, 328, 219–224. [Google Scholar]
- Dallinger, R.; Berger, B.; Hunziker, P.; Kägi, J.H. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 388, 237–238. [Google Scholar] [CrossRef]
- Höckner, M.; Stefanon, K.; de Vaufleury, A.; Monteiro, F.; Perez-Rafael, S.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R. Physiological relevance and contribution to metal balance of specific and non-specific metallothionein isoforms in the garden snail, Cantareus aspersus. Biometals 2011, 24, 1079–1092. [Google Scholar] [CrossRef]
- Palacios, O.; Pagani, A.; Perez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, e4. [Google Scholar] [CrossRef]
- Kröger, B.; Vinther, J.; Fuchs, D. Cephalopod origin and evolution: A congruent picture emergingfrom fossils, development and molecules: Extant cephalopods are younger than previously realised and were under major selection to become agile, shell-less predators. Bioessays 2011, 33, 602–613. [Google Scholar] [CrossRef]
- Tanaka, T.; Hayashi, Y.; Ishizawa, M. Subcellular distribution and binding of heavy metals in the untreated liver of the squid; comparison with data from the livers of cadmium and silver-exposed rats. Experientia 1983, 39, 746–748. [Google Scholar] [CrossRef]
- Carpenè, E.; Cattani, O.; Kindt, M.; Ventre, G.; Serra, R.; Isani, G. How does storage procedure influence the stability of metallothionein (MT) from different animal sources? In Proceedings of the Joint Symposia of Italian Biochemical Society and Gesellschaft fur Biochemie und Molekularbiologie, Bari, Italy, 27 September–1 October, 1998.
- Raimundo, J.; Vale, C.; Duarte, R.; Moura, I. Association of Zn, Cu, Cd and Pb with protein fractions and sub-cellular partitioning in the digestive gland of Octopus vulgaris living in habitats with different metal levels. Chemosphere 2010, 81, 1314–1319. [Google Scholar] [CrossRef]
- Craig, S.; Overnell, J. Metals in squid, Loligo forbesi, adults, eggs and hatchlings. No evidence for a role for Cu- or Zn-metallothionein. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2003, 134, 311–317. [Google Scholar] [CrossRef]
- Tomas, M.; Domenech, J.; Capdevila, M.; Bofill, R.; Atrian, S. The sea urchin metallothionein system: Comparative evaluation of the SpMTA and SpMTB metal-binding preferences. FEBS OpenBio 2013, 3, 89–100. [Google Scholar]
- Wang, Y.; Hess, D.; Hunziker, P.E.; Kägi, J.H. Separation and characterization of the metal-thiolate-cluster domains of recombinant sea urchin metallothionein. Eur. J. Biochem. 1996, 241, 835–839. [Google Scholar]
- Trinchella, F.; Riggio, M.; Filosa, S.; Parisi, E.; Scudiero, R. Molecular cloning and sequencing of metallothionein in squamates: New insights into the evolution of the metallothionein genes in vertebrates. Gene 2008, 423, 48–56. [Google Scholar]
- Trinchella, F.; Esposito, M.G.; Scudiero, R. Metallothionein primary structure in amphibians: Insights from comparative evolutionary analysis in vertebrates. C. R. Biol. 2012, 335, 480–487. [Google Scholar] [CrossRef]
- Overnell, J.; Coombs, T.L. Purification and properties of plaice metallothionein, a cadmium-binding protein from the liver of the plaice (Pleuronectes platessa). Biochem. J. 1979, 183, 277–283. [Google Scholar]
- Bonham, K.; Zafarullah, M.; Gedamu, L. The rainbow trout metallothioneins: Molecular cloning and characterization of two distinct cDNA sequences. DNA 1987, 6, 519–528. [Google Scholar]
- Zafarullah, M.; Olsson, P.E.; Gedamu, L. Rainbow trout metallothionein gene structure and regulation. Oxf. Surv. Eukaryot. Genes 1989, 6, 112–143. [Google Scholar]
- Zafarullah, M.; Olsson, P.E.; Gedamu, L. Differential regulation of metallothionein genes in rainbow trout fibroblasts, Rtg-2. Biochim. Biophys. Acta 1990, 1049, 318–323. [Google Scholar]
- Carpenè, E.; Vašák, M. Hepatic metallothioneins from goldfish (Carassius auratus L.). Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 92, 463–468. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, S.Y.; Kim, K.Y.; Nam, Y.K. wo metallothionein genes from mud loach Misgurnus mizolepis (Teleostei; Cypriniformes): Gene structure, genomic organization, and mRNA expression analysis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 317–326. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Vašák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta 1985, 827, 36–44. [Google Scholar] [CrossRef]
- Lazo, J.S.; Kondo, Y.; Dellapiazza, D.; Michalska, A.E.; Choo, K.H.; Pitt, B.R. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J. Biol. Chem. 1995, 270, 5506–5510. [Google Scholar]
- Wright, J.; George, S.; Martinez-Lara, E.; Carpenè, E.; Kindt, M. Levels of cellular glutathione and metallothionein affect the toxicity of oxidative stressors in an established carp cell line. Mar. Environ. Res. 2000, 50, 503–508. [Google Scholar] [CrossRef]
- Cirillo, T.; Cocchieri, R.A.; Fasano, E.; Lucisano, A.; Tafuri, S.; Ferrante, M.C.; Carpenè, E.; Andreani, G.; Isani, G. Cadmium accumulation and antioxidant responses in Sparus aurata exposed to waterborne cadmium. Arch. Environ. Contam. Toxicol. 2012, 62, 118–126. [Google Scholar]
