A Novel Diterpene Glycoside with Nine Glucose Units from Stevia rebaudiana Bertoni
Abstract
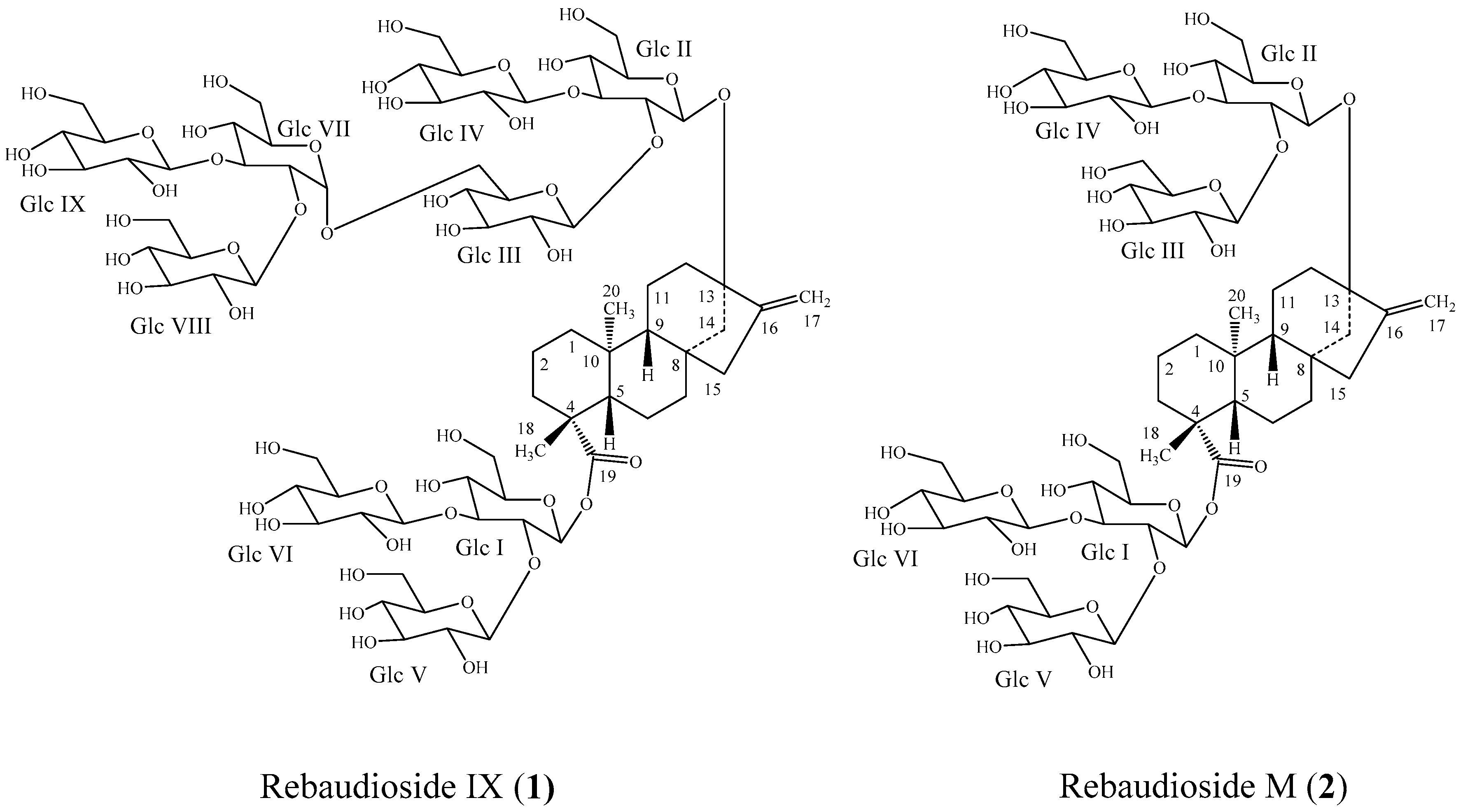
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures for Rebaudioside IX (1)
3.1.1. Isolation and Purification
3.1.2. Mass Spectrometry
3.1.3. Nuclear Magnetic Resonance Spectroscopy
3.2. Material Sources
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prakash, I.; DuBois, G.E.; King, G.A.; Upreti, M. Rebaudioside A Composition and Method for Purifying Rebaudioside A. U.S. Patent 2007/029582, 20 December 2007. [Google Scholar]
- Prakash, I.; DuBois, G.E.; Clos, J.F.; Wilkens, K.L.; Fosdick, L.E. Development of rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 2008, 46, S75–S82. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Chaturvedula, V.S.P.; Markosyan, A. Isolation, Characterization and Sensor Evaluation of a Hexa β-d-Glucopyranosyl Diterpene from Stevia rebaudiana. Nat. Prod. Commun. 2013, 8, 1523–1526. [Google Scholar] [PubMed]
- Soejarto, D.D.; Compadre, C.M.; Medon, P.J.; Kamath, S.K.; Kinghorn, A.D. Potential sweetening agents of plant origin. II. Field search for sweet-tasting Stevia species. Econ. Bot. 1983, 37, 71–79. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M.C. Steviol Glycosides: Chemical Diversity, Metabolism, and Function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of Novel Steviol Glycosides from Leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two Minor Diterpene Glycosides from the Leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar] [PubMed]
