Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach
Abstract
:1. Introduction
2. Results and Discussion
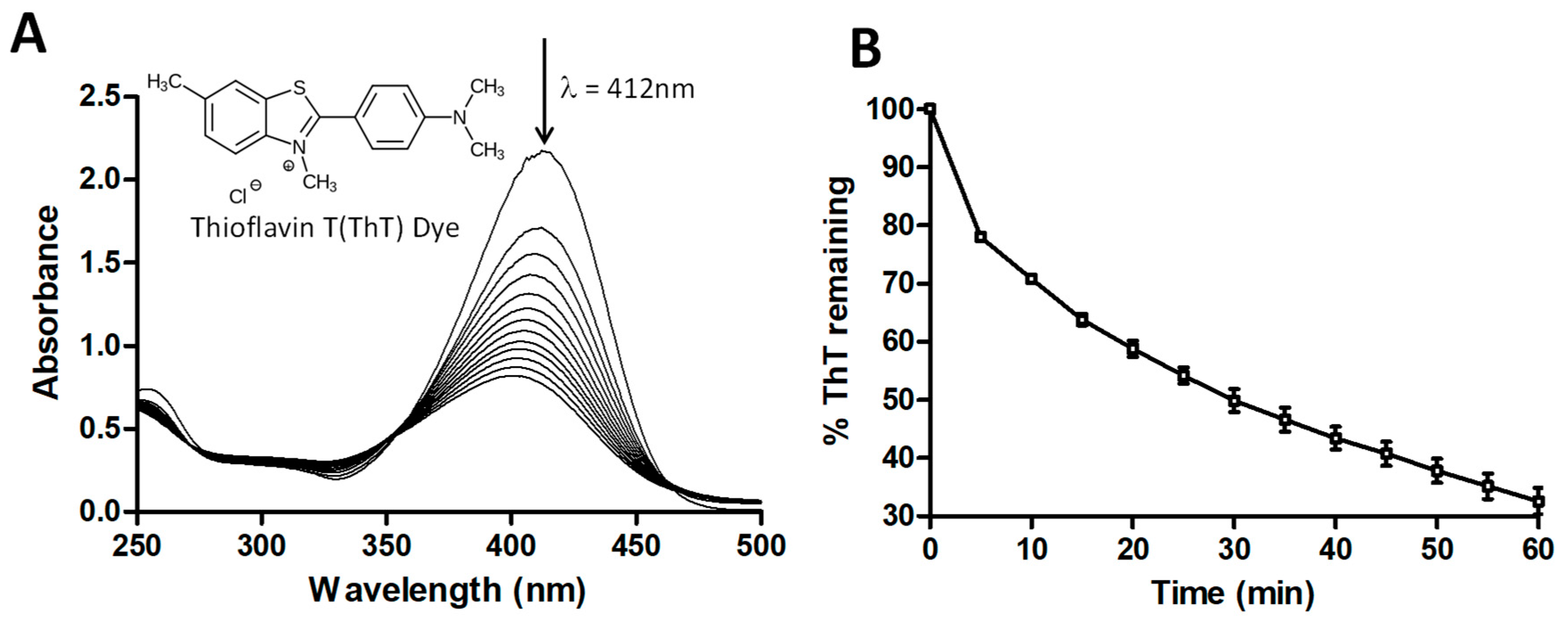
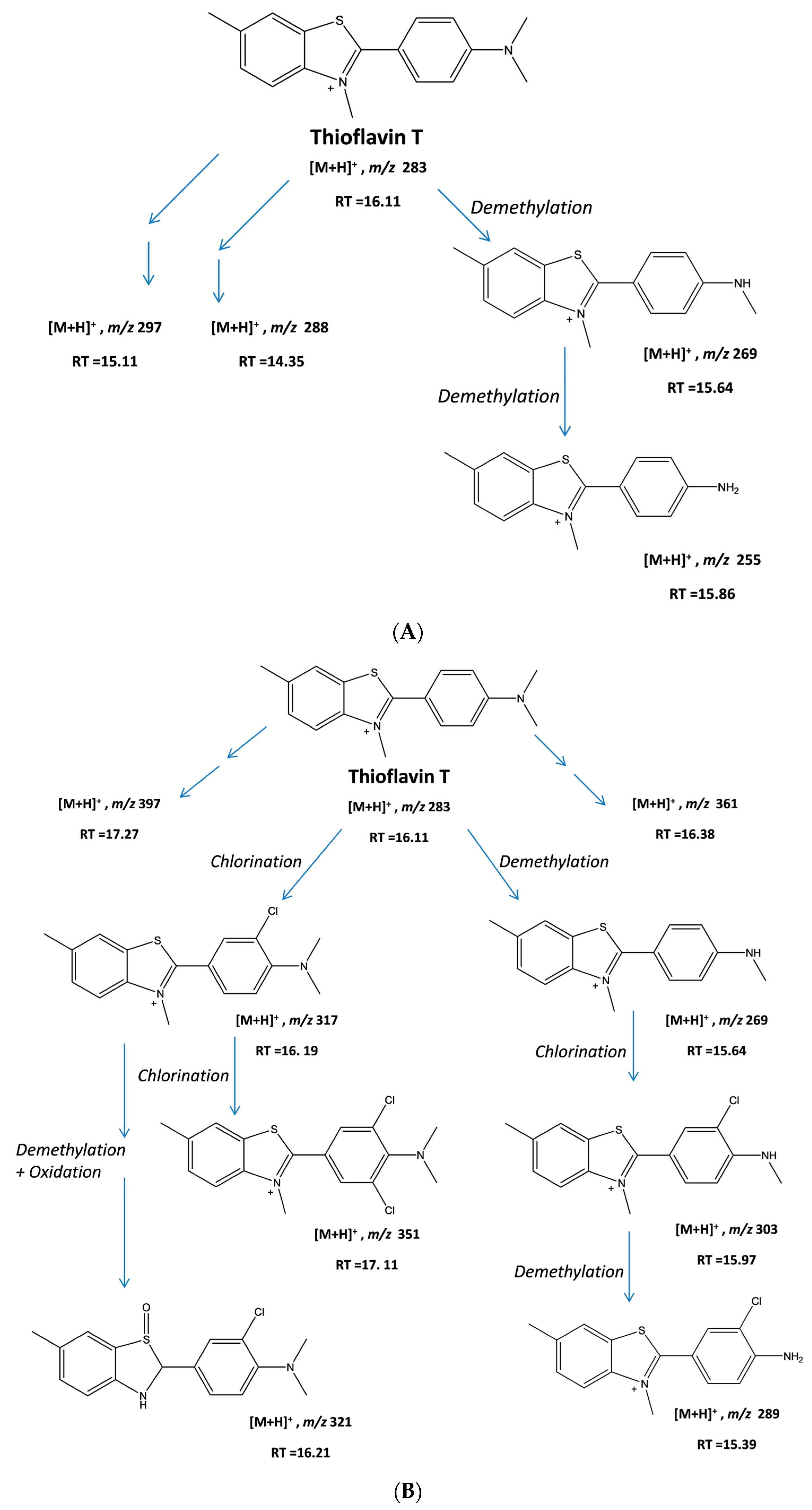
2.1. Degradation of ThT by UV + H2O2
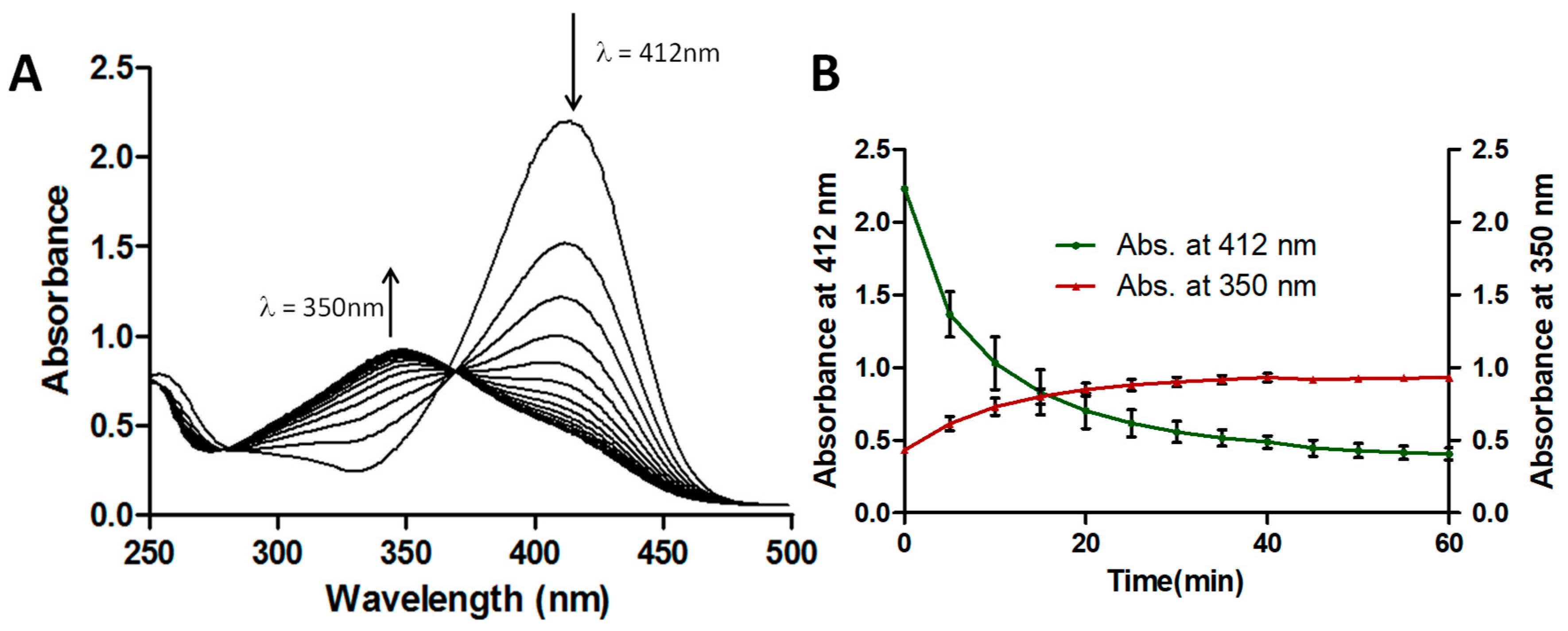
2.2. Degradation of ThT by CPO + H2O2
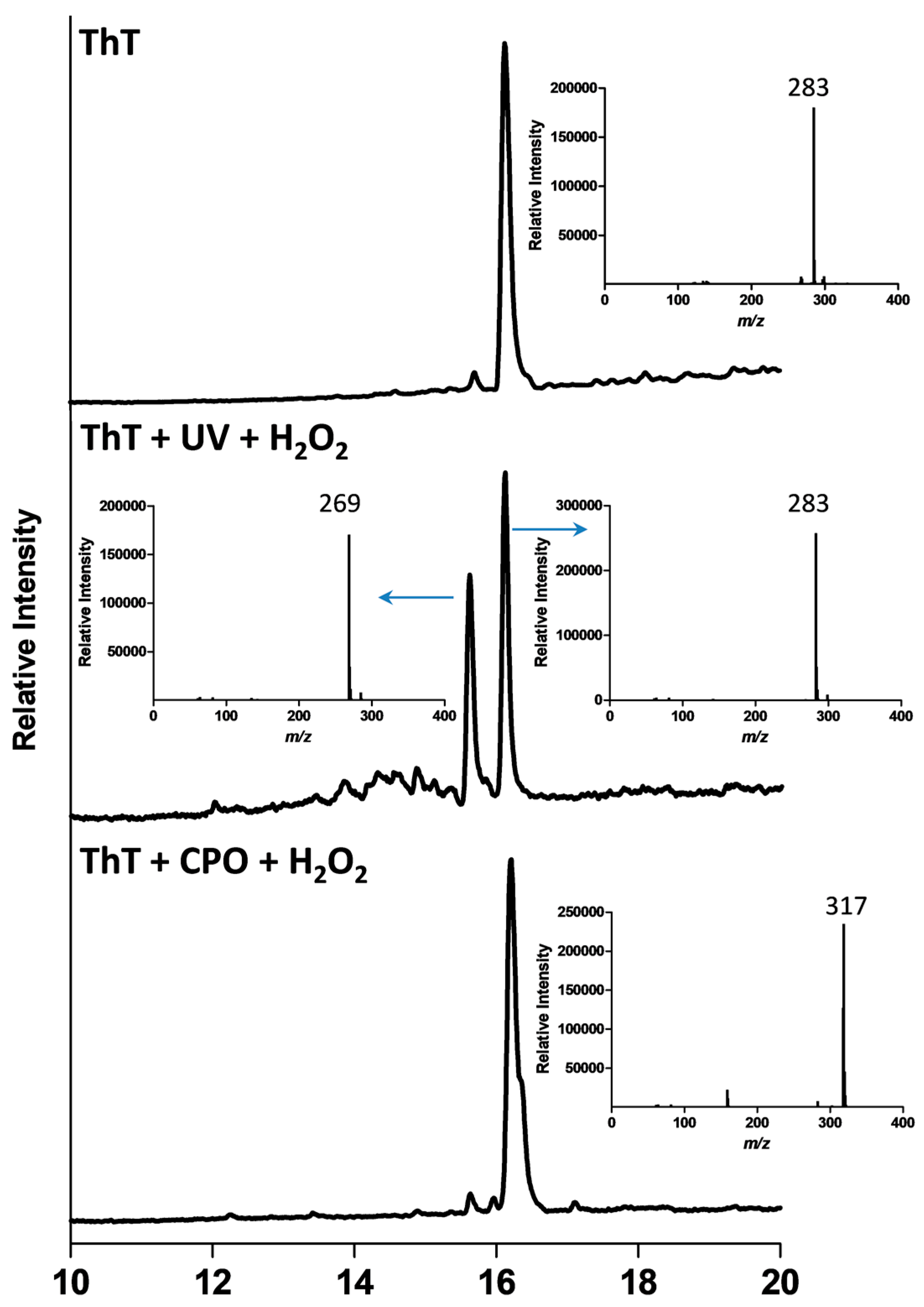
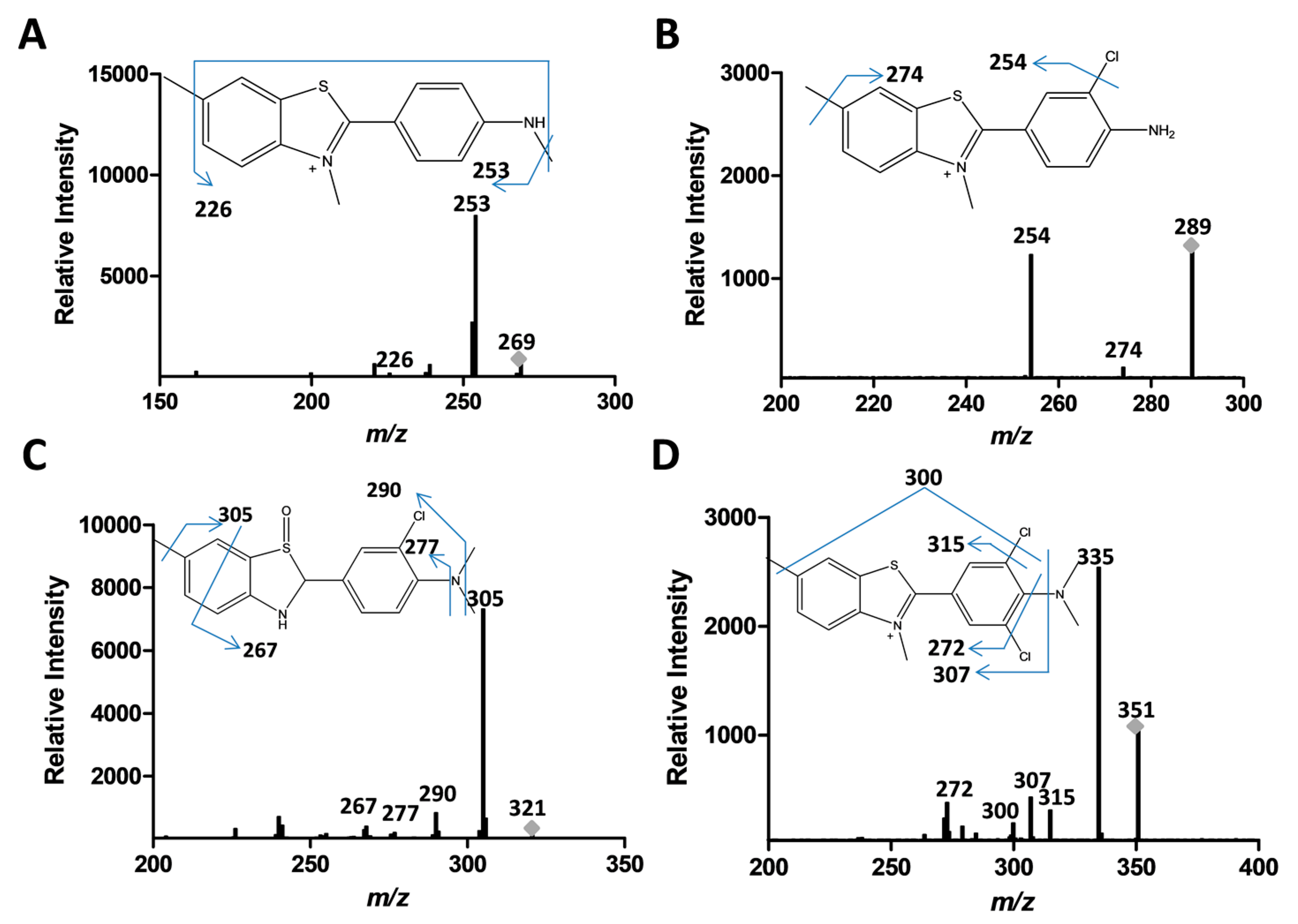
2.3. Analysis of Product Formation Using LC-MS
2.4. Mechanistic Studies
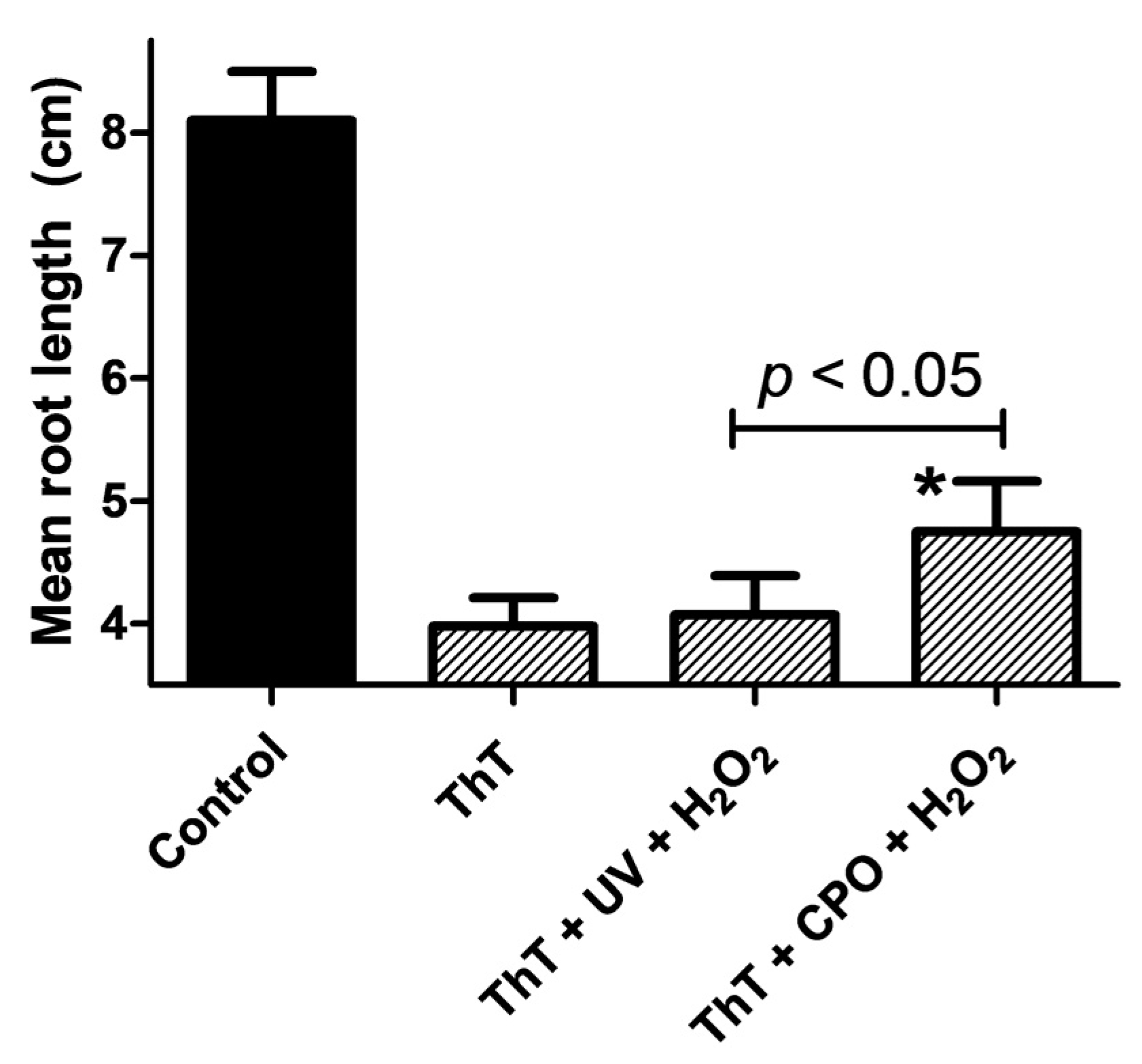
2.5. Toxicity Studies
3. Materials and Methods
3.1. Reagents
3.2. Thioflavin T Decolorization
3.3. LC-MS and MS-MS Analyses
3.4. Phytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kalsoom, U.; Ashraf, S.S.; Meetani, M.A.; Rauf, M.A.; Bhatti, H.N. Degradation and kinetics of H2O2 assisted photochemical oxidation of Remazol Turquoise Blue. Chem. Eng. J. 2012, 200, 373–379. [Google Scholar] [CrossRef]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Rauf, M.A.; Salman Ashraf, S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012, 209, 520–530. [Google Scholar] [CrossRef]
