Effect of the Long-Term Mean and the Temporal Stability of Water-Energy Dynamics on China’s Terrestrial Species Richness
Abstract
:1. Introduction
2. Materials and Methods
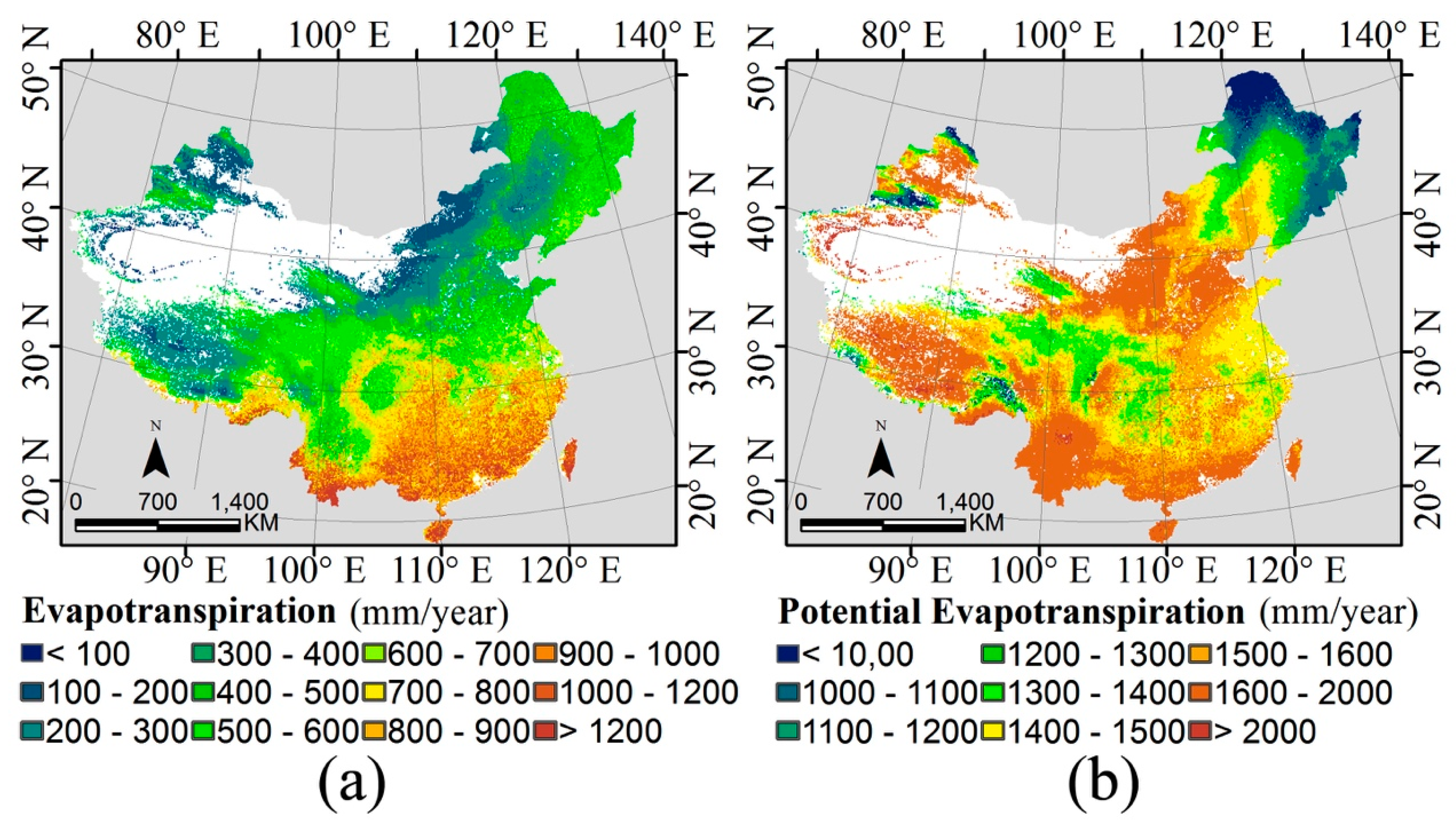
2.1. Water-Energy Dynamics and Remote Sensing Datasets
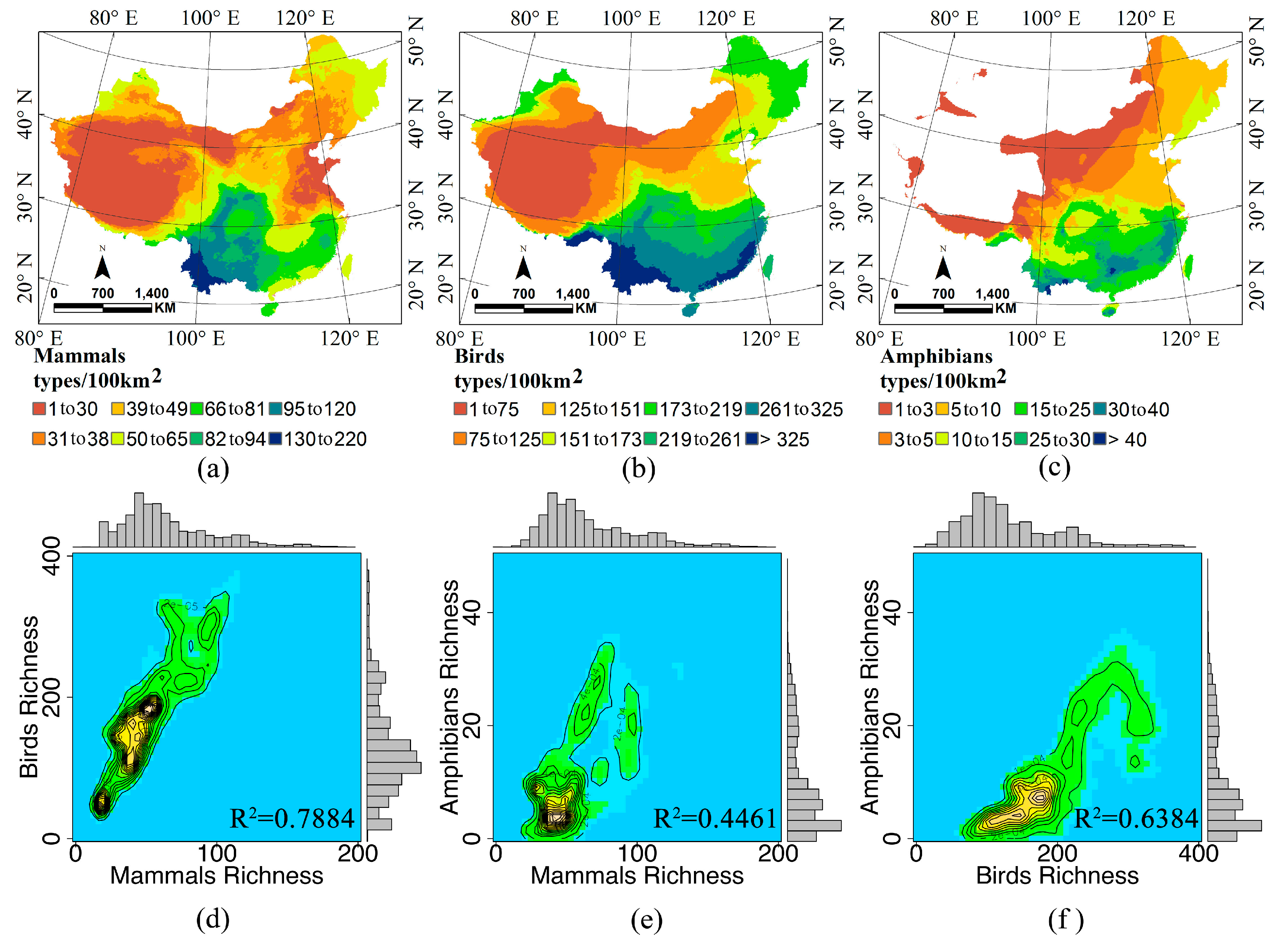
2.2. Species Richness and Remote Sensing Datasets
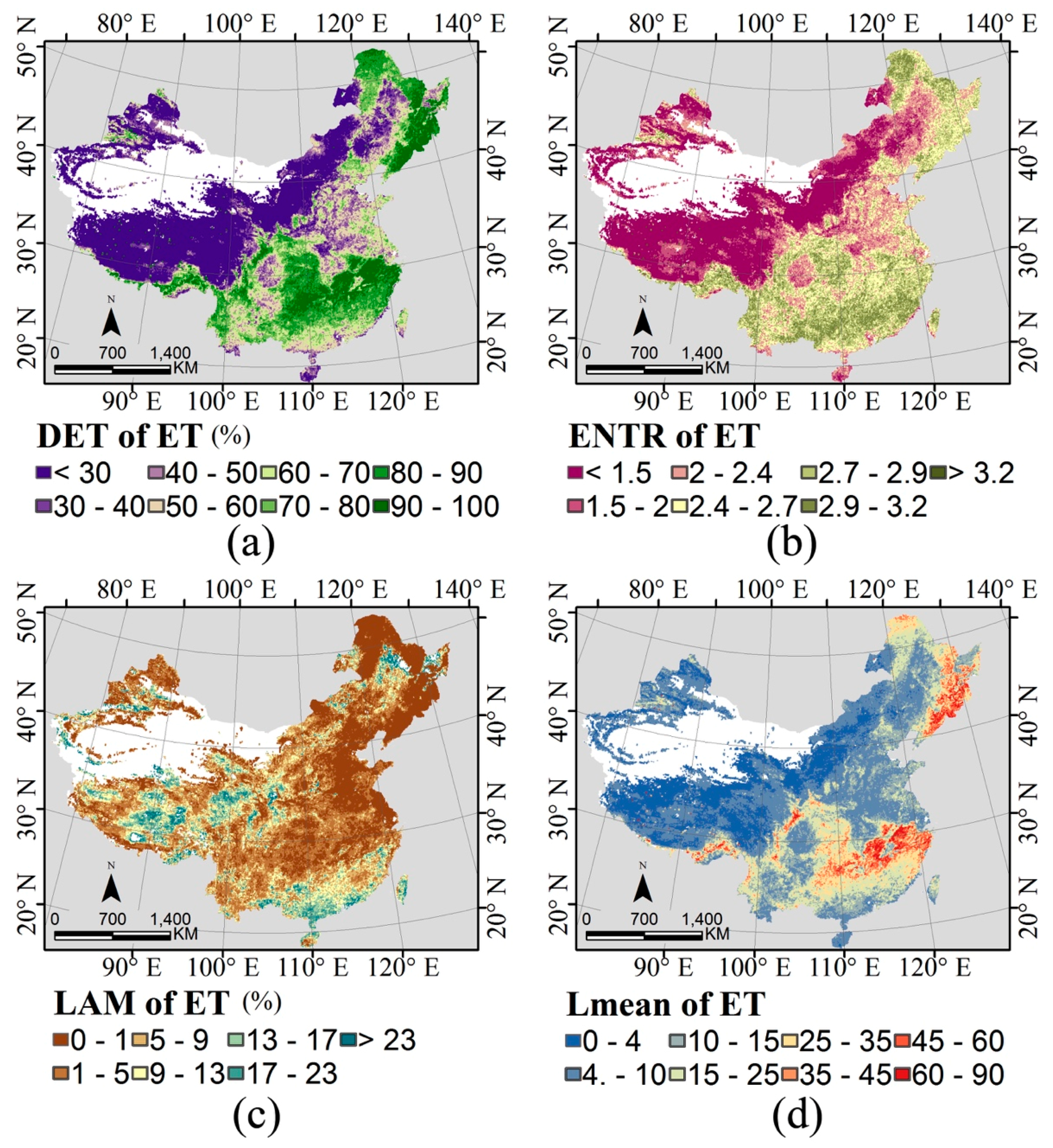
2.3. Indices of Temporal Stability
2.4. Tendency Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Water-Energy Dynamics and Species Richness
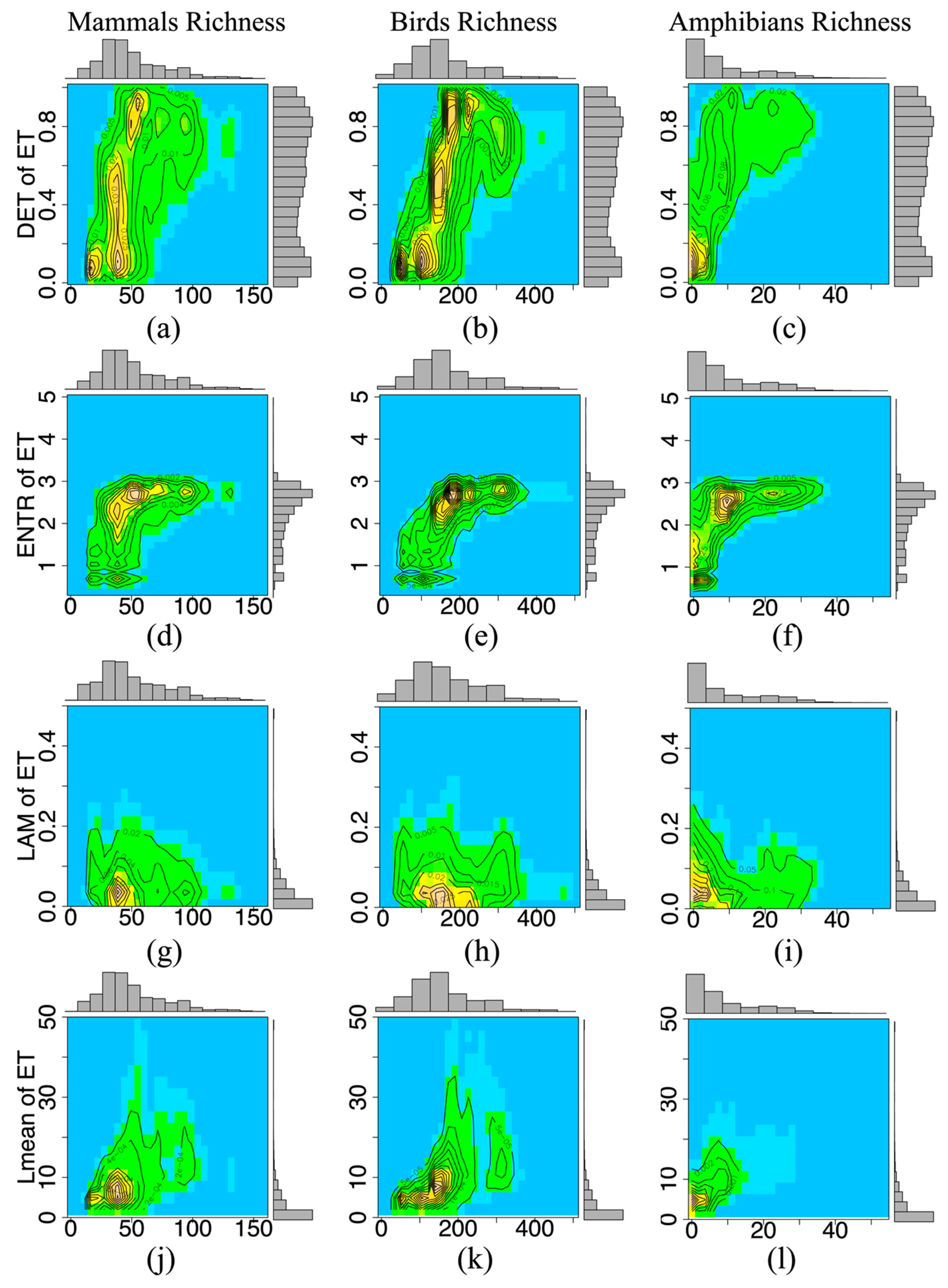
3.2. Temporal Stability and Species Richness
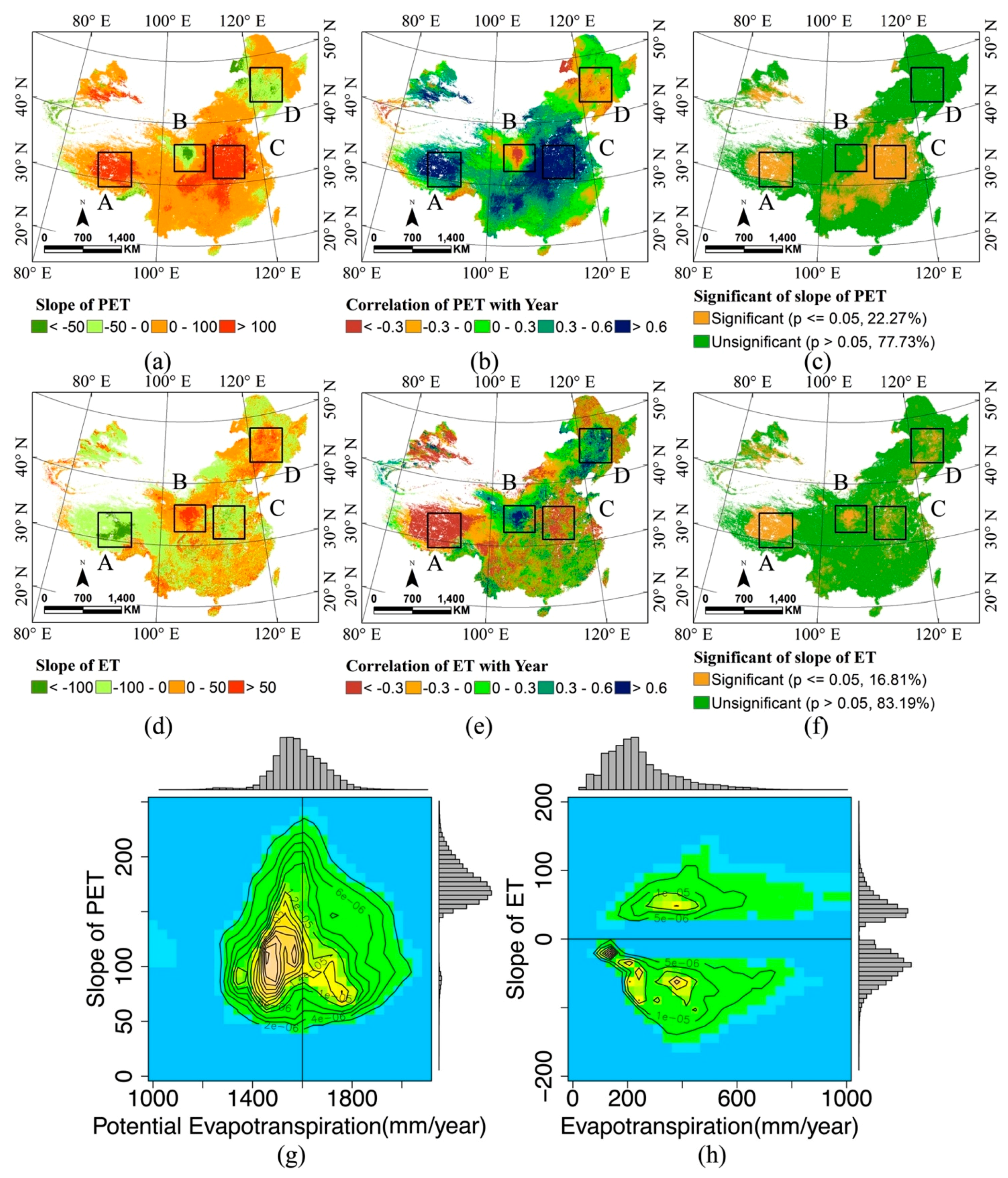
3.3. Tendency and Change
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mantyka-pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Case, M.J.; Lawler, J.J.; Tomasevic, J.A. Relative sensitivity to climate change of species in northwestern North America. Biol. Conserv. 2015, 187, 127–133. [Google Scholar] [CrossRef]
- Fritz, S.A.; Bininda-Emonds, O.R.; Purvis, A. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 2009, 12, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.-F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Zhang, K.; Kimball, J.S.; Mu, Q.; Jones, L.A.; Goetz, S.J.; Running, S.W. Satellite based analysis of Northern ET trends and associated changes in the regional water balance from 1983 to 2005. J. Hydrol. 2009, 379, 92–110. [Google Scholar] [CrossRef]
- O’Brien, E.M. Biological relativity to water–energy dynamics. J. Biogeogr. 2006, 33, 1868–1888. [Google Scholar] [CrossRef]