- Chaturvedula, V.S.P.; Prakash, I. Additional Minor Diterpene Glycosides from Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 1059–1062. [Google Scholar] [PubMed]
- Chaturvedula, V.S.P.; Prakash, I. Structures of the Novel Diterpene Glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Upreti, M.; Prakash, I. Structures of the Novel α-Glucosyl Linked Diterpene Glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Upreti, M.; Prakash, I. Diterpene Glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Markosyan, A.; Chaturvedula, V.S.P.; Campbell, M.; San Miguel, R.I.; Purkayastha, S.; Johnson, M. Methods for Purifying Steviol Glycosides. PCT Patent WO 2013/096420, 27 June 2013. [Google Scholar]
- Prakash, I.; Markosyan, A.; Bunders, C. Development of Next Generation Stevia Sweetener: Rebaudioside M. Foods 2014, 3, 162–175. [Google Scholar] [CrossRef]
- Prakash, I.; Ma, G.; Bunders, C.; Devkota, K.P.; Charan, R.D.; Ramirez, C.; Snyder, T.M.; Priedemann, C. A New Diterpene Glycoside: 15α-Hydroxy-Rebaudioside M Isolated from Stevia rebaudiana. Nat. Prod. Commun. 2015, 10, 1159–1161. [Google Scholar] [PubMed]
- Prakash, I.; Bunders, C.; Devkota, K.P.; Charan, R.D.; Ramirez, C.; Priedemann, C.; Markosyan, A. Isolation and Characterization of a Novel Rebaudioside M Isomer from a Bioconversion Reaction of Rebaudioside A and NMR comparison Studies of Rebaudioside M Isolated from Stevia rebaudiana Bertoni and Stevia rebaudiana Morita. Biomolecules 2014, 4, 374–389. [Google Scholar] [CrossRef] [PubMed]
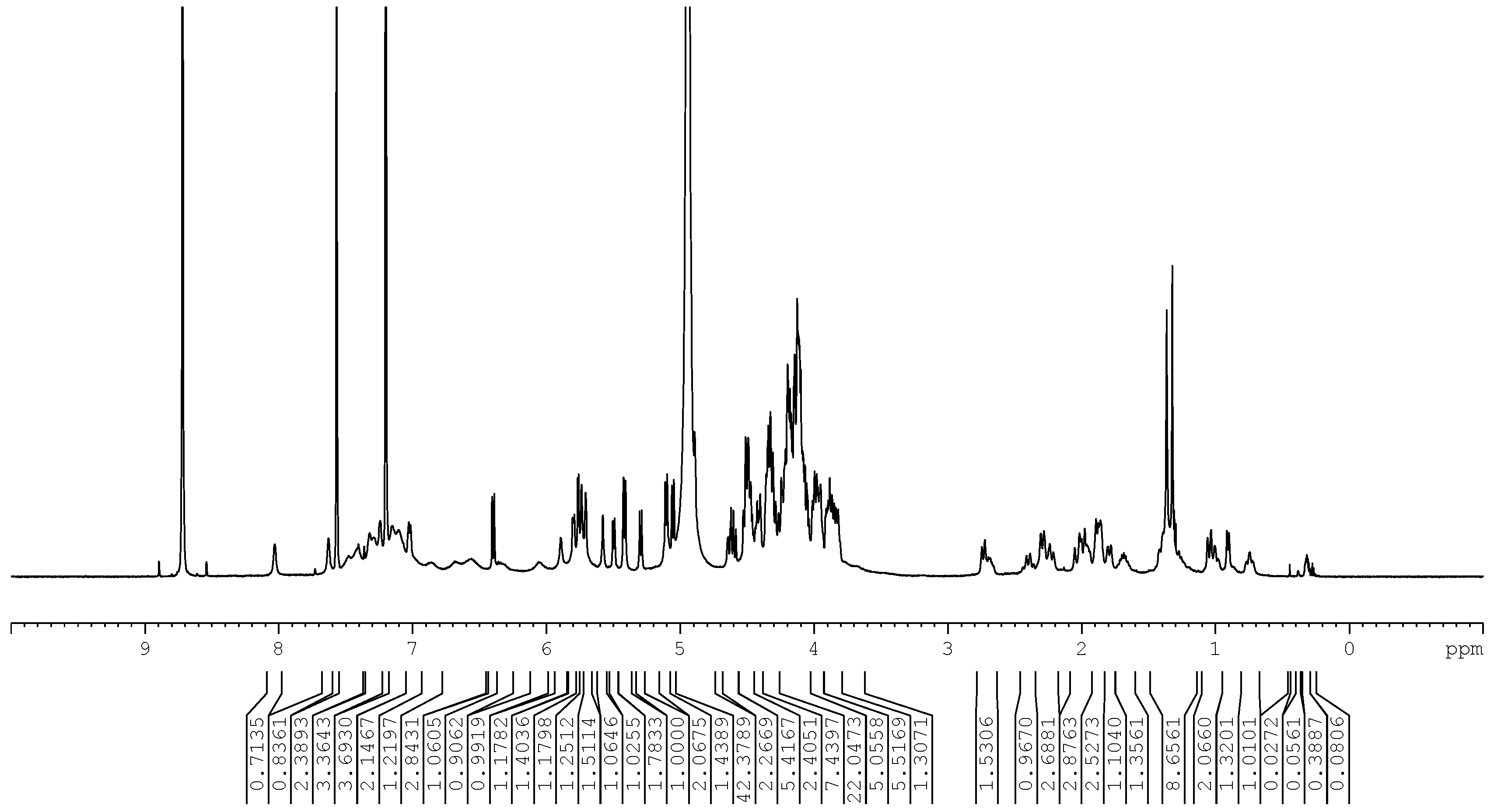

| Atom No. | 13C NMR | 1H NMR |
|---|---|---|
| 1 | 40.7 | 0.75 m, 1.86 m |