- Carpenè, E.; Camatti, A.; Isani, G.; Cattani, O.; Cortesi, P. Cd-metallothionein in liver and kidney of goldfish (Carassius auratus): Effects of temperature and salinity. Ital. J. Biochem. 1992, 41, 273–282. [Google Scholar]
- Tom, M.; Moran, O.; Jakubov, E.; Cavari, B.; Rinkevich, B. Molecular characterization of metallothionein cDNA of Sparus aurata used for detecting heavy metal pollution along the Mediterranean coast of Israel. Mar. Pollut. Bull. 1998, 36, 131–137. [Google Scholar] [CrossRef]
- Isani, G.; Andreani, G.; Monari, M.; Carpenè, E. Metal concentrations (Cu, Zn and Cd) and metallothionein expression in Sparus aurata exposed to waterborne copper. J. Trace Elem. Med. Biol. 2003, 17, 17–23. [Google Scholar]
- Villarreal, L.; Tio, L.; Capdevila, M.; Atrian, S. Comparative metal binding and genomic analysis of the avian (chicken) and mammalian metallothionein. FEBS J. 2006, 273, 523–535. [Google Scholar] [CrossRef]
- Andreani, G.; Santoro, M.; Cottignoli, S.; Fabbri, M.; Carpenè, E.; Isani, G. Metal distribution andmetallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci. Total Environ. 2008, 390, 287–294. [Google Scholar] [CrossRef]
- Weser, U.; Rupp, H.; Donay, F.; Linnemann, F.; Voelter, W.; Voetsch, W.; Jung, G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur. J. Biochem. 1973, 39, 127–140. [Google Scholar]
- McCormick, C.C.; Fullmer, C.S.; Garvey, J.S. Amino acid sequence and comparative antigenicity of chicken metallothionein. Proc. Natl. Acad. Sci. USA 1988, 85, 309–313. [Google Scholar] [CrossRef]
- Suzuki, K.T. Quantification and characterization of metallothioneins in tissues of lower vertebrates and invertebrates. Experientia Suppl. 1987, 52, 265–272. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, E.Y.; Iwata, H.; Tanabe, S. Molecular characterization of two metallothionein isoforms in avian species: Evolutionary history, tissue distribution profile, and expression associated with metal accumulation. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2007, 145, 295–305. [Google Scholar] [CrossRef]
- Kim, M.; Park, K.; Park, J.Y.; Kwak, I.S. Heavy metal contamination and metallothionein mRNA in blood and feathers of black-tailed gulls (Larus crassirostris) from South Korea. Environ. Monit. Assess. 2013, 185, 2221–2230. [Google Scholar] [CrossRef]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS One 2011, 6, e18487. [Google Scholar]
- García-Rico, L.; Frasquillo-Félix, C.; Robles-Burgueño, R.; Jara-Marini, M. Determination of cadmium and zinc and its relationship to metallothionein levels in swine kidney. Rev. Int. Contam. Ambient. 2002, 18, 157–162. [Google Scholar]
- Das, K.; Debacker, V.; Bouquegneau, J.M. Metallothioneins in marine mammals. Cell. Mol. Biol. 2000, 46, 283–294. [Google Scholar]
- Decataldo, A.; Leo, A.D.; Giandomenico, S.; Cardellicchio, N. Association of metals (mercury, cadmium and zinc) with metallothionein-like proteins in storage organs of stranded dolphins from the Mediterranean Sea (Southern Italy). J. Environ. Monit. 2004, 6, 361–367. [Google Scholar] [CrossRef]
- Das, K.; de Groof, A.; Jauniaux, T.; Bouquegneau, J.M. Zn, Cu, Cd and Hg binding to metallothioneins in harbour porpoises Phocoena phocoena from the southern North Sea. BMC Ecol. 2006. [Google Scholar] [CrossRef]
- Fritsch, C.; Cosson, R.P.; Coeurdassier, M.; Raoul, F.; Giraudoux, P.; Crini, N.; de Vaufleury, A.; Scheifler, R. Responses of wild small mammals to a pollution gradient: Host factors influence metal and metallothionein levels. Environ. Pollut. 2010, 158, 827–840. [Google Scholar] [CrossRef]
- Isani, G.; Andreani, G.; Gentilini, F.; Dondi, F.; Zambelli, D.; Carpenè, E. Metals-metallothionein interactions in biological systems. In Medicine and Diseases: A System Biology Perspective; Castellani, G., Franceschi, C., Alberghina, L., Milanesi, L., Eds.; Bononia University Press: Bologna, Italy, 2008; pp. 53–55. [Google Scholar]
- Swiergosz-Kowalewska, R.; Bednarska, A.; Callaghan, A. Expression of metallothionein genes I and II in bank vole Clethrionomys glareolus populations chronically exposed in situ to heavy metals. Environ. Sci. Technol. 2007, 41, 1032–1037. [Google Scholar] [CrossRef]
- Garcìa-Sevillano, M.A.; Jara-Biedma, R.; Gonzalez-Fernandez, M.; Garcia-Barrera, T.; Gomez-Ariza, J.L. Metal interactions in mice under environmental stress. Biometals 2013, 26, 651–666. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Isani, G.; Carpenè, E. Metallothioneins, Unconventional Proteins from Unconventional Animals: A Long Journey from Nematodes to Mammals. Biomolecules 2014, 4, 435-457. https://doi.org/10.3390/biom4020435
Isani G, Carpenè E. Metallothioneins, Unconventional Proteins from Unconventional Animals: A Long Journey from Nematodes to Mammals. Biomolecules. 2014; 4(2):435-457. https://doi.org/10.3390/biom4020435
Chicago/Turabian StyleIsani, Gloria, and Emilio Carpenè. 2014. "Metallothioneins, Unconventional Proteins from Unconventional Animals: A Long Journey from Nematodes to Mammals" Biomolecules 4, no. 2: 435-457. https://doi.org/10.3390/biom4020435