- O’Brien, E. Water-energy dynamics, climate, and prediction of woody plant species richness: An interim general model. J. Biogeogr. 1998, 25, 379–398. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D.; Cusens, J.; McBride, P.D.; Malhi, Y.; Whittaker, R.J. Latitude, productivity and species richness. Glob. Ecol. Biogeogr. 2015, 24, 107–117. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Porter, E.E. Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Glob. Ecol. Biogeogr. 2003, 12, 475–481. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Belmontes, J.A. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecol. 2005, 28, 65–70. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Nogués-Bravo, D.; Araújo, M.B. Geographical gradients of species richness: A test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob. Ecol. Biogeogr. 2007, 16, 76–89. [Google Scholar] [CrossRef]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V.; Field, R.; Guégan, J.F.; Hawkins, B.A.; Kaufman, D.M.; Kerr, J.T.; Oberdorff, T.; O’Brien, E. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Proulx, R.; Parrott, L.; Fahrig, L.; Currie, D.J. Long time-scale recurrences in ecology: Detecting relationships between climate dynamics and biodiversity along a latitudinal gradient. In Recurrence Quantification Analysis; Springer: Berlin, Germany, 2015; pp. 335–347. [Google Scholar]
- Bini, L.M.; Diniz-Filho, J.A.F.; Hawkins, B.A. Macroecological explanations for differences in species richness gradients: A canonical analysis of South American birds. J. Biogeogr. 2004, 31, 1819–1827. [Google Scholar] [CrossRef]
