The Many Hats of Sonic Hedgehog Signaling in Nervous System Development and Disease
Abstract
:1. Introduction
2. Neural Cell Proliferation
3. Neural Progenitor Specification
4. Neuronal and Glial Differentiation
5. Axon Guidance
6. Synapse Formation and Plasticity
7. Interactions of Shh with Other Signaling Pathways in the Nervous System
8. Neural Disease and Regeneration
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goodrich, L.V.; Milenkovic, L.; Higgins, K.M.; Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997, 277, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; Taylor, A.M.; Nakano, Y. Role of the Drosophila patched gene in positional signalling. Nature 1991, 353, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Alcedo, J.; Ayzenzon, M.; von Ohlen, T.; Noll, M.; Hooper, J.E. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the Hedgehog signal. Cell 1996, 86, 221–232. [Google Scholar] [CrossRef]
- Van den Heuvel, M.; Ingham, P.W. Smoothened encodes a receptor-like serpentine protein required for Hedgehog signalling. Nature 1996, 382, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Struhl, G. Dual roles for Patched in sequestering and transducing Hedgehog. Cell 1996, 87, 553–563. [Google Scholar] [CrossRef]
- Hynes, M.; Stone, D.M.; Dowd, M.; Pitts-Meek, S.; Goddard, A.; Gurney, A.; Rosenthal, A. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron 1997, 19, 15–26. [Google Scholar] [CrossRef]
- Dominguez, M.; Brunner, M.; Hafen, E.; Basler, K. Sending and receiving the Hedgehog signal: Control by the Drosophila Gli protein Cubitus interruptus. Science 1996, 272, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H.; St.-Jaques, B.; Lee, S.M.; Flax, J.D.; Snyder, E.Y.; McMahon, A.P. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci. 1999, 19, 8954–8965. [Google Scholar]
- Stecca, B.; Ruiz i Altaba, A. A Gli1–p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009, 28, 663–676. [Google Scholar]
- Wechsler-Reya, R.J.; Scott, M.P. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 1999, 22, 103–114. [Google Scholar] [CrossRef]
- Izzi, L.; Levesque, M.; Morin, S.; Laniel, D.; Wilkes, B.C.; Mille, F.; Krauss, R.S.; McMahon, A.P.; Allen, B.L.; Charron, F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev. Cell 2011, 20, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Kaspar, B.K.; Gage, F.H.; Schaffer, D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.; Han, Y.G.; Shah, J.K.; Obernier, K.; Guinto, C.D.; Alvarez-Buylla, A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl. Acad. Sci. USA 2014, 111, 12438–12443. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.; Garcia, A.D.; Joyner, A.L. Titration of GLI3 repressor activity by sonic hedgehog signaling is critical for maintaining multiple adult neural stem cell and astrocyte functions. J. Neurosci. 2013, 33, 17490–17505. [Google Scholar] [CrossRef] [PubMed]
- Cayuso, J.; Ulloa, F.; Cox, B.; Briscoe, J.; Marti, E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development 2006, 133, 517–528. [Google Scholar] [CrossRef] [PubMed]
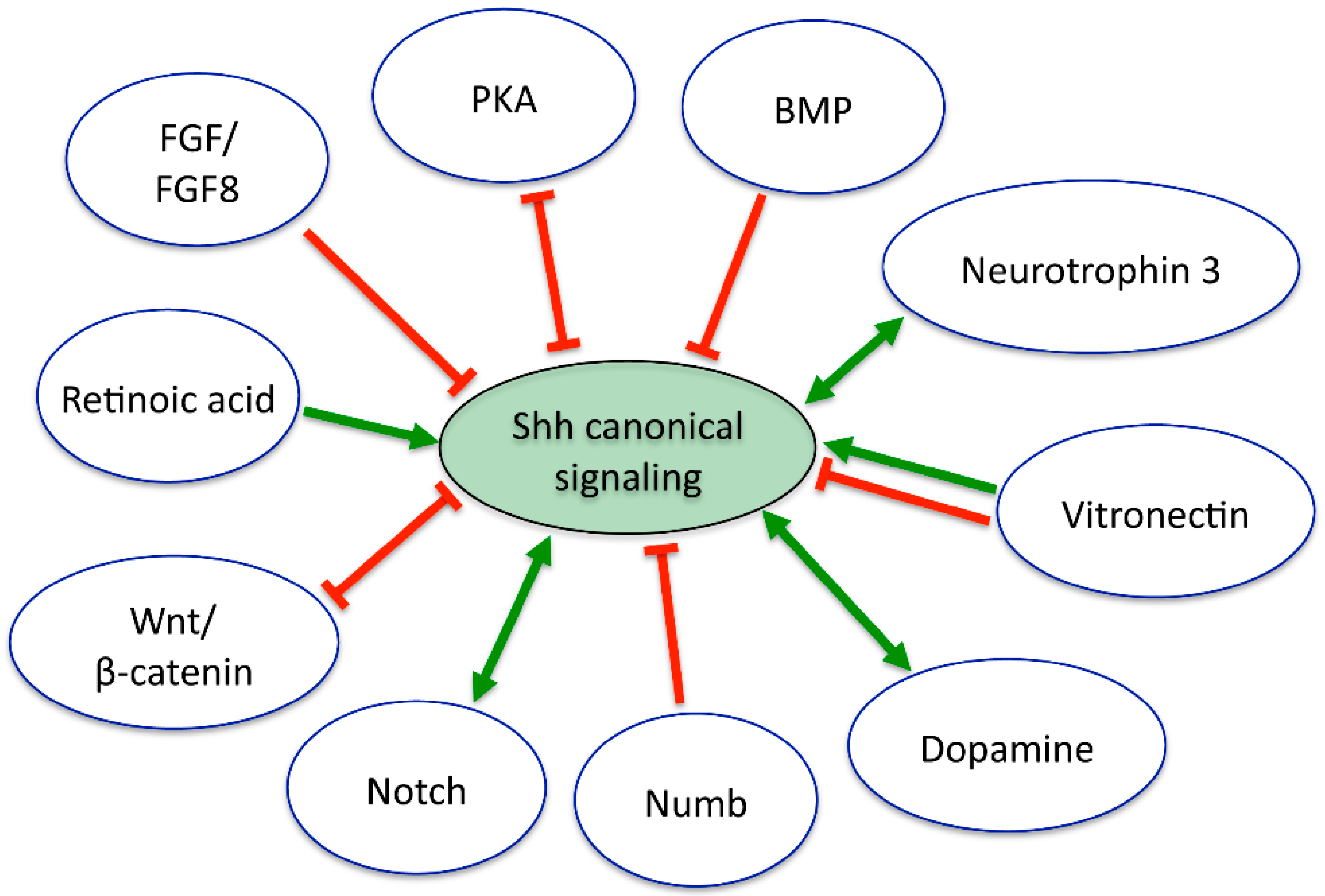
- Alvarez-Medina, R.; Le Dreau, G.; Ros, M.; Marti, E. Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development 2009, 136, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Locker, M.; Agathocleous, M.; Amato, M.A.; Parain, K.; Harris, W.A.; Perron, M. Hedgehog signaling and the retina: Insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006, 20, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.C.; Liu, Z.; Chia, W.; Cai, Y. Hedgehog signaling acts with the temporal cascade to promote neuroblast cell cycle exit. PLoS Biol. 2013, 11, e1001494. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Balasubramanian, S.; Witt, R.M.; Nazemi, K.J.; Choi, Y.; Pazyra-Murphy, M.F.; Walsh, C.O.; Thompson, M.; Segal, R.A. Proteoglycan interactions with Sonic Hedgehog specify mitogenic responses. Nat. Neurosci. 2009, 12, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wijgerde, M.; McMahon, J.A.; Rule, M.; McMahon, A.P. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002, 16, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Pierani, A.; Jessell, T.M.; Ericson, J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 2000, 101, 435–445. [Google Scholar] [CrossRef]
- Briscoe, J.; Sussel, L.; Serup, P.; Hartigan-O’Connor, D.; Jessell, T.M.; Rubenstein, J.L.; Ericson, J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 1999, 398, 622–627. [Google Scholar] [PubMed]
- Ericson, J.; Morton, S.; Kawakami, A.; Roelink, H.; Jessell, T.M. Two critical periods of Sonic hedgehog signaling required for the specification of motor neuron identity. Cell 1996, 87, 661–673. [Google Scholar] [CrossRef]
- Ericson, J.; Rashbass, P.; Schedl, A.; Brenner-Morton, S.; Kawakami, A.; van Heyningen, V.; Jessell, T.M.; Briscoe, J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 1997, 90, 169–180. [Google Scholar] [CrossRef]
- Ericson, J.; Thor, S.; Edlund, T.; Jessell, T.M.; Yamada, T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 1992, 256, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Tentner, A.R.; Huang, P.; Gelas, A.; Mosaliganti, K.R.; Souhait, L.; Rannou, N.; Swinburne, I.A.; Obholzer, N.D.; Cowgill, P.D.; et al. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell 2013, 153, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; Ribes, V.; Balaskas, N.; Yang, L.L.; Pierani, A.; Kicheva, A.; Novitch, B.G.; Briscoe, J.; Sasai, N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen Sonic hedgehog. PLoS Biol. 2010, 8, e1000382. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, N.; Ribeiro, A.; Panovska, J.; Dessaud, E.; Sasai, N.; Page, K.M.; Briscoe, J.; Ribes, V. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell 2012, 148, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Zhang, X.; Jeong, J.; Peterson, K.A.; Vedenko, A.; Bulyk, M.L.; Hide, W.A.; McMahon, A.P. A direct fate exclusion mechanism by Sonic hedgehog-regulated transcriptional repressors. Development 2015, 142, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Nishi, Y.; Ma, W.; Vedenko, A.; Shokri, L.; Zhang, X.; McFarlane, M.; Baizabal, J.M.; Junker, J.P.; van Oudenaarden, A.; et al. Neural-specific Sox2 input and differential Gli-binding affinity provide context and positional information in Shh-directed neural patterning. Genes Dev. 2012, 26, 2802–2816. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Jeong, Y.; Misra, K.; Li, S.; Zelman, A.K.; Epstein, D.J.; Matise, M.P. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic Shh-Gli signaling in the neural tube. Dev. Cell 2006, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Oosterveen, T.; Kurdija, S.; Alekseenko, Z.; Uhde, C.W.; Bergsland, M.; Sandberg, M.; Andersson, E.; Dias, J.M.; Muhr, J.; Ericson, J. Mechanistic differences in the transcriptional interpretation of local and long-range Shh morphogen signaling. Dev. Cell 2012, 23, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lei, Q.; Oosterveen, T.; Ericson, J.; Matise, M.P. Tcf/Lef repressors differentially regulate Shh-Gli target gene activation thresholds to generate progenitor patterning in the developing CNS. Development 2011, 138, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.P.; Yu, W.P.; Guthrie, S.; Roelink, H.; Lumsden, A.; Peterson, A.C.; Richardson, W.D. Determination of neuroepithelial cell fate: Induction of the oligodendrocyte lineage by ventral midline cells and Sonic hedgehog. Dev. Biol. 1996, 177, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Orentas, D.M.; Hayes, J.E.; Dyer, K.L.; Miller, R.H. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 1999, 126, 2419–2429. [Google Scholar] [PubMed]
