4.1. Human Vulnerability and Mosquito Vector Abundance
To assess the sensitivity and accuracy of the dasymetric mapping techniques, the raster surface of vulnerability can be compared to the data mapped by block groups. The dasymetric map was expected to provide a more spatially precise representation of population vulnerability as compared to the Census block group choropleths. Indeed, the block groups show few distinct patterns of vulnerability across Chesapeake (
Figure 4). In general, the block groups indicate vulnerability mapped at a coarse scale. Regions of high vulnerability are dispersed and highly concentrated at various locations throughout Chesapeake in block groups, primarily highlighting the urbanized northern and suburban central Chesapeake (
Figure 2). Land cover data corroborate this gradient between northern and central, suburban Chesapeake (
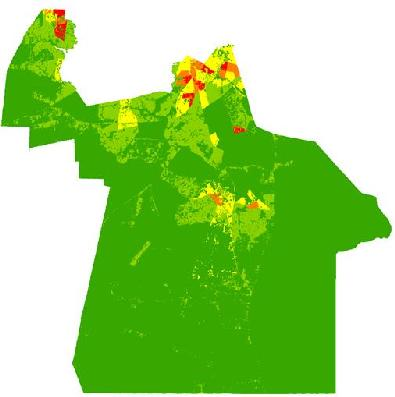
Figure 3). However, compared to the block group map, the dasymetric map shows a more detailed and fine-scale spatial representation of vulnerability (
Figure 7). Again, the highly vulnerable regions are concentrated in the northern portion of the city, but a greater fine-scale pattern and interspersion representing variations are found along arterial transportation routes, major suburban developments, and extensive creek and low floodplain areas. This pattern is mainly attributed to the clustering of vulnerable locations such as schools and daycare centers and surrounding neighborhoods in northern Chesapeake. Northern, urban Chesapeake is more developed than other regions of the city and consequently has a greater population density.
Figure 7.
Spatial overlay used to predict potential exposure to ephemeral species for June (a–c). The exposure in June (c) is the product of (a) ephemeral species abundance for that month; and (b) the dasymetric surface of vulnerable population in quantiles.
Figure 7.
Spatial overlay used to predict potential exposure to ephemeral species for June (a–c). The exposure in June (c) is the product of (a) ephemeral species abundance for that month; and (b) the dasymetric surface of vulnerable population in quantiles.
Two finer scale patterns of vulnerability are evident across the city. First, regions covered in water or rural areas are populated with a smaller number of vulnerable people. This was expected since these regions are less developed and contain lower disease susceptible populations. Mosquito control personnel interpret the fine-scale patterns of mosquito abundance, vulnerable populations, and composite exposure to assist operational surveillance and abatement activities. In some cases, the proximity of high abundance and high human populations may be amenable to spraying from trucks. In other cases where risk is low to moderate, less frequent spraying or larvicide applications are typically pursued. Second, the high risk indicated in the Western Branch area of
Figure 7c has typically spurred routine seasonal spraying, with particular attention to neighborhoods adjoining tidal creeks and the zone between this part of the city and the Great Dismal Swamp. High to very high risk is also noted in the north-central area of Chesapeake, the most urbanized area of the city adjoining the City of Norfolk. Although ephemeral species abundances in the month of June are low, the total population and areal density of human population is extremely high (much higher than the density in the western and southern suburbs.)
Public health and mosquito control specialists treat highly urbanized and dense population centers slightly differently from suburban populations. In this instance, the urbanized areas have permanent surveillance operations in light trapping and inspection of urban ditches, culverts, storm drains, and creeks prevalent in this area. In contrast, suburban surveillance typically comprises fewer and wider spread light traps and the use of periodic roving trapping efforts, as well as a greater dependence on nuisance reports and abatement requests to cover the much greater suburban area. The indices developed here are thus trending toward a persistently higher risk in the more populous urban centers, with more changeable risk in the suburban and rural zones. However, this index could indicate bias that could be construed as a false positive in low mosquito abundance periods or a lack of sensitivity during a rare high abundance bloom event. This concern is somewhat muted in the instance of northern Chesapeake, which also has not only the densest human population, but also a concentration of elderly. Such population densities can have profound effects on potential disease exposure [
3].