- Alemu, H.; Senay, G.B.; Kaptue, A.T.; Kovalskyy, V. Evapotranspiration variability and its association with vegetation dynamics in the Nile Basin, 2002–2011. Remote Sens. 2014, 6, 5885–5908. [Google Scholar] [CrossRef]
- Thomas, A. Spatial and temporal characteristics of potential evapotranspiration trends over China. Int. J. Climatol. 2000, 20, 381–396. [Google Scholar] [CrossRef]
- Currie, D.J. Energy and large-scale patterns of animal-and plant-species richness. Am. Nat. 1991, 137, 27–49. [Google Scholar] [CrossRef]
- Ruggiero, A.; Kitzberger, T. Environmental correlates of mammal species richness in South America: Effects of spatial structure, taxonomy and geographic range. Ecography 2004, 27, 401–417. [Google Scholar] [CrossRef]
- Mu, Q.; Zhao, M.; Running, S.W. Improvements to a MODIS global terrestrial evapotranspiration algorithm. Remote Sens. Env. 2011, 115, 1781–1800. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, Q.; Liu, J. The performances of MODIS-GPP and-ET products in China and their sensitivity to input data (fPAR/LAI). Remote Sens. 2014, 7, 135–152. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, D.; Guo, S.; Guan, Y.; Fraedrich, K.; Nie, Y.; Liu, X.; Bian, X. Spatial-temporal dynamics of China’s terrestrial biodiversity: A dynamic habitat index diagnostic. Remote Sens. 2016, 8, 227. [Google Scholar] [CrossRef]
- Parrott, L. Measuring ecological complexity. Ecol. Indic. 2010, 10, 1069–1076. [Google Scholar] [CrossRef]