- Lu, Q.R.; Yuk, D.; Alberta, J.A.; Zhu, Z.; Pawlitzky, I.; Chan, J.; McMahon, A.P.; Stiles, C.D.; Rowitch, D.H. Sonic hedgehog–regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 2000, 25, 317–329. [Google Scholar] [CrossRef]
- Takebayashi, H.; Nabeshima, Y.; Yoshida, S.; Chisaka, O.; Ikenaka, K.; Nabeshima, Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002, 12, 1157–1163. [Google Scholar] [CrossRef]
- Li, H.; de Faria, J.P.; Andrew, P.; Nitarska, J.; Richardson, W.D. Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron 2011, 69, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Sanders, T.A.; Ragsdale, C.W. Sonic hedgehog control of size and shape in midbrain pattern formation. Science 2001, 291, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Kiecker, C.; Lumsden, A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 2004, 7, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Garda, A.L.; Shimamura, K.; Martinez, S. Thalamic development induced by Shh in the chick embryo. Dev. Biol. 2005, 284, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Vue, T.Y.; Bluske, K.; Alishahi, A.; Yang, L.L.; Koyano-Nakagawa, N.; Novitch, B.; Nakagawa, Y. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J. Neurosci. 2009, 29, 4484–4497. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Dolson, D.K.; Waclaw, R.R.; Matise, M.P.; Sussel, L.; Campbell, K.; Kaestner, K.H.; Epstein, D.J. Spatial and temporal requirements for sonic hedgehog in the regulation of thalamic interneuron identity. Development 2011, 138, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Flandin, P.; Zhao, Y.; Vogt, D.; Jeong, J.; Long, J.; Potter, G.; Westphal, H.; Rubenstein, J.L. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 2011, 70, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.H.; Fishell, G. Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Curr. Opin. Genet. Dev. 2010, 20, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, V.; Yu, T.; Zaki, P.A.; Mason, J.O.; Price, D.J. Abnormal positioning of diencephalic cell types in neocortical tissue in the dorsal telencephalon of mice lacking functional Gli3. J. Neurosci. 2006, 26, 9282–9292. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Ruiz i Altaba, A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 2004, 131, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ge, G.; Uchida, Y.; Luu, B.; Ahn, S. Gli3 is required for maintenance and fate specification of cortical progenitors. J. Neurosci. 2011, 31, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Wilson, J.P.; Wang, C.; Wang, B.; McConnell, S.K. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev. Neurobiol. 2012, 72, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Yabut, O.R.; Fernandez, G.; Huynh, T.; Yoon, K.; Pleasure, S.J. Suppressor of fused is critical for maintenance of neuronal progenitor identity during corticogenesis. Cell Rep. 2015, 12, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, U.; Singh, C.M.; Luk, A.Y.; Donohoe, T.J. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature 1995, 373, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, U.; Wolff, T.; Rubin, G.M. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell 1993, 75, 913–926. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, Y.; Beachy, P.A.; Moses, K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell 1993, 75, 927–938. [Google Scholar] [CrossRef]