Other studies have used dasymetric techniques to map population density and have obtained similar results. Many studies have found that dasymetric mapping provides a more accurate representation of population distribution compared to conventional mapping techniques. One study used dasymetric mapping to map population density in five counties within southeast Pennsylvania [
16]. Using areal weighting, block group demographic data was mapped according to three classes of urban land cover. The dasymetric map raster was compared to vector population data within block groups. In a similar finding [
16], it was observed that within the urban core areas, the choroplethic and dasymetric maps did not differ significantly. However, in areas with parks and cemeteries, the dasymetric map was significantly more detailed. Similar methods were also used to map the San Francisco Bay population by land cover type [
13], but rather than using a three-tier classification system, that study used four classes of land cover. Correlation coefficients indicated that the daysmetric mapping method of representing block-group population density was more accurate than the choropleth mapping method. Recent developments have also used address weighting (AW) and parcel distribution (PD) to map rural populations within three North Carolina counties with a focus on using local sources of ancillary data [
25]. These mapping techniques are modifications of the existing dasymetric mapping techniques, street weighting (SW) and limiting variable (LV) algorithms. Statistical results indicated that AW and PD were appropriate for mapping rural populations, while SW and LV methods were more effective for mapping the population within urban areas. Yet another approach uses binary dasymetric mapping methods to interpolate populations across regions, such as demonstrated in the county of Leicestershire, England [
26]. ArcGIS spatial analyst tools were used to reclassify raster pixel maps as populated or unpopulated. Weighting methods to map Census data have also focused on streets to adjust population across areal units in Los Angeles County, California [
27]. Compared to simple aerial interpolation techniques, the dasymetric surface showed a 20% increase in population accuracy. An even finer scale and 3D cadastral-based data have been applied to map the population of New York City [
14]. This method disaggregates population data at a high spatial resolution using cadastral data. For this study, residential units (RU) and residential area (RA) were aggregated to the tax lot, block group, and census tract level. The census tract values were then disaggregated to the tax lot level and then re-aggregated to predict the values within each block group. Cadastral-based Expert Dasymetric System (CEDS) derived population values are thus more finely allocated population than census values within block groups. Our results using the dasymetric mapping method corroborate these previously published techniques, highlighting the technique’s potential to illustrate spatial variation and pattern among populations as compared to choroplethic mapping.
4.2. Risk Maps of Mosquito Vector Exposure
The risk of exposure to mosquito vectors across Chesapeake from both groups of mosquitoes is shown in
Figure 8. The risk indices were scaled to show the level of risk for each month. To provide an effective visualization of the risk indices, risk values were classified into quantiles while maintaining each monthly index on the same relative scale. The actual units of risk are arbitrary and represent a relative index ranging from low to high risk. It is apparent on examining the maps that the threat of disease does not change significantly from June through August of 2003. This is due to the relatively static climate (warm and humid) conditions that persisted throughout the summer of 2003. The threat of disease transmission was also very similar between the two species groups. A longer time series or a period with more variable precipitation or weather events inducing mosquito population cycles would have elicited greater temporal variability, as documented in preceding habitat suitability and environmental modeling [
4].
In particular, northern and central Chesapeake have a high risk of exposure to mosquitoes across all summer months. These high risk areas are reflective of the dasymetric map of vulnerability (
Figure 5). The high risk regions coincide with regions predicted to have a relatively high vulnerability to disease infection. Despite the elevated risk in these regions, absolute mosquito abundance was predicted to be low in northern Chesapeake (
Figure 6). The low number of mosquitoes may be due to the high level of urbanization and less prevalent suitable mosquito habitats or human adaptations to mitigate their abundance (especially reducing container breeding sites.) Although mosquito abundance was estimated to be low in northern Chesapeake, disease transmission levels may nonetheless be relatively high due the greater level of contact with humans in these developed areas. Indeed, cities may generally exacerbate disease transmission by bringing a large number of people into intimate contact with mosquito vectors [
28]. Even with a supply of clean water, adequate shelter, and access to health care, a high population density would greatly facilitate the spread of transmissible disease. Despite the low number of swamps and floodplains in these developed regions, mosquitoes may still come into close proximity of humans by other sources of standing water.
Ae. Vexans and
P. columbiae are typically found in flood plains where rivers overflow their banks, but significant numbers can be produced from virtually any area where water accumulates on an intermittent basis [
29]. These mosquitoes may breed in temporary water sources such as drainage ditches and tire ruts, which are extensive in low-lying coastal plains such as Chesapeake.
Figure 8.