- Marwan, N.; Romano, M.C.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Phys. Rev. E 2002, 66, 026702. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.; Toutenburg, H.; Shalabh, H.C.; Schomaker, M. Linear Models and Generalizations. Least Squares and Alternatives, 3rd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Abarbanel, H.D.; Brown, R.; Sidorowich, J.J.; Tsimring, L.S. The analysis of observed chaotic data in physical systems. Rev. Mod. Phys. 1993, 65, 1331. [Google Scholar] [CrossRef]
- Cao, L. Practical method for determining the minimum embedding dimension of a scalar time series. Physica D 1997, 110, 43–50. [Google Scholar] [CrossRef]
- Fraser, A.M.; Swinney, H.L. Independent coordinates for strange attractors from mutual information. Phys. Rev. A 1986, 33, 1134. [Google Scholar] [CrossRef]
- Qian, H.; Kissling, W.D. Spatial scale and cross-taxon congruence of terrestrial vertebrate and vascular plant species richness in China. Ecology 2010, 91, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.T.; Packer, L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 1997, 385, 252–254. [Google Scholar] [CrossRef]
- Qian, H.; Wang, X.; Wang, S.; Li, Y. Environmental determinants of amphibian and reptile species richness in China. Ecography 2007, 30, 471–482. [Google Scholar] [CrossRef]
- Hawkins, B.A.; McCain, C.M.; Davies, T.J.; Buckley, L.B.; Anacker, B.L.; Cornell, H.V.; Damschen, E.I.; Grytnes, J.A.; Harrison, S.; Holt, R.D. Different evolutionary histories underlie congruent species richness gradients of birds and mammals. J. Biogeogr. 2012, 39, 825–841. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Peng, J.; Li, S.; Wang, Y. Nonlinear features and complexity patterns of vegetation dynamics in the transition zone of North China. Ecol. Indic. 2015, 49, 237–246. [Google Scholar] [CrossRef]