- Neumann, C.J.; Nuesslein-Volhard, C. Patterning of the zebrafish retina by a wave of Sonic hedgehog activity. Science 2000, 289, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; William, C.; Jessell, T.M. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell 1998, 95, 67–80. [Google Scholar] [CrossRef]
- Eisen, J.S. Determination of primary motoneuron identity in developing zebrafish embryos. Science 1991, 252, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.G.; Landmesser, L.T. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004, 43, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.; Muhr, J.; Placzek, M.; Lints, T.; Jessell, T.M.; Edlund, T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: A common signal for ventral patterning within the neural tube. Cell 1995, 81, 747–756. [Google Scholar] [CrossRef]
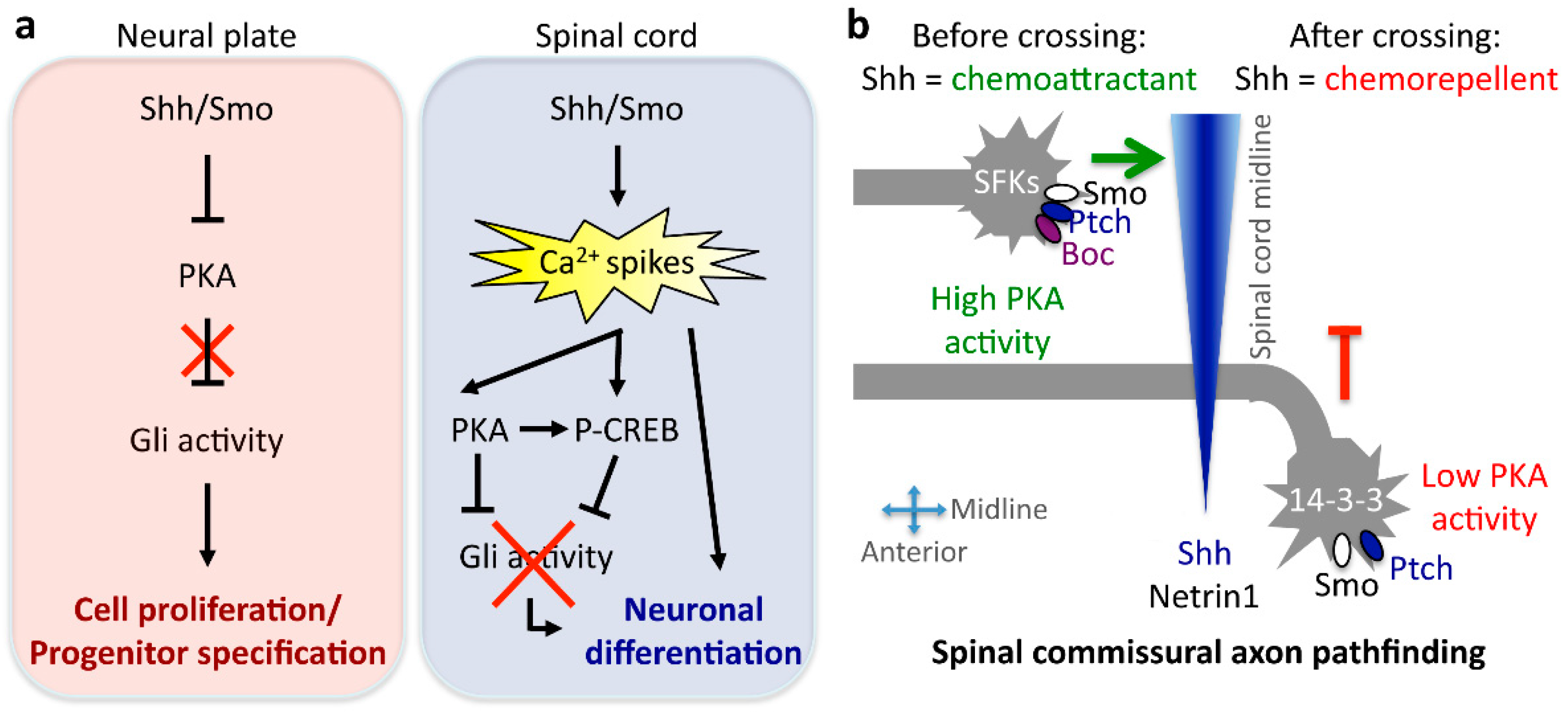
- Belgacem, Y.H.; Borodinsky, L.N. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc. Natl. Acad. Sci. USA 2011, 108, 4482–4487. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Root, C.M.; Cronin, J.A.; Sann, S.B.; Gu, X.; Spitzer, N.C. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 2004, 429, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Root, C.M.; Velazquez-Ulloa, N.A.; Monsalve, G.C.; Minakova, E.; Spitzer, N.C. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J. Neurosci. 2008, 28, 4777–4784. [Google Scholar] [CrossRef] [PubMed]
- Swapna, I.; Borodinsky, L.N. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 16336–16341. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.W.; Kurtz, L.M.; Spitzer, N.C. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat. Neurosci. 2010, 13, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Demarque, M.; Spitzer, N.C. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron 2010, 67, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Spitzer, N.C. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature 2008, 456, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Jamshidi, P.; Leutgeb, S.; Spitzer, N.C. Neurotransmitter switching in the adult brain regulates behavior. Science 2013, 340, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.T.; Abrahamsson, T.; Chierzi, S.; Lui, C.; Zaelzer, C.; Jones, E.V.; Bally, B.P.; Chen, G.G.; Theroux, J.F.; Peng, J.; et al. Neurons diversify astrocytes in the adult brain through Sonic hedgehog signaling. Science 2016, 351, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Strahle, U.; Fischer, N.; Blader, P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech. Dev. 1997, 62, 147–160. [Google Scholar] [CrossRef]
- Charron, F.; Stein, E.; Jeong, J.; McMahon, A.P.; Tessier-Lavigne, M. The morphogen Sonic hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell 2003, 113, 11–23. [Google Scholar] [CrossRef]
- Yam, P.T.; Langlois, S.D.; Morin, S.; Charron, F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 2009, 62, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Charron, F.; Morin, S.; Shin, D.S.; Wong, K.; Fabre, P.J.; Tessier-Lavigne, M.; McConnell, S.K. Boc is a receptor for Sonic hedgehog in the guidance of commissural axons. Nature 2006, 444, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Sloan, T.F.; Qasaimeh, M.A.; Juncker, D.; Yam, P.T.; Charron, F. Integration of shallow gradients of Shh and Netrin-1 guides commissural axons. PLoS Biol. 2015, 13, e1002119. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, D.; Pekarik, V.; Baeriswyl, T.; Grunditz, A.; Sadhu, R.; Nardo, M.; Stoeckli, E.T. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat. Neurosci. 2005, 8, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.T.; Kent, C.B.; Morin, S.; Farmer, W.T.; Alchini, R.; Lepelletier, L.; Colman, D.R.; Tessier-Lavigne, M.; Fournier, A.E.; Charron, F. 14-3-3 proteins regulate a cell-intrinsic switch from Sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron 2012, 76, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Trousse, F.; Marti, E.; Gruss, P.; Torres, M.; Bovolenta, P. Control of retinal ganglion cell axon growth: A new role for Sonic hedgehog. Development 2001, 128, 3927–3936. [Google Scholar] [PubMed]
