Pinocembrin–Lecithin Complex: Characterization, Solubilization, and Antioxidant Activities
Abstract
:1. Introduction
2. Results
2.1. Solubility Analysis
2.2. Ultraviolet and Infrared Analysis
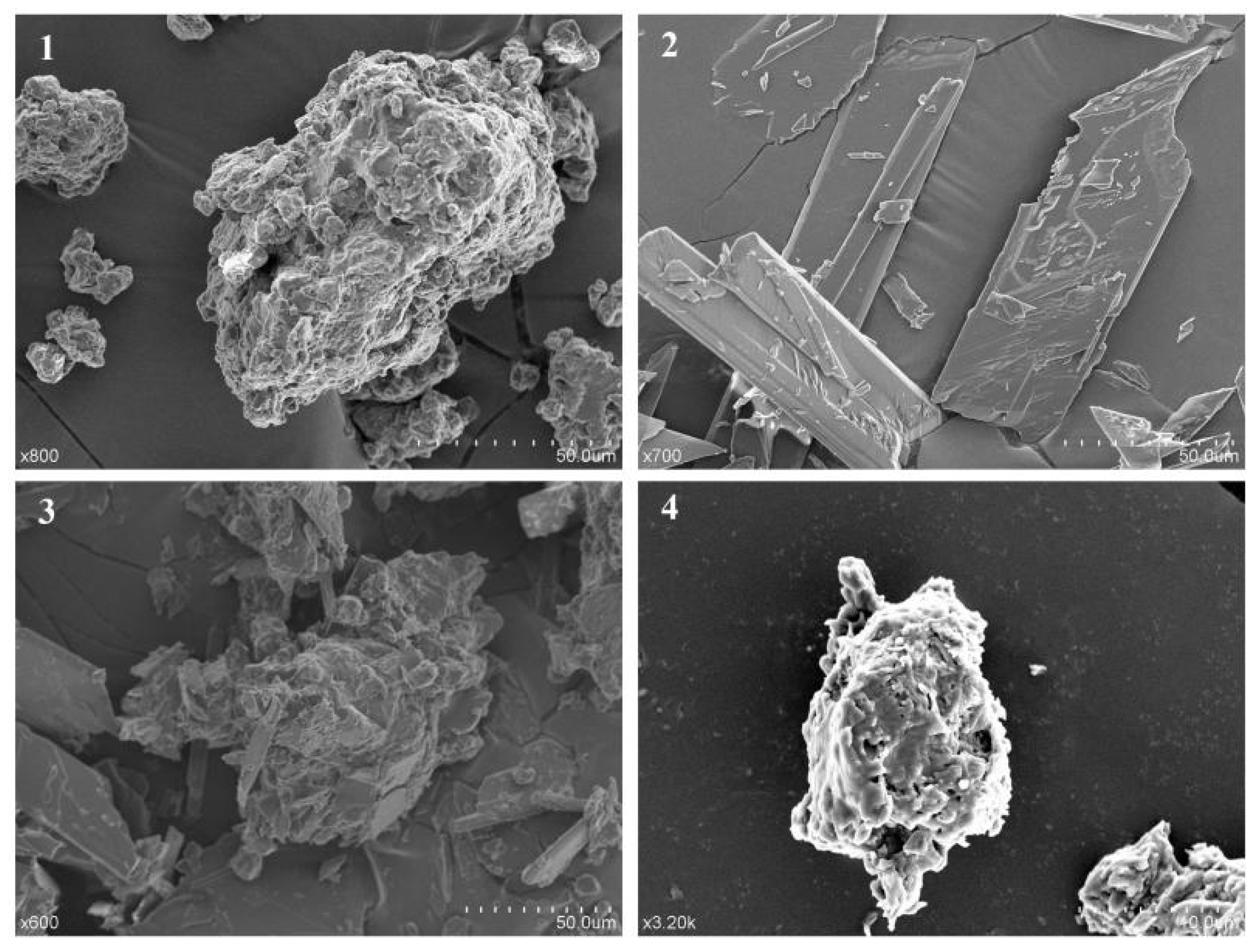
2.3. Scanning Electron Microscopy Analysis
2.4. Differential Scanning Calorimetry Analysis
2.5. Antioxidant Activities In Vitro
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of Pinocembrin–Lecithin Complex
4.3. Preparation of Physical Mixture of Pinocembrin and Lecithin
4.4. Determination of Solubility in Water and n-octane
4.5. Ultraviolet-Vis Spectroscopy
4.6. Fourier-Transform Infrared Spectroscopy
4.7. Scanning Electron Microscopy
4.8. Differential Scanning Calorimetry
4.9. Determination of Antioxidant Activity In Vitro
4.9.1. 2,2′-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity
4.9.2. 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) Radical Cation Scavenging Activity
4.9.3. Hydroxyl Radical Scavenging Activity
4.9.4. Superoxide-Anion Radical Scavenging Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lan, X.; Wang, W.Z.; Li, Q.; Wang, J. The natural flavonoid pinocembrin: Molecular targets and potential therapeutic applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Armentano, B.; Polerà, N.; Carullo, G.; Loizzo, M.R.; Bonesi, M.; Cappello, M.S.; Capobianco, L.; Tundis, R. From vegetable waste to new agents for potential health applications: Antioxidant properties and effects of extracts, fractions and pinocembrin from Glycyrrhiza glabra L. aerial parts on viability of five human cancer cell lines. J. Agric. Food Chem. 2017, 65, 7944–7954. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, Q.Y.; Tong, Y.F.; Chen, F.; Qi, Y.; Duan, Y.B.; Wu, S. Synthesis and enantiomeric resolution of (±)-pinocembrin. J. Asian Nat. Prod. Res. 2008, 10, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, H.J.; Ahn, J.H. Biosynthesis of pinocembrin from glucose using engineered Escherichia coli. J. Microbiol. Biotechnol. 2014, 24, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Ferreira, G.C.; Brasil, F.B.; Peres, A. Pinocembrin suppresses H2O2-induced mitochondrial dysfunction by a mechanism dependent on the Nrf2/HO-1 axis in SH-SY5Y cells. Mol. Neurobiol. 2018, 55, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gutiérrez, S.L.; Nieto-Camacho, A.; Castillo-Arellano, J.I.; Huerta-Salazar, E.; Hernández-Pasteur, G.; Silva-Miranda, M.; Argüello-Nájera, O.; Sepúlveda-Robles, O.; Espitia, C.I.; Reyes-Chilpa, R. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ɛ-caprolactone derivative. Molecules 2018, 23, 334. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Frattaruolo, L.; Carullo, G.; Armentano, B.; Badolato, M.; Loizzo, M.R.; Aiello, F.; Cappello, A.R. An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Nat. Prod. Res. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Fang, L.H.; Li, Y.J.; Du, G.H. Endothelium-dependent and-independent relaxation induced by pinocembrin in rat aortic rings. Vasc. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 830616–830618. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Sun, J. Pinocembrin alleviates cognition deficits by inhibiting inflammation in diabetic mice. J. Neuroimmunol. 2018, 314, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, R.; Zhu, S.Y.; Du, G.H. Acute neurovascular unit protective action of pinocembrin against permanent cerebral ischemia in rats. J. Asian Nat. Prod. Res. 2008, 10, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Guang, H.M.; Du, G.H. Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2006, 542, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.X.; Rui, L.; Gao, M.; Zhao, G.; Wu, S.; Wu, C.F.; Du, G.H. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci. Lett. 2013, 546, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.R.; Rui, L.; Gao, M.; Wang, Y.H.; Yu, X.Y.; Xuan, Z.H.; Sun, J.L.; Yang, F.; Wu, C.F.; Du, G.H. Pinocembrin attenuates blood–brain barrier injury induced by global cerebral ischemia–reperfusion in rats. Brain Res. 2013, 546, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Gao, G.Y.; Sun, T.H.; Zhao, X.; Hu, X.; Yan, J.L.; Peng, Y.Y.; Shi, A.X.; Li, Y.; Xue, W.; et al. Determination of pinocembrin in human plasma by solid-phase extraction and LC/MS/ MS: Application to pharmacokinetic studies. Biomed. Chromatogr. 2014, 28, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nair, M.; Hema, P.S.; Mohan, J.; Santhoshkumar, T.R. Pinocembrin triggers bax-dependent mitochondrial apoptosis in colon cancer cells. Mol. Carcinogen. 2007, 46, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Napal, G.N.D.; Carpinella, M.C.; Palacios, S.M. Antifeedant activity of ethanolic extract from Flourensia oolepis and isolation of pinocembrin as its active principle compound. Bioresour. Technol. 2007, 46, 231–241. [Google Scholar]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.Y.; Ma, S.X.; Cheng, H.L.; Yang, L.J.; Chen, W.; Yin, Y.Q.; Shi, Y.M. Host–guest interaction between pinocembrin and cyclodextrins: Characterization, solubilization and stability. J. Mol. Struct. 2014, 1058, 181–188. [Google Scholar] [CrossRef]
- Cautelaa, J.; Giustinia, M.; Pavela, N.V.; Palazzob, G.; Galantini, L. Wormlike reverse micelles in lecithin/bile salt/water mixtures in oil. Colloids Surf. A 2017, 532, 411–419. [Google Scholar] [CrossRef]
