Imaging Neuronal Activity in the Optic Tectum of Late Stage Larval Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Generation of the NBT:GCaMP3 Transgenic Line
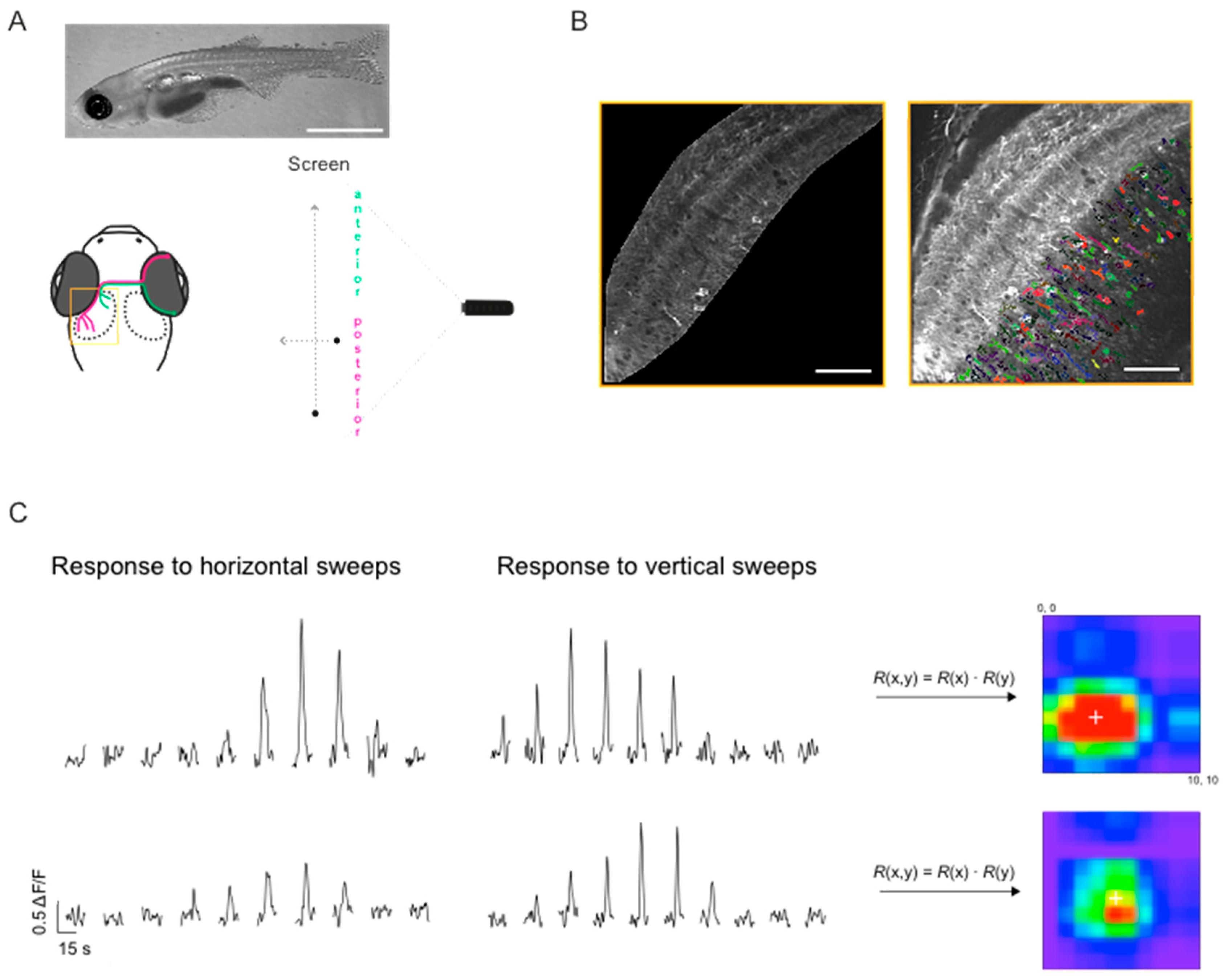
2.3. Imaging and Visual Stimulation
2.4. Image Analysis