- Kolpak, A.; Zhang, J.; Bao, Z.Z. Sonic hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration. J. Neurosci. 2005, 25, 3432–3441. [Google Scholar] [CrossRef] [PubMed]
- Fabre, P.J.; Shimogori, T.; Charron, F. Segregation of ipsilateral retinal ganglion cell axons at the optic chiasm requires the Shh receptor Boc. J. Neurosci. 2010, 30, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Barresi, M.J.; Hutson, L.D.; Chien, C.B.; Karlstrom, R.O. Hedgehog regulated Slit expression determines commissure and glial cell position in the zebrafish forebrain. Development 2005, 132, 3643–3656. [Google Scholar] [CrossRef] [PubMed]
- Stacher Horndli, C.; Chien, C.B. Sonic hedgehog is indirectly required for intraretinal axon pathfinding by regulating chemokine expression in the optic stalk. Development 2012, 139, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Martinelli, D.C.; Zheng, X.; Tessier-Lavigne, M.; Fan, C.M. Gas1 is a receptor for Sonic hedgehog to repel enteric axons. Proc. Natl. Acad. Sci. USA 2015, 112, E73–E80. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Chiu, M.; Zhang, E.; Kunes, S. A C-terminal motif targets Hedgehog to axons, coordinating assembly of the Drosophila eye and brain. Dev. Cell 2006, 10, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.; Murakami, S.; Sato, M.; Tabata, T. The highly ordered assembly of retinal axons and their synaptic partners is regulated by Hedgehog/Single-minded in the Drosophila visual system. Development 2006, 133, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Zheng, X.; Beachy, P.A.; Luo, L. Patterning axon targeting of olfactory receptor neurons by coupled hedgehog signaling at two distinct steps. Cell 2010, 142, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Masdeu, C.; Bernard, V.; Faure, H.; Traiffort, E.; Ruat, M. Distribution of Smoothened at hippocampal mossy fiber synapses. Neuroreport 2007, 18, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Schwartz, C.M.; Wang, Y.X.; Mattson, M.P.; Yao, P.J. Subcellular localization of Patched and Smoothened, the receptors for Sonic hedgehog signaling, in the hippocampal neuron. J. Comp. Neurol. 2011, 519, 3684–3699. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Mattson, M.P.; Yao, P.J. Sonic hedgehog distribution within mature hippocampal neurons. Commun. Integr. Biol. 2011, 4, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Harwell, C.C.; Parker, P.R.; Gee, S.M.; Okada, A.; McConnell, S.K.; Kreitzer, A.C.; Kriegstein, A.R. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron 2012, 73, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Petralia, R.S.; Currier, D.G.; Wang, Y.X.; Kim, A.; Mattson, M.P.; Yao, P.J. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. J. Cell Sci. 2012, 125, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.K.; Vesque, C.; Lints, T.J.; Sampath, T.K.; Furley, A.; Dodd, J.; Placzek, M. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 1997, 90, 257–269. [Google Scholar] [CrossRef]
- Liem, K.F., Jr.; Jessell, T.M.; Briscoe, J. Regulation of the neural patterning activity of Sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development 2000, 127, 4855–4866. [Google Scholar] [PubMed]