- Liu, B.G.; Du, J.Q.; Zeng, J.; Chen, C.G.; Niu, S.Y. Characterization and antioxidant activity of dihydromyricetin-lecithin complex. Eur. Food Res. Technol. 2009, 230, 325–331. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zhang, M.Y.; Liu, Z.Y.; Zhang, Y.Y.; Gu, L.Q.; Hu, G.S.; Chen, X.H.; Jia, J.M. Development of quercetin-phospholipid complex to improve the bioavailability and protection effects against carbon tetrachloride-induced hepatotoxicity in SD rats. Fitoterapia 2016, 113, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Luo, Z.G.; Xiao, Z.G. Preparation, characterization, and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Dong, X.D.; Ji, H.Y. Research on characteristics, antioxidant and antitumor activities of dihydroquercetin and its complexes. Molecules 2017, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.G.; Zhang, Q.; Liu, K.F.; Wu, Q.X. Physicochemical properties and lipophilicity of polydatin-lecithin complex. Trop. J. Pharm. Res. 2015, 14, 2217–2222. [Google Scholar] [CrossRef]
- Brad, K.; Zhang, Y.; Gao, J. Extraction and purification of formonometin from Trifolium pratense L: Physicochemical properties of its complex with lecithin. Trop. J. Pharm. Res. 2017, 16, 1757–1763. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Wang, J.T.; Liu, X.L.; Sun, Y.; Zheng, Y.J.; Wang, X.J.; Liu, Y. Effect of hydrolysis time on the physicochemical and functional properties of corn glutelin by Protamex hydrolysis. Food Chem. 2015, 172, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Oke, M.; Jacob, J.K.; Paliyath, G. Effect of soy lecithin in enhancing fruit juice/sauce quality. Food Res. Int. 2010, 43, 232–240. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.J.; Han, J.J.; Shim, I.; Her, S.; Lee, Y.S.; Lee, H.J.; Hahm, D.H. Oral administration of squid lecithin-transphosphatidylated phosphatidylserine improves memory impairment in aged rats. Prog. Neuro-Psychophysiol. 2015, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lalhminghlui, K.; Jagetia, G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA 2018, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Reejamol, M.K.; Swaminathan, M. Estimation of lipid peroxides and antioxidants in smokers and non-smokers with periodontitis. King Saud Univ. J. Dent. Sci. 2013, 4, 53–56. [Google Scholar] [CrossRef]
- Siahpoosh, A.; Alikhani, K. Evaluation of antioxidant capacity and free radical scavenging activities of pepsin extract of cuttlefish (Sepia pharaonis) from Persian Gulf. Indian J. Tradit. Knowl. 2016, 15, 604–610. [Google Scholar]