2.5. Mapping Receptive Field Properties
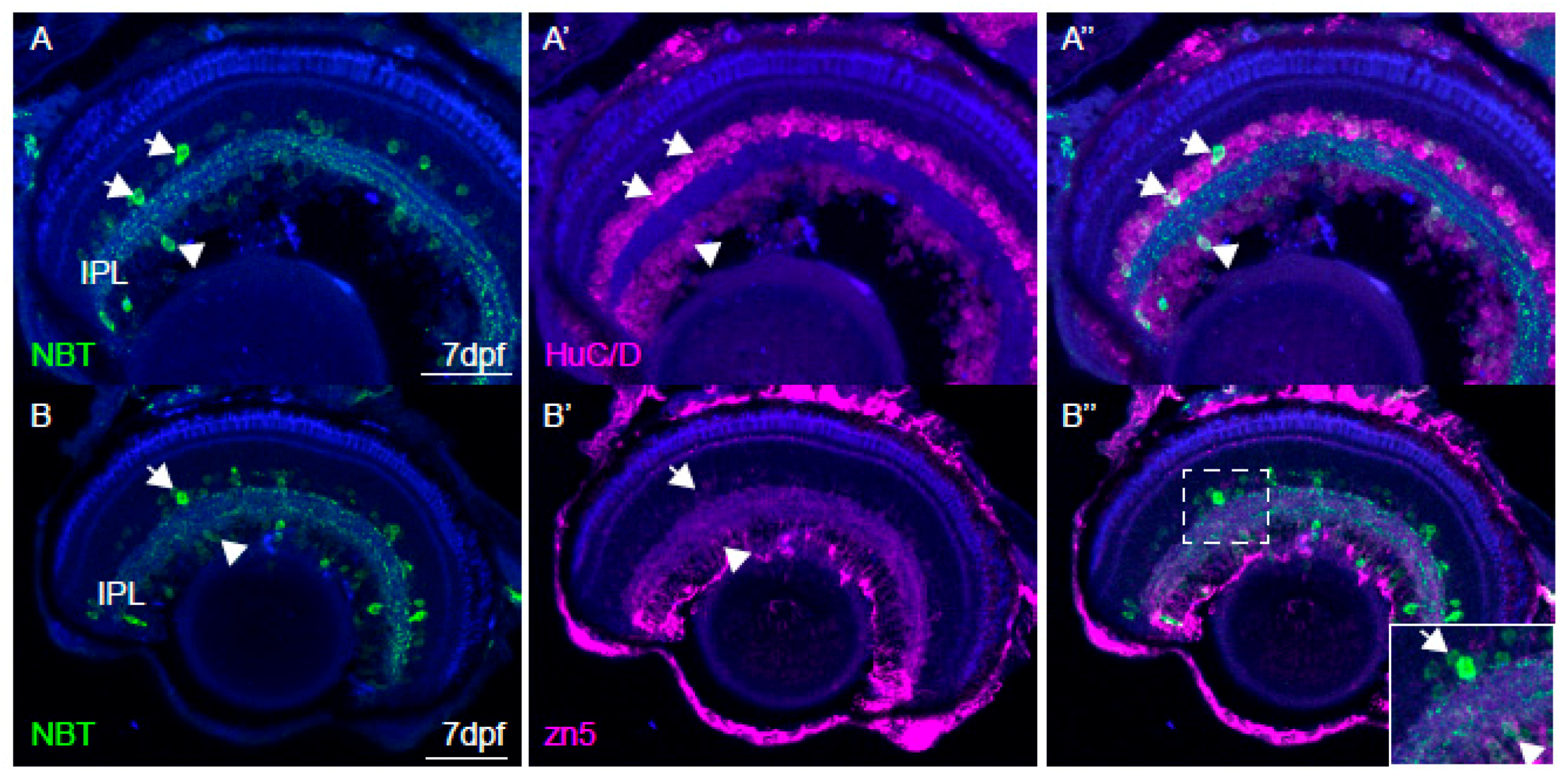
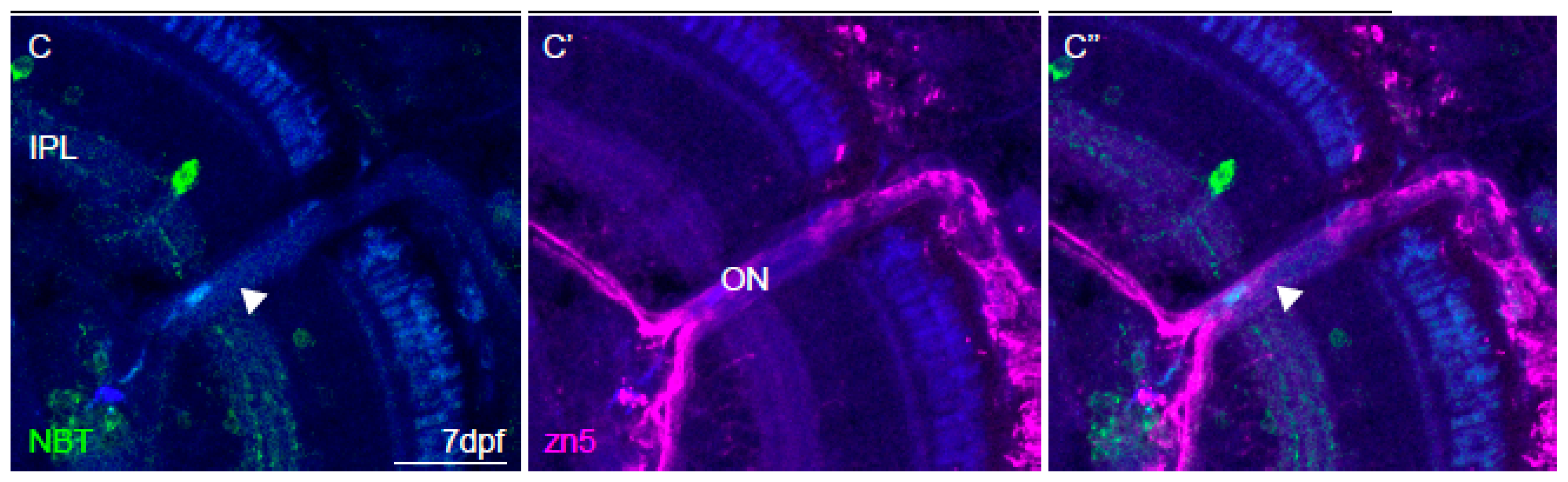
2.6. Immunohistochemistry and Imaging
3. Results
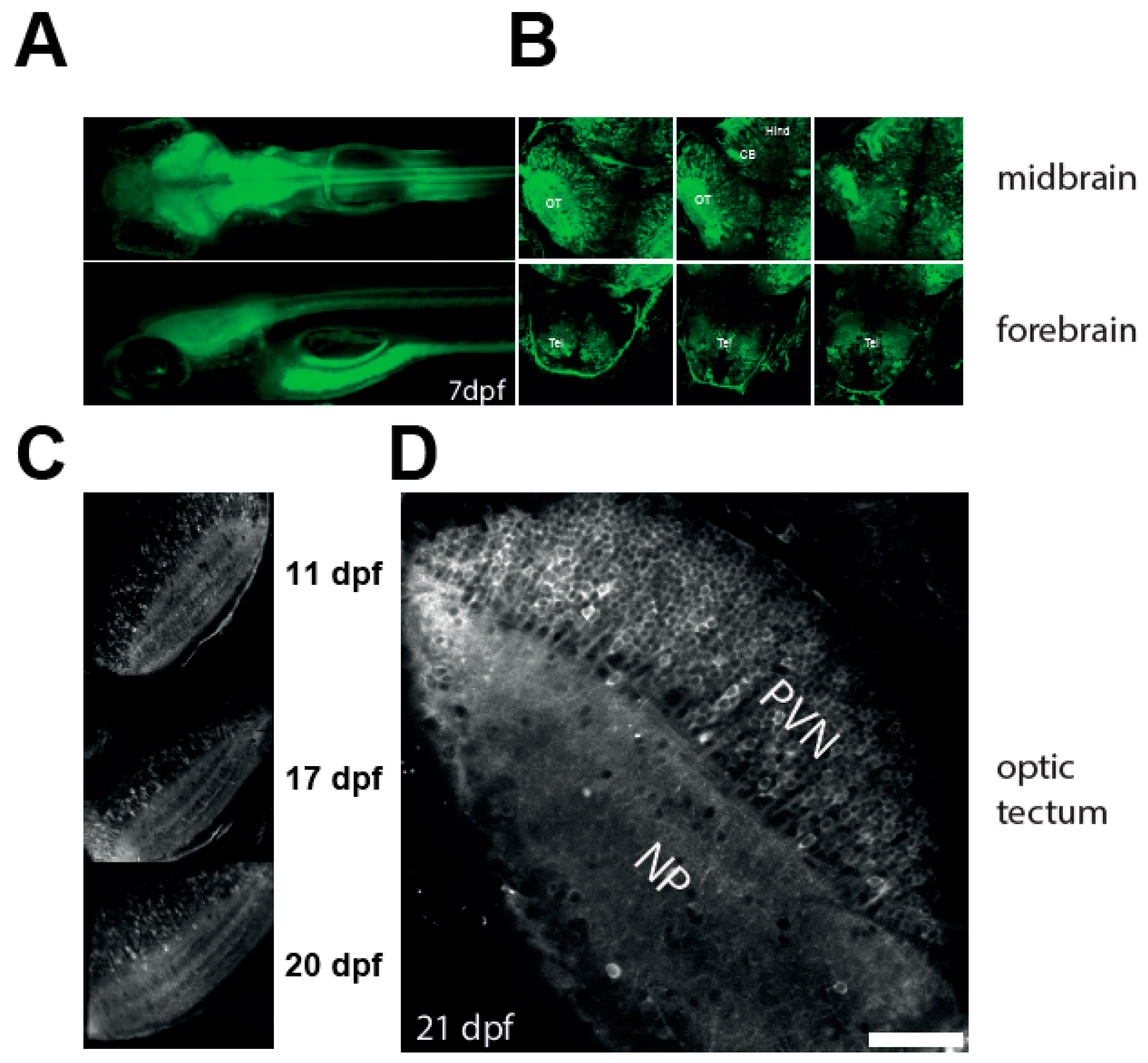