- Dutton, R.; Yamada, T.; Turnley, A.; Bartlett, P.F.; Murphy, M. Sonic hedgehog promotes neuronal differentiation of murine spinal cord precursors and collaborates with neurotrophin 3 to induce Islet-1. J. Neurosci. 1999, 19, 2601–2608. [Google Scholar] [PubMed]
- Kucera, J.; Fan, G.; Jaenisch, R.; Linnarsson, S.; Ernfors, P. Dependence of developing group Ia afferents on neurotrophin-3. J. Comp. Neurol. 1995, 363, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Novitch, B.G.; Wichterle, H.; Jessell, T.M.; Sockanathan, S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 2003, 40, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Marti, E. Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation. Development 2000, 127, 333–342. [Google Scholar] [PubMed]
- Pons, S.; Trejo, J.L.; Martinez-Morales, J.R.; Marti, E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development 2001, 128, 1481–1492. [Google Scholar] [PubMed]
- Fogarty, M.P.; Emmenegger, B.A.; Grasfeder, L.L.; Oliver, T.G.; Wechsler-Reya, R.J. Fibroblast growth factor blocks Sonic hedgehog signaling in neuronal precursors and tumor cells. Proc. Natl. Acad. Sci. USA 2007, 104, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Bluske, K.K.; Vue, T.Y.; Kawakami, Y.; Taketo, M.M.; Yoshikawa, K.; Johnson, J.E.; Nakagawa, Y. β-Catenin signaling specifies progenitor cell identity in parallel with Shh signaling in the developing mammalian thalamus. Development 2012, 139, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Tuson, M.; He, M.; Anderson, K.V. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development 2011, 138, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Norris, A.; Ohnmacht, J.; Patani, R.; Zhong, Z.; Dias, T.B.; Kuscha, V.; Scott, A.L.; Chen, Y.C.; Rozov, S.; et al. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev. Cell 2013, 25, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.H.; Yang, L.; Dessaud, E.; Chuang, K.; Moore, D.M.; Rohatgi, R.; Briscoe, J.; Novitch, B.G. Notch activity modulates the responsiveness of neural progenitors to Sonic hedgehog signaling. Dev. Cell 2015, 33, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Di Marcotullio, L.; Ferretti, E.; Greco, A.; de Smaele, E.; Po, A.; Sico, M.A.; Alimandi, M.; Giannini, G.; Maroder, M.; Screpanti, I.; et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch- dependent ubiquitination. Nat. Cell Biol. 2006, 8, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Sakamoto, M.; Yamaguchi, M.; Mori, K.; Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010, 30, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Stasiulewicz, M.; Gray, S.D.; Mastromina, I.; Silva, J.C.; Bjorklund, M.; Seymour, P.A.; Booth, D.; Thompson, C.; Green, R.J.; Hall, E.A.; et al. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development 2015, 142, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Platt, K.A.; Censullo, P.; Ruiz i Altaba, A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 1997, 124, 2537–2552. [Google Scholar] [PubMed]
- Belgacem, Y.H.; Borodinsky, L.N. Inversion of Sonic hedgehog action on its canonical pathway by electrical activity. Proc. Natl. Acad. Sci. USA 2015, 112, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, E.H.; Walterhouse, D.O.; Iannaccone, P.M. The Sonic hedgehog–Patched–Gli pathway in human development and disease. Am. J. Hum. Genet. 2000, 67, 1047–1054. [Google Scholar] [CrossRef]
- Pietsch, T.; Waha, A.; Koch, A.; Kraus, J.; Albrecht, S.; Tonn, J.; Sorensen, N.; Berthold, F.; Henk, B.; Schmandt, N.; et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997, 57, 2085–2088. [Google Scholar] [PubMed]
