Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria
Abstract
:1. Introduction
2. Results
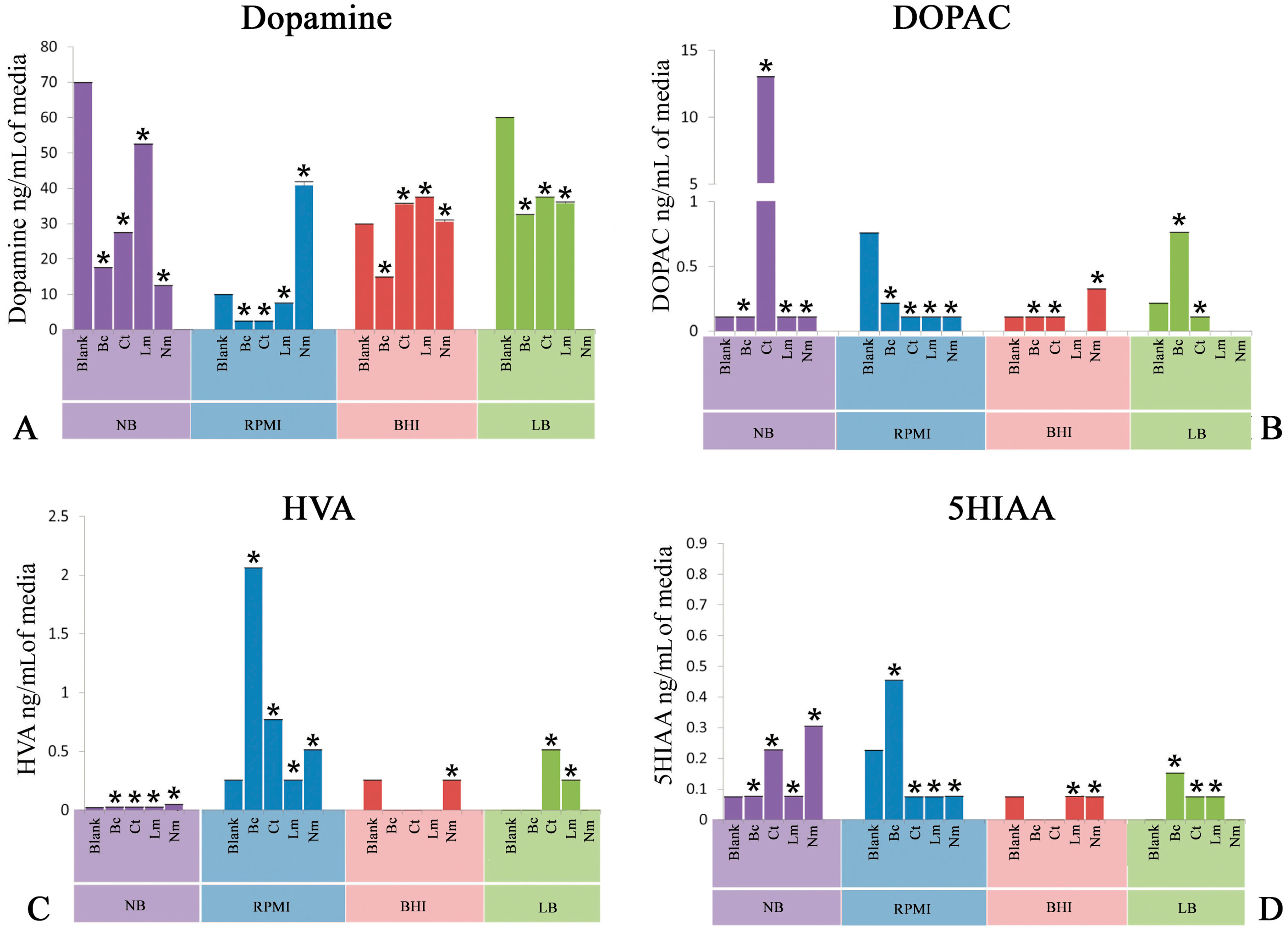
2.1. Quantification of Dopamine
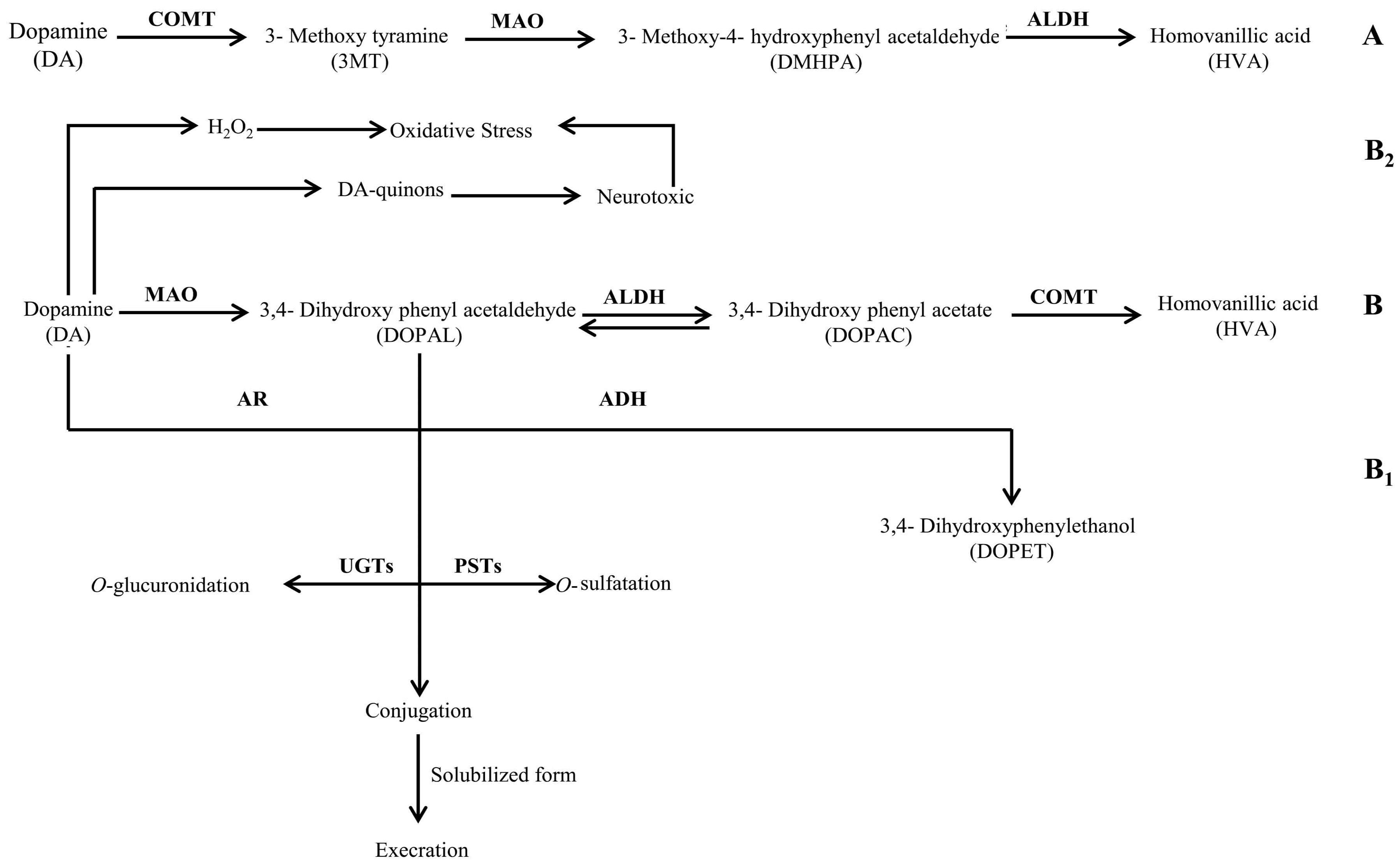
2.2. Quantification of 3,4-Dihydroxyphenylacetic Acid
2.3. Quantification of Homovanillic Acid
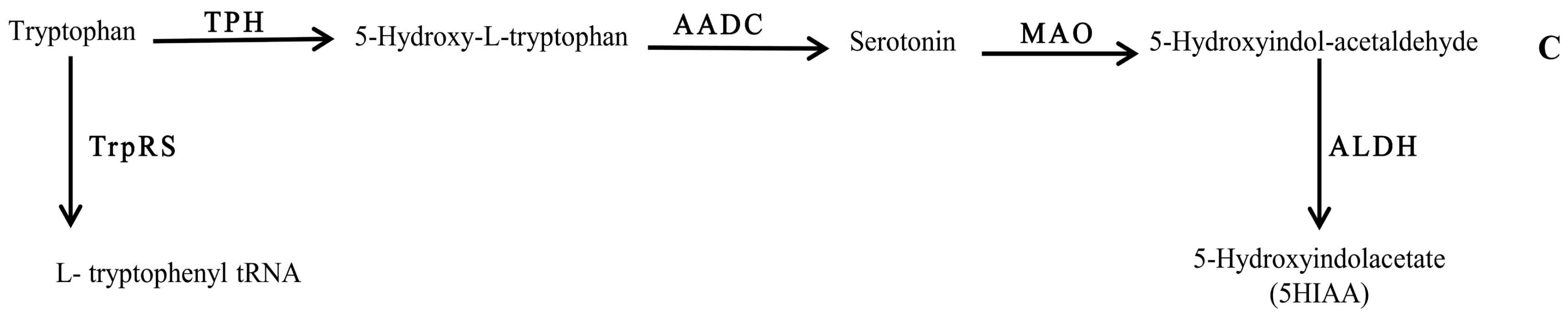
2.4. Quantification of 5-Hydroxyindoleacetic Acid
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacteria
4.3. Microbiological Media
4.4. Experimental Procedure
4.5. Sterility Check
4.6. HPLC-EC
4.7. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lyte, M. Microbial endocrinology and the microbiota-gut-brain axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 3–24. [Google Scholar]
- Lyte, M. Microbial Endocrinology in the Pathogenesis of Infectious Disease. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Fink, G.; Pfaff, D.W.; Levine, J.E. Handbook of Neuroendocrinology, 1st ed.; Academic Press: Boston, MA, USA, 2012. [Google Scholar]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell–cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.; Sandrini, S.M.; Haigh, R.D.; Lyte, M. Microbial endocrinology: How stress influences susceptibility to infection. Trends Microbiol. 2008, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004, 12, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. The role of microbial endocrinology in infectious disease. J. Endocrinol. 1993, 137, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Lyte, M.; Ernst, S. Alpha and beta adrenergic receptor involvement in catecholamine-induced growth of gram-negative bacteria. Biochem. Biophys. Res. Commun. 1993, 190, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Frank, C.D.; Green, B.T. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157: H7. FEMS Microbiol. Lett. 1996, 139, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Erickson, A.K.; Arulanandam, B.P.; Frank, C.D.; Crawford, M.A.; Francis, D.H. Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli. Biochem. Biophys. Res. Commun. 1997, 232, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Arulanandam, B.P.; Frank, C.D. Production of Shiga-like toxins by Escherichia coli O157: H7 can be influenced by the neuroendocrine hormone norepinephrine. J. Lab. Clin. Med. 1996, 128, 392–398. [Google Scholar] [CrossRef]