- Fjeldså, J.; Bowie, R.C.K.; Rahbek, C. The role of mountain ranges in the diversification of birds. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 249–265. [Google Scholar] [CrossRef]
- Hortal, J.; Carrascal, L.M.; Triantis, K.A.; Thébault, E.; Meiri, S.; Sfenthourakis, S. Species richness can decrease with altitude but not with habitat diversity. Proc. Natl. Acad. Sci. USA 2013, 110, 2149–2150. [Google Scholar] [CrossRef] [PubMed]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Coristine, L.E.; Kerr, J.T. Temperature-related geographical shifts among passerines: Contrasting processes along poleward and equatorward range margins. Ecol. Evol. 2015, 5, 5162–5176. [Google Scholar] [CrossRef]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Thuiller, W. Biodiversity: Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Fraedrich, K.; Sielmann, F.; Zhang, L.; Zhu, X.; Guo, S.; Guan, Y. Vegetation dynamics on the Tibetan Plateau (1982 to 2006): An attribution by eco-hydrological diagnostics. J. Clim. 2015, 4576–4584. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J.; et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Chang. Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, C.; Wu, L. Aridity pattern of Tibetan Plateau and its influential factors in 2001–2010. Adv. Clim. Chang. Res. 2012, 8, 320–326. [Google Scholar]