| 2 | 20.1 | 1.36 m, 2.29 m |
| 3 | 38.9 | 1.01 m, 2.30 m |
| 4 | 44.7 | - |
| 5 | 57.9 | 1.05 d (13.1) |
| 6 | 23.9 | 2.23 m, 2.39 m |
| 7 | 43.0 | 1.40 m, 1.80 m |
| 8 | 41.6 | - |
| 9 | 54.8 | 0.91 d (7.9) |
| 10 | 40.2 | - |
| 11 | 20.6 | 1.68 m, 1.88 m |
| 12 | 39.0 | 1.95 m, 2.69 m |
| 13 | 88.0 | - |
| 14 | 43.9 | 1.99 m, 2.74 d (10.6) |
| 15 | 46.9 | 1.87 m, 2.02 m |
| 16 | 153.7 | - |
| 17 | 105.4 | 4.89 bs, 5.71 bs |
| 18 | 28.7 | 1.33 s |
| 19 | 177.3 | - |
| 20 | 17.1 | 1.37 s |
| Sugar | Atom No. | 13C NMR | 1H NMR |
|---|---|---|---|
| Glc I | 1 | 95.3 | 6.40 d (8.2) |
| 2 | 77.2 | 4.51 m | |
| 3 | 89.0 | 5.11 m | |
| 4 | 70.5 or 70.6 or 70.7 | 4.20 m | |
| 5 | 78.9 | 4.14 m | |
| 6 | 62.2 | 4.20 m, 4.32 m | |
| Glc V | 1 | 104.6 | 5.80 d (7.4) |
| 2 | 75.8 | 4.19 m | |
| 3 | 78.0–78.5 ¥ | 4.20 m | |
| 4 | 71.5 or 71.6 or 72.1 | 4.11 m | |
| 5 | 78.0–78.5 ¥ | 3.90 m | |
| 6 | 64.4 | 4.33 m, 4.63 m | |
| Glc VI | 1 | 104.6 | 5.29 d (7.9) |
| 2 | 75.9 | 3.95 m | |
| 3 | 78.0–78.5 ¥ | 4.35 m | |
| 4 | 71.5 or 71.6 | 4.09 m | |
| 5 | 78.0–78.5 ¥ | 3.86 m | |
| 6 | 62.2–63.4 ¥ | 4.10 m or 4.36 m, 4.30 m |
| Sugar | Atom No. | 13C NMR | 1H NMR |
|---|---|---|---|
| Glc II | 1 | 96.6 | 5.42 d (8.0) |
| 2 | 81.2 | 4.19 m | |
| 3 | 88.6 | 4.93 m # | |
| 4 | 70.5 or 70.6 or 70.7 | 4.09 m | |
| 5 | 78.0–78.5 ¥ | 3.88 m | |
| 6 | 62.2–63.4 ¥ | 4.19 m or 4.22 m, 4.33 m | |
| Glc III | 1 | 104.8 | 5.48 d (7.4) |
| 2 | 75.9 or 76.0 | 4.11 m | |
| 3 | 78.7–79.0 ¥ | 4.13 m | |
| 4 | 74.3 | 3.87 m | |
| 5 | 76.5 | 3.97 m | |
| 6 | 72.1 | 4.44 m, 4.50 m | |
| Glc IV | 1 | 104.3 | 5.40 d (8.0) |