- Freestone, P.P.; Haigh, R.D.; Lyte, M. Specificity of catecholamine-induced growth in Escherichia coli O157: H7, Salmonella enterica and Yersinia enterocolitica. FEMS Microbiol. Lett. 2007, 269, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Anuchin, A.M.; Chuvelev, D.I.; Kirovskaya, T.A.; Oleskin, A.V. Effects of monoamine neuromediators on the growth-related variables of Escherichia coli K-12. Microbiology 2008, 77, 674–680. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Kirovskaia, T.A.; Botvinko, I.V.; Lysak, L.V. Effect of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms. Mikrobiologiia 1997, 67, 305–312. [Google Scholar]
- Strakhovskaia, M.G.; Ivanova, E.V.; Fraĭnkin, G. Stimulatory effect of serotonin on the growth of the yeast Candida guilliermondii and the bacterium Streptococcus faecalis. Mikrobiologiia 1993, 62, 46–49. [Google Scholar] [PubMed]
- Malikina, K.D.; Shishov, V.A.; Chuvelev, D.I.; Kudrin, V.S.; Oleskin, A.V. Regulatory role of monoamine neurotransmitters in Saccharomyces cerevisiae cells. Appl. Biochem. Microbiol. 2010, 46, 620–625. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Role of neuromediators in the functioning of the human microbiota: “Business talks” among microorganisms and the microbiota-host dialogue. Microbiology 2016, 85, 1–22. [Google Scholar] [CrossRef]
- Averina, O.V.; Danilenko, V.N. Human intestinal microbiota: Role in development and functioning of the nervous system. Microbiology 2017, 86, 1–8. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Zhilenkova, O.G.; Shenderov, B.A.; Amerhanova, A.M.; Kudrin, V.S.; Klodt, P.M. Lactic-acid bacteria supplement fermented dairy products with human behavior-modifying neuroactive compounds. J. Pharm. Nutr. Sci. 2014, 4, 199–206. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shishov, V.I.; Malikina, K.D. Symbiotic Biofilms and Brain Neurochemistry; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O’Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 2017, 28, 1353881. [Google Scholar]