- Fan, L.; Lu, C.; Yang, B.; Chen, Z. Long-term trends of precipitation in the North China Plain. J. Geogr. Sci. 2012, 22, 989–1001. [Google Scholar] [CrossRef]
- Meng, S.; Fei, Y.; Zhang, Z.; Lei, T.; Qian, Y.; Li, Y. Research on spatial and temporal distribution of the precipitation infiltration amount over the past 50 years in North China Plain. Adv. Earth Sci. 2013, 8, 9. [Google Scholar]
- Uchida, E.; Xu, J.; Rozelle, S. Grain for green: Cost-effectiveness and sustainability of China’s conservation set-aside program. Land Econ. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, J.; Deng, X.; Huang, J.; Uchida, E.; Rozelle, S. Grain for green versus grain: Conflict between food security and conservation set-aside in China. World Dev. 2006, 34, 130–148. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lin, Y.; Shi, W.; Song, Y.; He, X. Balancing green and grain trade. Nat. Geosci. 2015, 8, 739–741. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, F.L.; Liu, W.Z.; Flanagan, D.C. Spatial distribution and temporal trends of extreme temperature and precipitation events on the Loess Plateau of China during 1961–2007. Quatern. Int. 2010, 226, 92–100. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, X.; Wang, M. Precipitation trends during 1961–2010 in the Loess Plateau region of China. Acta Ecol. Sin. 2011, 31, 5512–5523. [Google Scholar]
- Qi, T.; Zhang, Q.; Wang, Y.; Xiao, M.; Liu, J.; Sun, P. Spatiotemporal patterns of pan evaporation in 1960–2005 in China: Changing properties and possible causes. Sci. Geograp. Sin. 2015, 35, 1599–1606. [Google Scholar]