Monthly indices representing the risk of exposure to mosquito vectors for C. melanura and ephemeral species (values classified using natural breaks and fixed class breaks through time).
Figure 8.
Monthly indices representing the risk of exposure to mosquito vectors for C. melanura and ephemeral species (values classified using natural breaks and fixed class breaks through time).
Prior to estimating the vulnerable population, it was expected that regions of high mosquito abundance would coincide with regions of high risk. However, many regions observed to have a high number of mosquitoes such as southern Chesapeake were predicted to have a lower relative risk of exposure. The area surrounding the Great Dismal Swamp, for instance, was observed, as expected, to exhibit high mosquito abundance. Nonetheless, this area exhibits a relatively low disease risk owing to low resident populations. Recreational activities in such swamplands should be considered a significant caveat, as recreational activity spaces (boaters, hikers, bird-watchers, and paddlers) are not characterized in this modeling. Such mosquito-abundant areas are mostly undeveloped and therefore capable of supporting mosquito populations. The climatic variables such as temperature and rainfall were calculated to have a greater effect on the mosquito trap counts in western Chesapeake compared to the rest of the city [
4]. These western areas are covered in either wetlands or croplands. The immature stages of mosquitoes require water and therefore are often found in wetlands [
30]. The runoff from croplands can also support mosquito presence [
31]. Despite these favorable conditions, many of these rural areas have a low human population density and exhibit less total population at risk of disease exposure. Hence, these results highlight that although mosquitoes can transmit disease to humans, without exposure to the pathogen, the likelihood of disease transmission is decreased.
In order to compare the variation in risk from June through August, the differences in the monthly risk indices were calculated. Each month’s risk values were subtracted from the following month’s values to calculate the difference over each month. The results of this calculation are shown in
Figure 9 It is clear that the most significant changes in risk occurred in northern Chesapeake. The remaining portion of the city did not show a significant change in risk over the three-month period. From June through July, the risk of exposure from
C. melanura increased drastically in northern Chesapeake. Conversely, the risk values for the ephemeral species decreased over this time period. Modest and opposite trends are evident from July through August. For
C. melanura, risk decreased in northern Chesapeake, while the risk values for the ephemeral species increased. Overall, there was little variation in risk between June and August.
C. melanura showed minimal change, while the ephemeral species showed almost no change in risk values.
Validation of trends in abundance and population exposure is a challenging proposition in the instance of rare disease and a complex mosaic landscape such as Chesapeake. No human cases of West Nile Virus or EEE were reported in Chesapeake during the season studied. However, The Virginia Department of Public Health later reported (2004) [
32] that 20 pools of mosquitoes were found to be positive for
C. melanura infected with EEE, while 10 pools were positive for West Nile. Additional surveillance and nuisance abatement request data were provided by the City of Chesapeake and offer limited affirmation of the risk mapped. While not validated, some studies have found that animal cases can be accurate indicators of human disease prevalence [
33].
Figure 10 depicts a composite of available information, including total vector species trap counts over the season, dead bird reports (a majority of crows), and cases of Eastern Equine Encephalitis (EEE) among horse farms. Although an exhaustive map of horse farms was not available, EEE incidents are coincident with the highest vector abundance data. Further, the abatement service requests and pattern of dead birds collected (initially many of these were tested for WNV), also spatially conforms to the dasymetric population map and human exposure risk.
Figure 9 also illustrates the dilemma of solely relying upon discovery of surveillance tools such as dead birds or resident reported abatement requests, as these patterns reflect the abundance of humans and not necessarily the abundance of mosquito vector species.
It is difficult to validate such risk models due to the low number of disease cases and underreporting. Disease surveillance data do not record any actual human cases of WNV or EEE occurring in Chesapeake in 2003. However, in 2003, 20 pools of mosquitoes were found to test positive for
C. melanura infected with EEE, while 10 pools were infected with
C. melanura positive for WNV [
32]. These corroborating data, combined with the usual under-reported and underdiagnosed nature of WNV, suggest that
C. melanura mosquitoes were an emerging health threat to Chesapeake in 2003. In addition, bird, equine, and sentinel flock cases of WNV and EEE were reported for this year. These cases may not provide a validation of the model since the model estimates risk to humans, rather than animal hosts, but they do lend some credence and affirmation to the risk mapping approach. A plausible spatial relationship can be seen between EEE cases and
C. melanura abundance. In 2003, the majority of EEE cases occurred in western Chesapeake, surrounding the Dismal Swamp, and our abundance mapping of the EEE vector
C. melanura was also predicted to be high in western Chesapeake from June through August. The high predicted abundance of mosquito vectors, observations in traps, and resident abatement requests also lend credence to inference of higher risk exposure.