- Koedel, U.; Pfister, H.W. Oxidative stress in bacterial meningitis. Brain Pathol. 1999, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kastenbauer, S.; Koedel, U.; Becker, B.F.; Pfister, H.W. Oxidative stress in bacterial meningitis in humans. Neurology 2002, 58, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Schaper, M.; Lykkesfeldt, J.; Siegenthaler, C.; Bifrare, Y.D.; Banič, S.; Leib, S.L.; Täuber, M.G. Oxidative stress in brain during experimental bacterial meningitis: Differential effects of α-phenyl-tert-butyl nitrone and N-acetylcysteine treatment. Free Radic. Biol. Med. 2001, 31, 754–762. [Google Scholar] [CrossRef]
- De Menezes, C.C.; Dorneles, A.G.; Sperotto, R.L.; Duarte, M.M.; Schetinger, M.R.; Loro, V.L. Oxidative stress in cerebrospinal fluid of patients with aseptic and bacterial meningitis. Neurochem. Res. 2009, 34, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Scheld, W.M.; Koedel, U.; Nathan, B.; Pfister, H.W. Pathophysiology of bacterial meningitis: Mechanism (s) of neuronal injury. J. Infect. Dis. 2002, 186, S225–S233. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Klein, M.; Pfister, H.W. New understandings on the pathophysiology of bacterial meningitis. Curr. Opin. Infect. Dis. 2010, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Koedel, U.; Pfister, H.W. Oxidative stress in pneumococcal meningitis: A future target for adjunctive therapy? Prog. Neurobiol. 2006, 80, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Heggers, J.P.; Robson, M.C.; Phillips, L.G. Quantitative Bacteriology: Its Role in the Armamentarium of the Surgeon; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Parkinson’s Disease and its Management. BMJ Br. Med. J. 1994, 308, 281. [Google Scholar] [CrossRef]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. 2011, 27. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yu, W.; Wu, J.; Chen, C.; Lou, Z.; Zhang, Q.; Zhao, J.; Wang, J.; Xiao, B. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS ONE 2013, 8, e78418. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Herrera, A.J.; Venero, J.L.; Santiago, M.; De Pablos, R.M.; Villarán, R.F.; Espinosa-Oliva, A.M.; Argüelles, S.; Sarmiento, M.; Delgado-Cortés, M.J.; et al. Inflammatory animal model for Parkinson’s disease: The intranigral injection of LPS induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Boltz, D.A.; Webster, R.G.; Smeyne, R.J. Viral Parkinsonism. Biochim. Biophys. Acta 2009, 1792, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Leask, B.G.; Ruthven, C.R.; Sandler, M.; Southgate, J. Investigation of 5-hydroxytryptamine production by Candida albicans in vitro and in vivo. Microbios 1971, 4, 133–143. [Google Scholar]