- Liu, J.; Kuang, W.; Zhang, Z.; Xu, X.; Qin, Y.; Ning, J.; Zhou, W.; Zhang, S.; Li, R.; Yan, C. Spatiotemporal characteristics, patterns, and causes of land-use changes in China since the late 1980s. J. Geogr. Sci. 2014, 24, 195–210. [Google Scholar] [CrossRef]
- He, T.; Shao, Q. Spatial-temporal variation of terrestrial evapotranspiration in China from 2001 to 2010 using MOD16 products. J. Geo-Inform. Sci 2014, 16, 979–988. [Google Scholar]
- Mayden, R.L. A hierarchy of species concepts: The denouement in the saga of the species problem. In Species: The Units of Biodiversity; Claridge, M.F., Dawah, H.A., Wilson, M.R., Eds.; Chapman and Hall: London, UK, 1997; Volume 54, pp. 381–423. [Google Scholar]
- Jiang, Z.; Ma, Y.; Wu, Y.; Wang, Y.; Zhou, K.; Liu, S.; Feng, Z.; Li, L. China’s Mammals Diversity and Geographic Distribution; China Science Publishing: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L. Biological identifications through DNA barcodes. Proc. R Soc. Lond B Bio. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; McCallum, I.; Schill, C.; Perger, C.; See, L.; Schepaschenko, D.; van der Velde, M.; Kraxner, F.; Obersteiner, M. Geo-wiki: An online platform for improving global land cover. Environ. Model. Softw. 2012, 31, 110–123. [Google Scholar] [CrossRef]
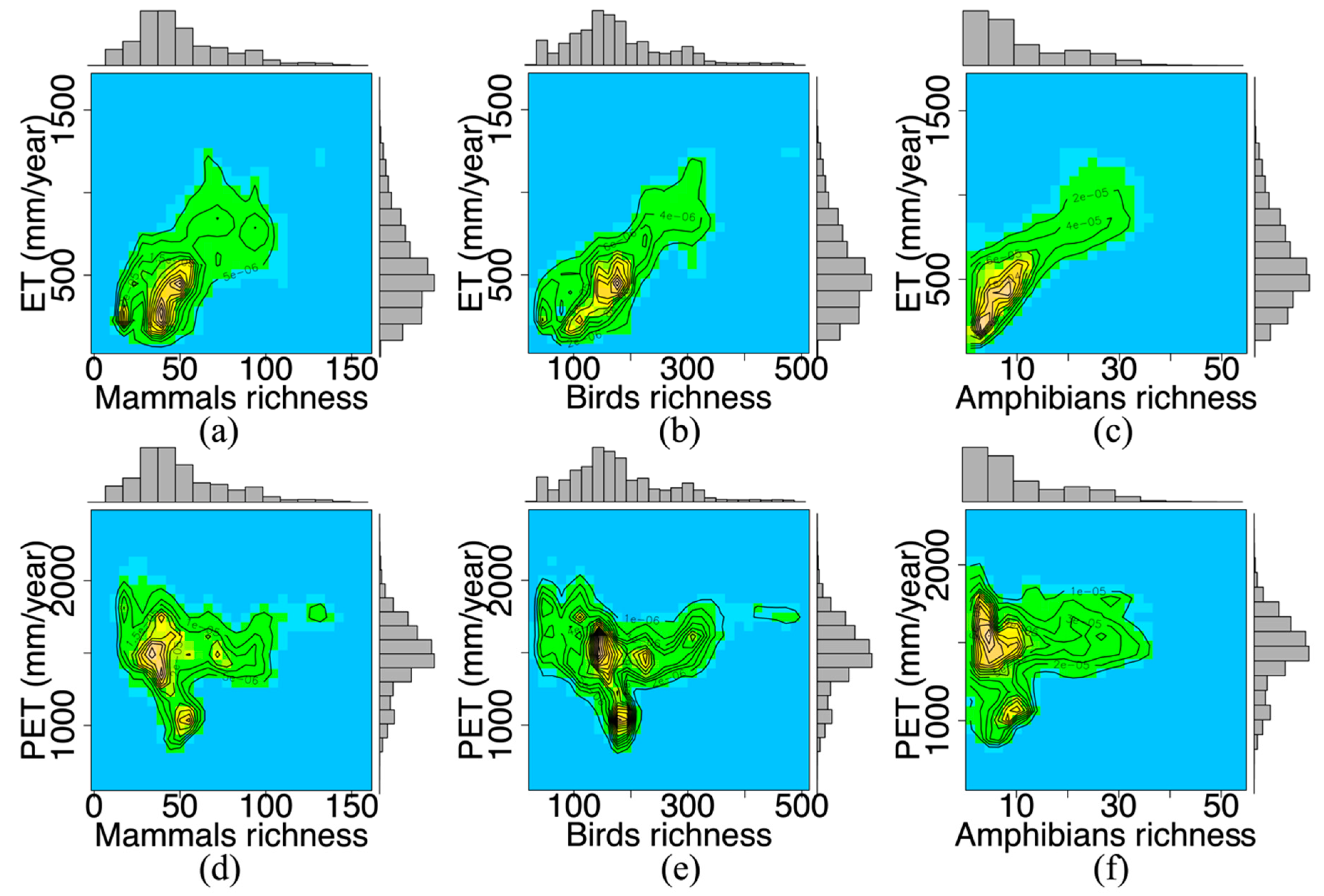
| Species Richness | Slopes | R2 | p Value | DF | ||
|---|---|---|---|---|---|---|
| ET | PET | ET | PET | |||
| Mammals | 0.0617 | 0.0030 | 0.4028 | 0.004 | <0.05 | 65,135 |
| Birds | 0.2517 | 0.0063 | 0.5735 | 0.003 | <0.05 | 65,135 |