Figure 9.
Change in monthly exposure risk values through the summer derived by calculating the difference in the risk indices shown in
Figure 8.
Figure 9.
Change in monthly exposure risk values through the summer derived by calculating the difference in the risk indices shown in
Figure 8.
Dasymetric mapping is shown in this investigation to be effective at representing population data as compared to mapping by areal choropleths such as Census block groups for population vulnerability and exposure assessment. The choroplethic map of vulnerability in Census units (
Figure 9) gives the impression that the population is homogenously distributed within each block group, yet proportions of each block group are extensively uninhabited. The dasymetric map on the other hand (
Figure 8), shows vulnerability at a finer scale and with many localized patterns and concentrations among urban, suburban, and rural settlements. By using pixels as the areal units rather than block groups, the raster shows a continuous and variable surface of vulnerability. By integrating the “nighttime” population values from the Census data with the point-based nodes of vulnerability to produce the vulnerability index, the resulting population risk map is likely to have a higher accuracy than results generated from considering the Census data alone.
Figure 10.
Seasonal mosquito vector trap counts, enzootic disease reports (dead birds and veterinary surveillance of positive EEE horses), and public abatement service requests within Chesapeake, over Landsat TM tasseled cap wetness index image for 29 July 2002.
Figure 10.
Seasonal mosquito vector trap counts, enzootic disease reports (dead birds and veterinary surveillance of positive EEE horses), and public abatement service requests within Chesapeake, over Landsat TM tasseled cap wetness index image for 29 July 2002.
This study affirms the role of demographic data and spatial analysis for mapping and predicting the risk of mosquito vector exposure. Due to the many factors affecting disease transmission, vector abundance and exposure may not be positively correlated with general areal risk of exposure. Although exposure to mosquitoes may be high in some regions across Chesapeake, disease transmission may not necessarily be high due to the low population density in these regions. This study supports the importance of incorporating human data in addition to climate data when predicting risk and may be used as a guide to strategically allocate surveillance and control activities over space. Ancillary enzootic data from dead birds and EEE cases as well as nuisance abatement requests support the inference of geographic gradients of vector abundance and possible exposure risk. Future studies may consider incorporating other human factors such as behavior, disease resistance, and socioeconomic data. According to Daily and Ehrlich [
28], “human demographic factors are key variables in epidemiology, influencing the rate at which a population is invaded by new parasites, their chances of becoming established, the rate of their spread, the evolution of their virulence, and the capacity of human social structures (and other cultural traits) to coevolve in defense”. The lack of fine-scale human population data was considered a limiting factor in the initial development of dasymetric maps, yet future studies should more carefully assess the role of scale in both vector abundance as well as susceptible populations.
Further considerations of disease transmission factors could improve similar research. For instance, it is possible that scale issues of human settlements and activity species and the vectors could be individually better understood and that fine-scale data (of either factor) may not add additional value to surveillance or control. Other factors, which influence vector exposure and disease transmission, may also be incorporated into future studies. Deforestation, for instance, can lead to environmental changes conducive to mosquito survival [
30]. Vector competence, for example, could also be used to help predict the rate of transmission, and studies have begun to explore the relationship between West Nile virus, climate change [
34], and specific vector competence of
Culiseta incidens and
Culex thriambus [
35] and the variation in vector competence of
Culex pipens across California [
36]. Further, climate change, particularly extreme heat, rainfall, and rising sea levels, is poised to alter the habitats, suitability, and potential human settlement patterns in low-lying areas such as Chesapeake.
The geospatial methods used in this study may be beneficial to other cities afflicted by vector-borne diseases and extreme weather events. In rich and poor nations alike, cities are afflicted by a lack of control over disease reservoirs and vectors [
28]. Predicting where mosquito-borne diseases pose the greatest risk to human health could improve spatial precision of mosquito control efforts and thereby reduce costs and impacts of broader insecticide use. GIS has been demonstrated as highly useful in emergency operational assessment of control methods, such as estimating areal needs for aerial or ground-based spraying, including distance and drive-times [
37].