- Stecca, B.; Ruiz i Altaba, A. Brain as a paradigm of organ growth: Hedgehog–Gli signaling in neural stem cells and brain tumors. J. Neurobiol. 2005, 64, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Schuller, U.; Heine, V.M.; Mao, J.; Kho, A.T.; Dillon, A.K.; Han, Y.G.; Huillard, E.; Sun, T.; Ligon, A.H.; Qian, Y.; et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 2008, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Ellis, T.; Markant, S.L.; Read, T.A.; Kessler, J.D.; Bourboulas, M.; Schuller, U.; Machold, R.; Fishell, G.; Rowitch, D.H.; et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 2008, 14, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Swartling, F.J.; Savov, V.; Persson, A.I.; Chen, J.; Hackett, C.S.; Northcott, P.A.; Grimmer, M.R.; Lau, J.; Chesler, L.; Perry, A.; et al. Distinct neural stem cell populations give rise to disparate brain tumors in response to N-MYC. Cancer Cell 2012, 21, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Klisch, T.J.; Schuster, G.; Zoghbi, H.Y. Deletion of Atoh1 disrupts Sonic hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science 2009, 326, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Bihannic, L.; Cigna, S.M.; Lefevre, C.; Remke, M.; Barnat, M.; Dodier, S.; Shirvani, H.; Mercier, A.; Mensah, A.; et al. Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors. Dev. Cell 2014, 29, 649–661. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, L.; Chen, Y.; Remke, M.; Shih, D.; Lu, F.; Wang, H.; Deng, Y.; Yu, Y.; Xia, Y.; et al. The G protein αsubunit Gαs is a tumor suppressor in Sonic hedgehog-driven medulloblastoma. Nat. Med. 2014, 20, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Karhadkar, S.S.; Hallahan, A.R.; Pritchard, J.I.; Eberhart, C.G.; Watkins, D.N.; Chen, J.K.; Cooper, M.K.; Taipale, J.; Olson, J.M.; et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 2002, 297, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.G.; Read, T.A.; Kessler, J.D.; Mehmeti, A.; Wells, J.F.; Huynh, T.T.; Lin, S.M.; Wechsler-Reya, R.J. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development 2005, 132, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, S.; Tanori, M.; Mancuso, M.; Gessi, M.; Pasquali, E.; Leonardi, S.; Oliva, M.A.; Rebessi, S.; di Majo, V.; Covelli, V.; et al. Two-hit model for progression of medulloblastoma preneoplasia in Patched heterozygous mice. Oncogene 2006, 25, 5575–5580. [Google Scholar] [CrossRef] [PubMed]
- Mille, F.; Tamayo-Orrego, L.; Levesque, M.; Remke, M.; Korshunov, A.; Cardin, J.; Bouchard, N.; Izzi, L.; Kool, M.; Northcott, P.A.; et al. The Shh receptor Boc promotes progression of early medulloblastoma to advanced tumors. Dev. Cell 2014, 31, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Crome, L.; Cowie, V.; Slater, E. Statistical Note on Cerebellar and Brain-Stem Weight in Mongolism. J. Ment. Defic. Res. 1966, 10, 69–72. [Google Scholar] [CrossRef]
- Baxter, L.L.; Moran, T.H.; Richtsmeier, J.T.; Troncoso, J.; Reeves, R.H. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum. Mol. Genet. 2000, 9, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.E.; Roper, R.J.; Baxter, L.L.; Carlson, E.J.; Epstein, C.J.; Reeves, R.H. Down syndrome mouse models Ts65Dn, Ts1Cje, and Ms1Cje/Ts65Dn exhibit variable severity of cerebellar phenotypes. Dev. Dyn. 2004, 230, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Roper, R.J.; Baxter, L.L.; Saran, N.G.; Klinedinst, D.K.; Beachy, P.A.; Reeves, R.H. Defective cerebellar response to mitogenic Hedgehog signaling in Down’s syndrome mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Trazzi, S.; Mitrugno, V.M.; Valli, E.; Fuchs, C.; Rizzi, S.; Guidi, S.; Perini, G.; Bartesaghi, R.; Ciani, E. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Hum. Mol. Genet. 2011, 20, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Park, J.M.; Shin, J.H.; Jeon, S.K.; Lorenzi, H.; Linden, D.J.; Worley, P.F.; Reeves, R.H. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci. Transl. Med. 2013, 5, 201ra120. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, C.; Tsuzuki, H.; Nakamura, Y.; Sasaki, Y.; Ohsaki, K.; Nakamura, S.; Arakawa, Y.; Kohsaka, S. The upregulated expression of Sonic hedgehog in motor neurons after rat facial nerve axotomy. J. Neurosci. 2004, 24, 7923–7930. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Kuscha, V.; Wyatt, C.; Sorensen, I.; Frank, R.E.; Knuwer, M.; Becker, T.; Becker, C.G. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J. Neurosci. 2009, 29, 15073–15082. [Google Scholar] [CrossRef] [PubMed]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to Sonic hedgehog. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Amankulor, N.M.; Hambardzumyan, D.; Pyonteck, S.M.; Becher, O.J.; Joyce, J.A.; Holland, E.C. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 2009, 29, 10299–10308. [Google Scholar] [CrossRef] [PubMed]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Krishnamurty, R.G.; Bannerman, P.; Pleasure, D.E.; Deng, W. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014, 5, e1481. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Grund, E.M.; Silva, H.M.; Lafaille, J.J.; Fishell, G.; Salzer, J.L. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015, 526, 448–452. [Google Scholar] [CrossRef] [PubMed]
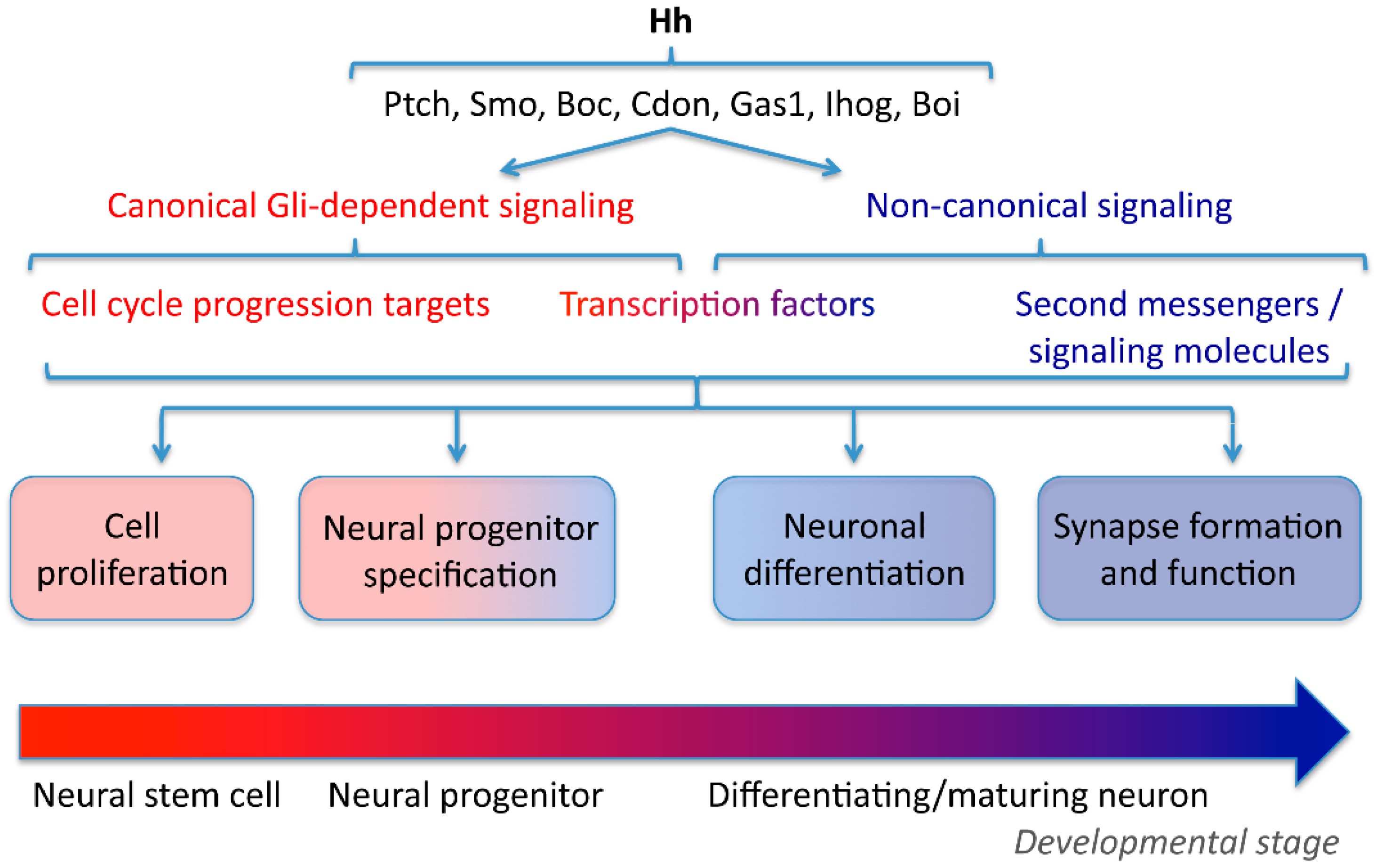
| Canonical | Non-Canonical | |||||
|---|---|---|---|---|---|---|
| Neural Cell Proliferation | Neural Progenitor Specification | Axon Guidance | Neuronal Differentiation | Axon Guidance | Axon Guidance | |
| Receptors | Ptch, Smo (Gas1, Boc) | Ptch, Smo | Ptch, Smo | Ptch, Smo | Smo, Boc | (1) Smo (mouse), Hhip (chick) (2) Boc (3) Smo, Gas1 |
| Second messengers | IP3, Ca2+ | (1) 14-4-3, cAMP (3) Gnaz | ||||
| Transcription factors | Gli | Gli | Gli | cJun | Transcription independent | Transcription independent |
| Targets | Bcl2, P53, cyclin A, B, E, D1 | Nkx2.2, Nkx6.1, Pax6, Evx1, Phox2A, Gata2, Fox2A, etc. | (1) Slit (2) Stromal cell-derived factor 1 | Tlx3 | Src | |
| Subcellular needs for Hh signaling | Nucleus Primary cilium? | Nucleus Primary cilium | Nucleus | Nucleus Primary cilium? | Axonal growth cone | Axonal growth cone |
| Main roles | Regulation of cell cycle progression and cell survival | Spinal cord and brain patterning during morphogenesis | (1) Midline crossing forebrain commissural axons (2) Retinal ganglion cell axon guidance | Specification of spinal neuron transmitter phenotype | Attractant guidance commissural spinal axons | (1) Repulsive guidance commissural spinal axons (2) Repulsive retinal ganglion cell axon guidance (3) Repulsive guidance enteric axons |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belgacem, Y.H.; Hamilton, A.M.; Shim, S.; Spencer, K.A.; Borodinsky, L.N. The Many Hats of Sonic Hedgehog Signaling in Nervous System Development and Disease. J. Dev. Biol. 2016, 4, 35. https://doi.org/10.3390/jdb4040035
Belgacem YH, Hamilton AM, Shim S, Spencer KA, Borodinsky LN. The Many Hats of Sonic Hedgehog Signaling in Nervous System Development and Disease. Journal of Developmental Biology. 2016; 4(4):35. https://doi.org/10.3390/jdb4040035
Chicago/Turabian StyleBelgacem, Yesser H., Andrew M. Hamilton, Sangwoo Shim, Kira A. Spencer, and Laura N. Borodinsky. 2016. "The Many Hats of Sonic Hedgehog Signaling in Nervous System Development and Disease" Journal of Developmental Biology 4, no. 4: 35. https://doi.org/10.3390/jdb4040035