3.1. Robust Expression of a Calcium Reporter in the Optic Tectum of NBT:GCaMP3 Fish
3.2. Imaging Neuronal Activity in the Optic Tectum of 3 Weeks Old Larvae
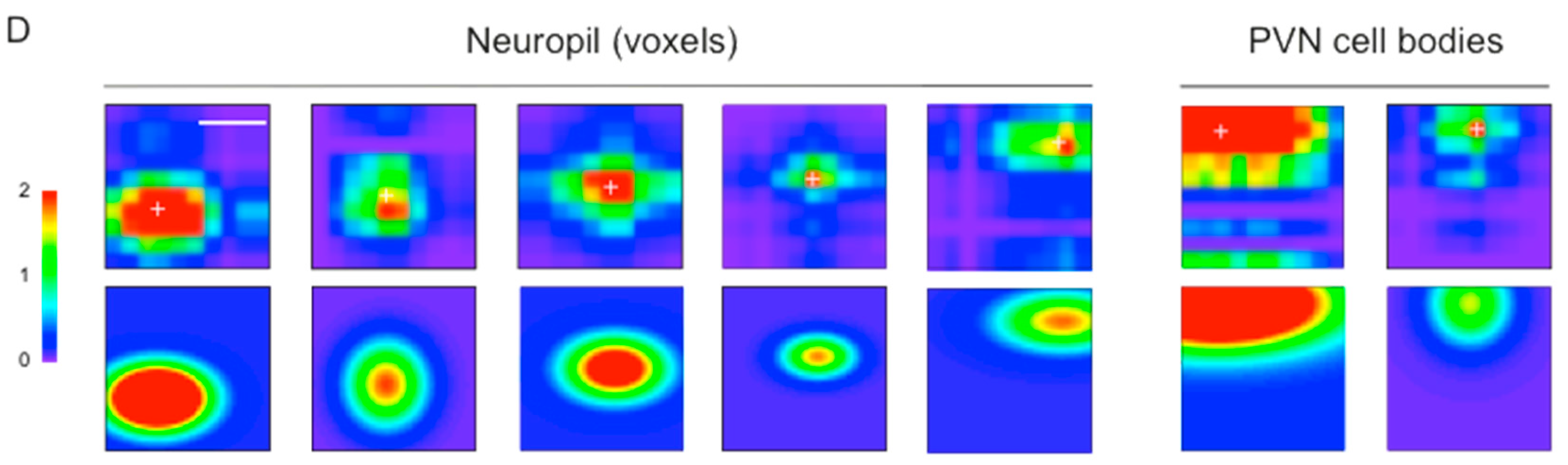
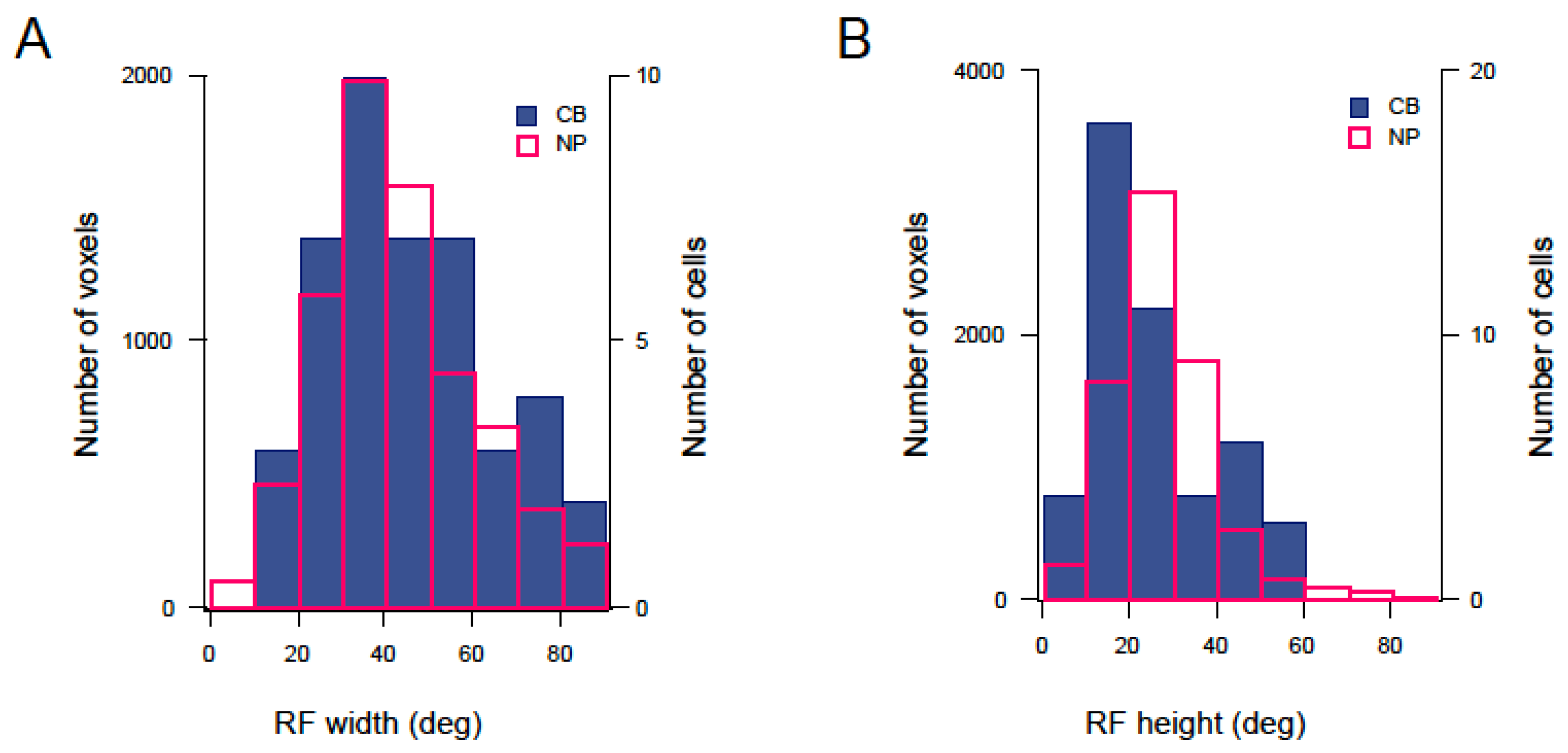
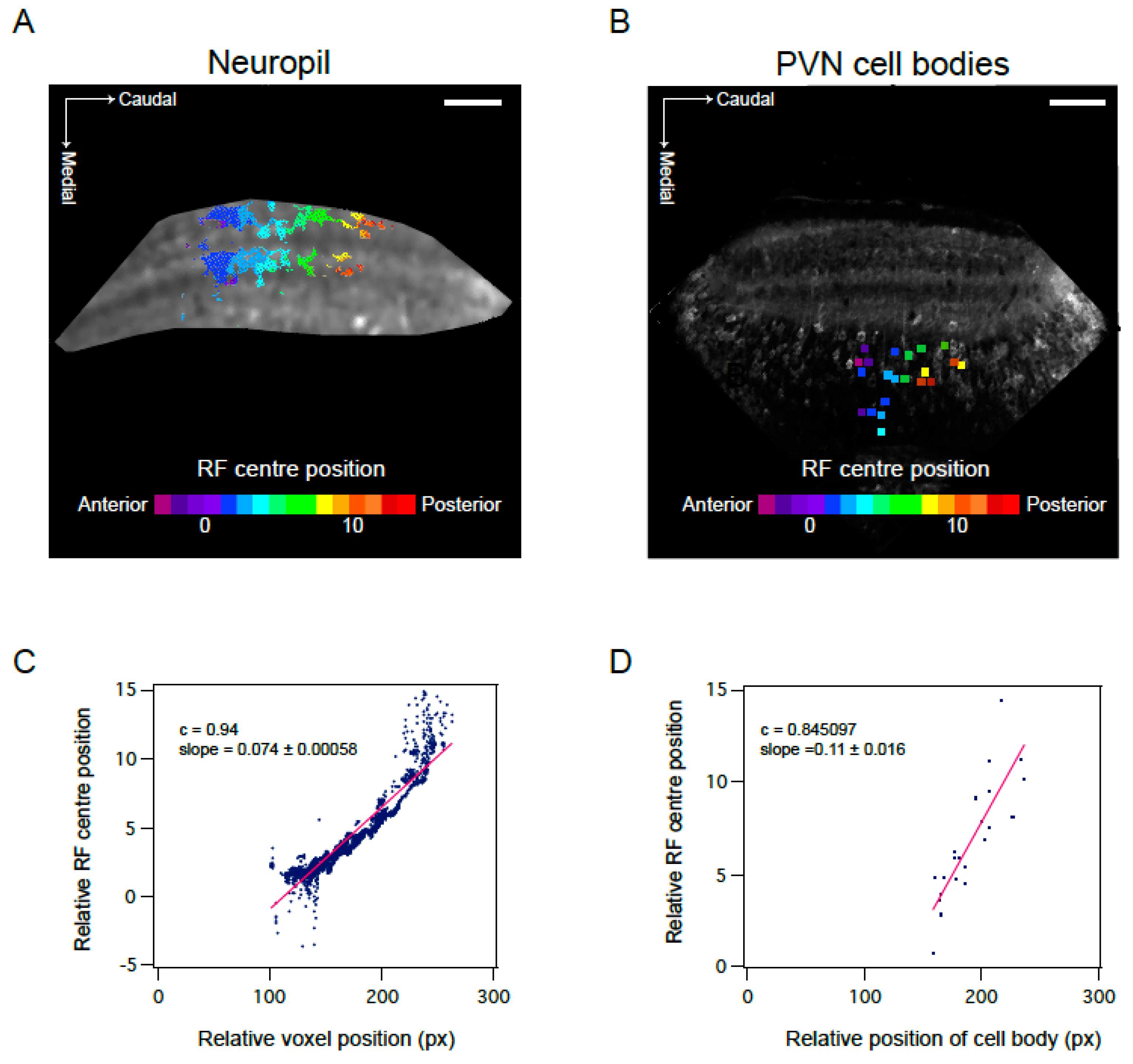