- Ali, L.; Algaithi, R.; Habib, H.M.; Souka, U.; Rauf, M.A.; Ashraf, S.S. Soybean peroxidase-mediated degradation of an azo dye– a detailed mechanistic study. BMC Biochem. 2013, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Bibi, I.; Bhatti, H.N.; Asgher, M. Comparative study of natural and synthetic phenolic compounds as efficient laccase mediators for the transformation of cationic dye. Biochem. Eng. J. 2011, 56, 225–231. [Google Scholar] [CrossRef]
- Kalsoom, U.; Ashraf, S.S.; Meetani, M.A.; Rauf, M.A.; Bhatti, H.N. Mechanistic study of a diazo dye degradation by Soybean Peroxidase. Chem. Cent. J. 2013, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Franciscon, E.; Piubeli, F.; Fantinatti-Garboggini, F.; Ragagnin de Menezes, C.; Serrano Silva, I.; Cavaco-Paulo, A.; Grossman, M.J.; Durrant, L.R. Polymerization study of the aromatic amines generated by the biodegradation of azo dyes using the laccase enzyme. Enzym. Microb. Technol. 2010, 46, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Ryan, B.J.; Carolan, N.; Ó’Fágáin, C. Horseradish and soybean peroxidases: Comparable tools for alternative niches? Trends Biotechnol. 2006, 24, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.-B.; Jia, R.; Li, P.-S.; Zhu, Q.; Tu, S.-Q.; Tang, W.-Z. Studies on the Properties and Co-immobilization of Manganese Peroxidase. Chin. J. Biotechnol. 2007, 23, 90–96. [Google Scholar] [CrossRef]