| 2 | 75.9 or 76.0 | 3.97 m | |
| 3 | 78.7–79.0 ¥ | 4.47 m | |
| 4 | 71.5 or 71.6 | 4.13 m or 4.15 m | |
| 5 | 78.0–78.5 ¥ | 3.97 m | |
| 6 | 62.2–63.4 ¥ | 4.19 m or 4.22 m, 4.32 m | |
| Glc VII | 1 | 100.6 | 5.76 d (3.5) |
| 2 | 81.4 | 4.13 m | |
| 3 | 84.7 | 4.60 m | |
| 4 | 70.5 or 70.6 or 70.7 | 4.16 m | |
| 5 | 74.0 | 4.41 m | |
| 6 | 62.2–63.4 ¥ | 4.36 m, ~4.5 m | |
| Glc VIII | 1 | 106.5 | 5.10 d (7.7) |
| 2 | 75.7 | 4.06 m | |
| 3 | 78.7–79.0 ¥ | 4.17 m | |
| 4 | 72.1 or 74.0 | 4.10 m or 4.16 m | |
| 5 | 78.7–79.0 ¥ | 3.81 m or 3.85 m | |
| 6 | 62.2–63.4 ¥ | ~4.2 m to ~4.5 m | |
| Glc IX | 1 | 105.4 | 5.06 d (7.8) |
| 2 | 76.1 | 3.98 m | |
| 3 | 78.7–79.0 ¥ | 4.13 m | |
| 4 | 72.1 or 74.0 | 4.10 m or 4.16 m | |
| 5 | 78.7–79.0 ¥ | 3.81 m or 3.85 m | |
| 6 | 62.2–63.4 ¥ | ~4.2 m to ~4.5 m |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, I.; Ma, G.; Bunders, C.; Charan, R.D.; Ramirez, C.; Devkota, K.P.; Snyder, T.M. A Novel Diterpene Glycoside with Nine Glucose Units from Stevia rebaudiana Bertoni. Biomolecules 2017, 7, 10. https://doi.org/10.3390/biom7010010
Prakash I, Ma G, Bunders C, Charan RD, Ramirez C, Devkota KP, Snyder TM. A Novel Diterpene Glycoside with Nine Glucose Units from Stevia rebaudiana Bertoni. Biomolecules. 2017; 7(1):10. https://doi.org/10.3390/biom7010010
Chicago/Turabian StylePrakash, Indra, Gil Ma, Cynthia Bunders, Romila D. Charan, Catherine Ramirez, Krishna P. Devkota, and Tara M. Snyder. 2017. "A Novel Diterpene Glycoside with Nine Glucose Units from Stevia rebaudiana Bertoni" Biomolecules 7, no. 1: 10. https://doi.org/10.3390/biom7010010