- Ozogul, F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogens. Int. J. Food Sci. Technol. 2011, 46, 478–484. [Google Scholar] [CrossRef]
- Shishov, V.A.; Kirovskaia, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl. Biokhim. Mikrobiol. 2009, 45, 550–554. (In Russian) [Google Scholar] [PubMed]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [Green Version]
- Tsavkelova, E.A.; Botvinko, I.V.; Kudrin, V.S.; Oleskin, A.V. Detection of neurotransmitter amines in microorganisms with the use of high performance liquid chromatography. Dokl. Biochem. 2000, 372, 115–117. [Google Scholar] [PubMed]
- Taj, A.; Jamil, N. Implications of Neuroinvasive Bacterial Peptides on Rodents Behaviour and Neurotransmission. Pathogens 2017, 6, 27. [Google Scholar]
- Taj, A.; Jamil, N. Detection of meningococcal meningitis in cerebrospinal fluid of patients with neurological disorders in government hospitals of Karachi. J. Pak. Med. Assoc. 2016, 66, 1418–1421. [Google Scholar]
| Serial Number | Bacteria | Media Used for Filter- Sterilized Cell-Free Cultural Broths (SCFBs) Preparation |
|---|---|---|
| 01 | Cl. tetani | Nutrient Broth |
| Luria Basal Broth | ||
| Brain Heart Infusion Broth | ||
| RPMI 1640 with human serum | ||
| 02 | L. monocytogenes | Nutrient Broth |
| Luria Basal Broth | ||
| Brain Heart Infusion Broth | ||
| RPMI 1640 with human serum | ||
| 03 | B. cereus | Nutrient Broth |
| Luria Basal Broth | ||
| Brain Heart Infusion Broth | ||
| RPMI 1640 with human serum | ||
| 04 | N. meningitides | Nutrient Broth |
| Luria Basal Broth | ||
| Brain Heart Infusion Broth | ||
| RPMI 1640 with human serum | ||
| 05 | Control | Blank Nutrient Broth |
| Blank Luria Basal Broth | ||
| Blank Brain Heart Infusion Broth | ||
| Blank RPMI 1640 with human serum |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taj, A.; Jamil, N. Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria. Biomolecules 2018, 8, 10. https://doi.org/10.3390/biom8010010
Taj A, Jamil N. Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria. Biomolecules. 2018; 8(1):10. https://doi.org/10.3390/biom8010010
Chicago/Turabian StyleTaj, Aneela, and Nusrat Jamil. 2018. "Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria" Biomolecules 8, no. 1: 10. https://doi.org/10.3390/biom8010010