- Adrio, J.L.; Demain, A.L. Microbial Enzymes: Tools for Biotechnological Processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Tan, Y.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Rapid decolorization of anthraquinone and triphenylmethane dye using chloroperoxidase: Catalytic mechanism, analysis of products and degradation route. Chem. Eng. J. 2014, 244, 9–18. [Google Scholar] [CrossRef]
- Alneyadi, A.H.; Ashraf, S.S. Differential enzymatic degradation of thiazole pollutants by two different peroxidases—A comparative study. Chem. Eng. J. 2016, 303, 529–538. [Google Scholar] [CrossRef]
- Alneyadi, A.H.; Shah, I.; AbuQamar, S.F.; Ashraf, S.S. Differential Degradation and Detoxification of an Aromatic Pollutant by Two Different Peroxidases. Biomolecules 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. The crystal structure of chloroperoxidase: A heme peroxidase—cytochrome P450 functional hybrid. Structure 1995, 3, 1367–1378. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. Heidelb. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, W.H.M.; He, X.; Komy, Z.R.; Ismail, N.M.; Dionysiou, D.D. Revealing the mechanism, pathways and kinetics of UV254nm/H2O2-based degradation of model active sunscreen ingredient PBSA. Chem. Eng. J. 2016, 288, 824–833. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Removal of Endocrine Disruptors from Urban Wastewater by Advanced Oxidation Processes (AOPs): A Review. Open Biotechnol. J. 2016, 10. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.; Ornelas-Soto, N. Laccases: A Blue Enzyme for Greener Alternative Technologies in the Detection and Treatment of Emerging Pollutants. In Green Technologies and Environmental Sustainability; Springer: Cham, Switzerland, 2017; pp. 45–65. ISBN 978-3-319-50653-1. [Google Scholar]
- Dhillon, G.S.; Kaur, S. Agro-Industrial Wastes as Feedstock for Enzyme Production: Apply and Exploit the Emerging and Valuable Use Options of Waste Biomass; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-802612-0. [Google Scholar]
- Calza, P.; Avetta, P.; Rubulotta, G.; Sangermano, M.; Laurenti, E. TiO2-soybean peroxidase composite materials as a new photocatalytic system. Chem. Eng. J. 2014, 239, 87–92. [Google Scholar] [CrossRef]
- Nogueira, V.; Lopes, I.; Freitas, A.C.; Rocha-Santos, T.A.P.; Gonçalves, F.; Duarte, A.C.; Pereira, R. Biological treatment with fungi of olive mill wastewater pre-treated by photocatalytic oxidation with nanomaterials. Ecotoxicol. Environ. Saf. 2015, 115, 234–242. [Google Scholar] [CrossRef] [PubMed]