| Amphibians | 0.0288 | 0.0740 | 0.6639 | 0.0352 | <0.05 | 54,738 |
| Species Richness | R2 | p Value | DF | |||
|---|---|---|---|---|---|---|
| DET | ENTR | LAM | Lmean | |||
| Mammals | 0.2493 | 0.2332 | 0.0001 | 0.1276 | <0.05 | 65,135 |
| Birds | 0.3564 | 0.3643 | 0.0007 | 0.1758 | <0.05 | 65,135 |
| Amphibians | 0.3106 | 0.2840 | 0.0039 | 0.2053 | <0.05 | 54,738 |
| Variations | R2 | ||
|---|---|---|---|
| Mammals | Birds | Amphibians | |
| DET | 0.4250 | 0.6057 | 0.6794 |
| DET + ENTR | 0.4301 | 0.6132 | 0.6859 |
| DET + ENTR + LAM | 0.4302 | 0.6137 | 0.6860 |
| DET + ENTR + LAM + Lmean | 0.4357 | 0.6234 | 0.6882 |
| DET + ENTR + Lmean | 0.4357 | 0.6231 | 0.6881 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Cai, D.; Li, W.; Guo, S.; Guan, Y.; Bian, X.; Yao, W. Effect of the Long-Term Mean and the Temporal Stability of Water-Energy Dynamics on China’s Terrestrial Species Richness. ISPRS Int. J. Geo-Inf. 2017, 6, 58. https://doi.org/10.3390/ijgi6030058
Zhang C, Cai D, Li W, Guo S, Guan Y, Bian X, Yao W. Effect of the Long-Term Mean and the Temporal Stability of Water-Energy Dynamics on China’s Terrestrial Species Richness. ISPRS International Journal of Geo-Information. 2017; 6(3):58. https://doi.org/10.3390/ijgi6030058
Chicago/Turabian StyleZhang, Chunyan, Danlu Cai, Wang Li, Shan Guo, Yanning Guan, Xiaolin Bian, and Wutao Yao. 2017. "Effect of the Long-Term Mean and the Temporal Stability of Water-Energy Dynamics on China’s Terrestrial Species Richness" ISPRS International Journal of Geo-Information 6, no. 3: 58. https://doi.org/10.3390/ijgi6030058