- Jasprica, I.; Bojic, M.; Mornar, A.; Besic, E.; Bucan, K.; Medic-Saric, M. Evaluation of antioxidative activity of Croatian propolis samples using DPPH· and ABTS+ stable free radical assays. Molecules 2007, 12, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Laskar, R.A.; SK, I.; Roy, N.; Begum, N.A. Antioxidant activity of Indian propolis and its chemical constituents. Food Chem. 2010, 122, 233–237. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Mendoza-Wilson, A.M.; Balandrán-Quintana, R.R.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Study of the molecular structure and chemical reactivity of pinocembrin by DFT calculations. Comput. Theor. Chem. 2015, 1058, 21–27. [Google Scholar] [CrossRef]
- Guan, M.; Ren, X.J.; Li, Y.P.; Kulisong, H. Preparation and physico-chemical properties of quercetin-phosphatidylcholine Compound. Food Sci. 2010, 31, 51–53. [Google Scholar]
- Kang, C.C.; Hao, L.M.; Zhang, L.M.; Zheng, Z.Q.; Yang, Y.W. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar]
- Liu, Z.J.; Li, G.; Long, C.; Xu, J.; Cen, J.R.; Yang, X.B. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Chi, Y.J.; Cheng, Y.; Zhao, Y. Physicochemical properties, in vitro digestibility and antioxidant activity of dry-heated egg white protein. Food Chem. 2018, 246, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Feng, H.B.; Yu, Y.; Sun, M.X.; Liu, Y.R.; Li, T.Z.; Sun, X.; Liu, S.J.; Sun, M.D. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr. Polym. 2017, 157, 629–636. [Google Scholar] [CrossRef] [PubMed]






| No. | Characterization Methods |
|---|---|
| 1 | Ultraviolet spectrum (UV) |
| 2 | Fourier transform infrared spectroscopy (FTIR) |
| 3 | Scanning electron microscope (SEM) |
| 4 | Differential scanning calorimetry (DSC) |
| Solvent | Apparent Solubility (μg/mL) | ||
|---|---|---|---|
| Pinocembrin | Physical Mixture | Complex | |
| Water | 48.33 | 178.33 | 265.00 |
| n-Octane | 65.24 | 84.29 | 165.24 |
| Antioxidant Test | Best Fitting Function | Determination Coefficient (R2) |
|---|---|---|
| DPPH | Y = 0.0264X + 0.2801 | 0.9266 |
| ABTS | Y = 0.0417X + 0.0440 | 0.9906 |
| Hydroxyl | Y = 0.0754Ln(X) + 0.7120 | 0.9236 |
| Superoxide-anion | Y = 0.0379X + 0.4076 | 0.9839 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, X.; Chen, X.-Y.; Ji, H.-Y.; Zhang, Y.; Liu, A.-J. Pinocembrin–Lecithin Complex: Characterization, Solubilization, and Antioxidant Activities. Biomolecules 2018, 8, 41. https://doi.org/10.3390/biom8020041
Yang X, Wang X, Chen X-Y, Ji H-Y, Zhang Y, Liu A-J. Pinocembrin–Lecithin Complex: Characterization, Solubilization, and Antioxidant Activities. Biomolecules. 2018; 8(2):41. https://doi.org/10.3390/biom8020041
Chicago/Turabian StyleYang, Xu, Xin Wang, Xiao-Yu Chen, Hai-Yu Ji, Yan Zhang, and An-Jun Liu. 2018. "Pinocembrin–Lecithin Complex: Characterization, Solubilization, and Antioxidant Activities" Biomolecules 8, no. 2: 41. https://doi.org/10.3390/biom8020041