- García-Montaño, J.; Domènech, X.; García-Hortal, J.A.; Torrades, F.; Peral, J. The testing of several biological and chemical coupled treatments for Cibacron Red FN-R azo dye removal. J. Hazard. Mater. 2008, 154, 484–490. [Google Scholar] [CrossRef] [PubMed]
- García-Montaño, J.; Pérez-Estrada, L.; Oller, I.; Maldonado, M.I.; Torrades, F.; Peral, J. Pilot plant scale reactive dyes degradation by solar photo-Fenton and biological processes. J. Photochem. Photobiol. Chem. 2008, 195, 205–214. [Google Scholar] [CrossRef]
- Sánchez Peréz, J.A.; Carra, I.; Sirtori, C.; Agüera, A.; Esteban, B. Fate of thiabendazole through the treatment of a simulated agro-food industrial effluent by combined MBR/Fenton processes at μg/L scale. Water Res. 2014, 51, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.O.; Smith, S.R. Review of “emerging” organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef] [PubMed]
- Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; Rubirola, A. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Georgiou, D.; Melidis, P.; Aivasidis, A.; Gimouhopoulos, K. Degradation of azo-reactive dyes by ultraviolet radiation in the presence of hydrogen peroxide. Dyes Pigments 2002, 52, 69–78. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ali, L.; Sadig, M.S.A.Y.; Ashraf, S.S.; Hisaindee, S. Comparative degradation studies of Malachite Green and Thiazole Yellow G and their binary mixture using UV/H2O2. Desalination Water Treat. 2016, 57, 8336–8342. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Li, T. UV/H2O2 Process Under High Intensity UV Irradiation: A Rapid and Effective Method for Methylene Blue Decolorization. CLEAN Soil Air Water 2013, 41, 1201–1207. [Google Scholar] [CrossRef]
- Hisaindee, S.; Meetani, M.A.; Rauf, M.A. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC Trends Anal. Chem. 2013, 49, 31–44. [Google Scholar] [CrossRef]
- He, Y.; Grieser, F.; Ashokkumar, M. The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 2011, 18, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Meetani, M.A.; Hisaindee, S.M.; Abdullah, F.; Ashraf, S.S.; Rauf, M.A. Liquid chromatography tandem mass spectrometry analysis of photodegradation of a diazo compound: A mechanistic study. Chemosphere 2010, 80, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, P.E.M.; Blomberg, M.R.A. Mechanisms for enzymatic reactions involving formation or cleavage of O-O bonds. Theor. Comput. Chem. 2001, 9, 95–143. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Combination of enzymatic degradation by chloroperoxidase with activated sludge treatment to remove sulfamethoxazole: Performance, and eco-toxicity assessment. J. Chem. Technol. Biotechnol. 2016, 91, 2802–2809. [Google Scholar] [CrossRef]
- Hager, L.P.; Morris, D.R.; Brown, F.S.; Eberwein, H. Chloroperoxidase II. Utilization of Halogen Anions. J. Biol. Chem. 1966, 241, 1769–1777. [Google Scholar] [PubMed]
- Correia, V.M.; Stephenson, T.; Judd, S.J. Characterisation of textile wastewaters—A review. Environ. Technol. 1994, 15, 917–929. [Google Scholar] [CrossRef]
- Silva, M.C.; Corrêa, A.D.; Amorim, M.T.S.P.; Parpot, P.; Torres, J.A.; Chagas, P.M.B. Decolorization of the phthalocyanine dye reactive blue 21 by turnip peroxidase and assessment of its oxidation products. J. Mol. Catal. B Enzym. 2012, 77, 9–14. [Google Scholar] [CrossRef]
- Ecological Effects Test Guidelines OCSPP 850.4100: Seedling Emergence and Seedling Growth; United States Environmental Protection Agency, National Service Center for Environmental Publications (NSCEP): Cincinnati, OH, USA, 2012.
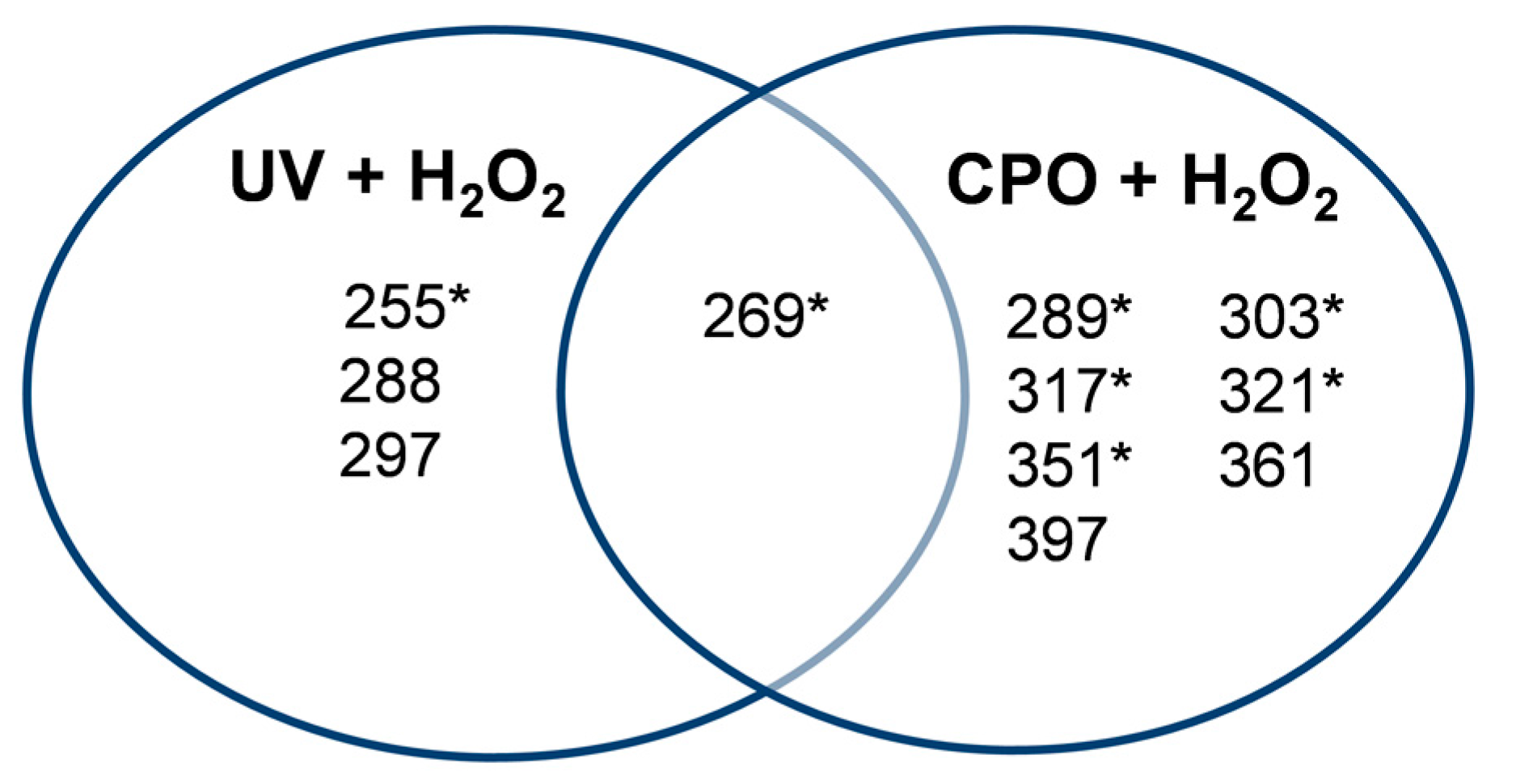
| Retention Time (min) | ThT + UV + H2O2 | ThT + CPO + H2O2 | ||
|---|---|---|---|---|
| m/z | Peak Area | m/z | Peak Area | |
| 14.35 | 288 | 24,398 | - | - |
| 14.86 | 255 | 161,335 | - | - |
| 15.11 | 297 | 70,562 | - | - |
| 15.39 | - | - | 289 | 27,169 |
| 15.64 | 269 | 1,216,238 | 269 | 300,854 |
| 15.97 | - | - | 303 | 190,007 |
| 16.11 | 283 | 1,789,254 | 283 | 249,499 |
| 16.19 | - | - | 317 | 6,147,872 |
| 16.21 | - | - | 321 | 138,735 |
| 16.38 | - | - | 361 | 639,968 |
| 17.11 | - | - | 351 | 81,812 |
| 17.27 | - | - | 397 | 9,472 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules 2017, 7, 64. https://doi.org/10.3390/biom7030064
Al-Maqdi KA, Hisaindee SM, Rauf MA, Ashraf SS. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules. 2017; 7(3):64. https://doi.org/10.3390/biom7030064
Chicago/Turabian StyleAl-Maqdi, Khadega A., Soleiman M. Hisaindee, Muhammad A. Rauf, and Syed Salman Ashraf. 2017. "Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach" Biomolecules 7, no. 3: 64. https://doi.org/10.3390/biom7030064