3.3. Mapping RF Properties in the Tectal Neuropil and the Cell Bodies
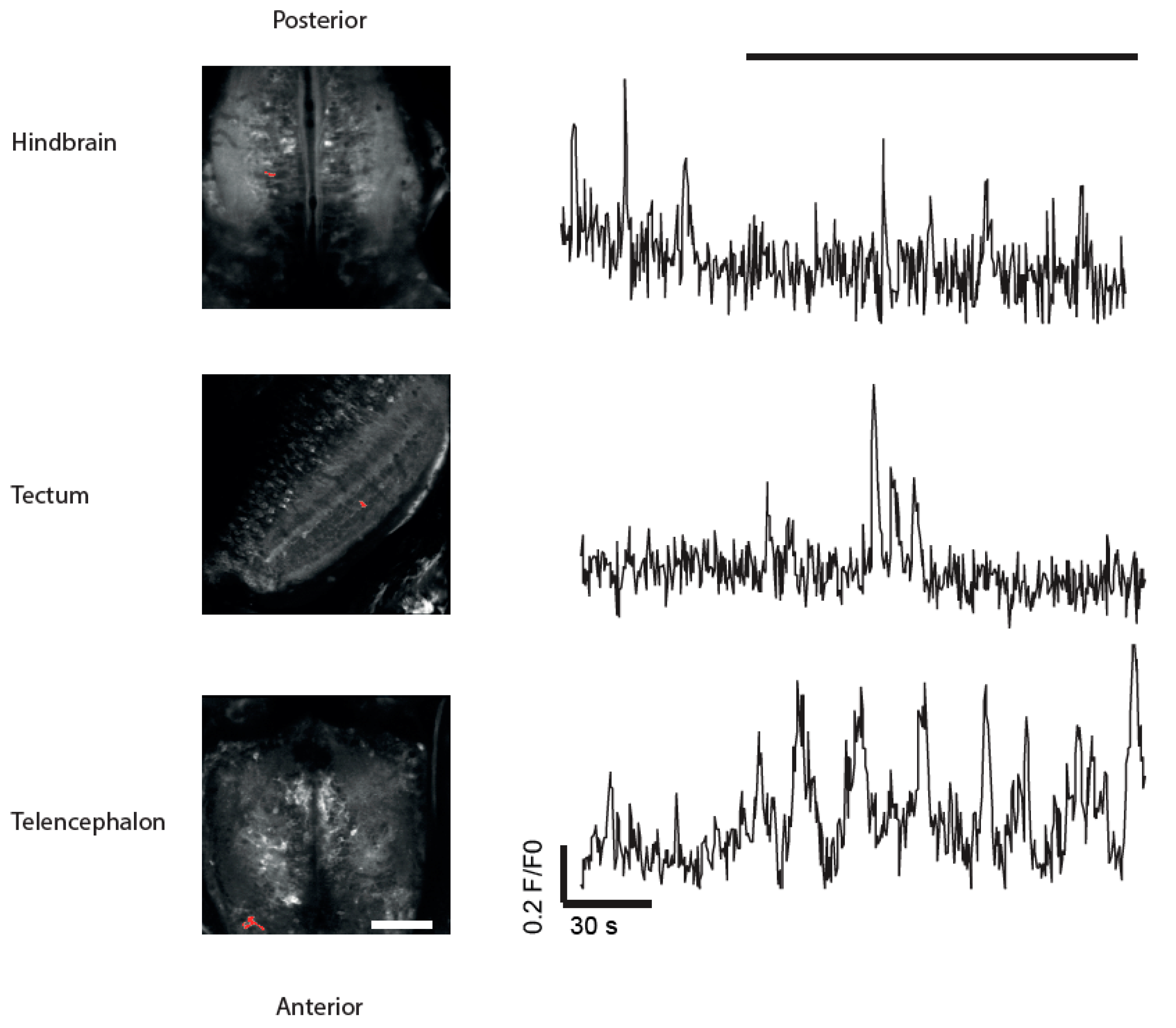
3.4. Imaging of Neuronal Activity in Other Brain Areas
4. Discussion
4.1. Imaging 2–3 Weeks Old Larvae
4.2. RF Properties in Late Stage Larvae
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Del Bene, F.; Wyart, C.; Robles, E.; Tran, A.; Looger, L.; Scott, E.K.; Isacoff, E.Y.; Baier, H. Filtering of Visual Information in the Tectum by an Identified Neural Circuit. Science 2010, 330, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Filosa, A.; Barker, A.J.; Maschio, M.D.; Baier, H. Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Neuron 2016, 90, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.P.; Trivedi, C.A.; Maurer, C.M.; Ryu, S.; Bollmann, J.H. Layer-Specific Targeting of Direction-Selective Neurons in the Zebrafish Optic Tectum. Neuron 2012, 76, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Preuss, S.J.; Trivedi, C.A.; Berg-Maurer, C.M.V.; Ryu, S.; Bollmann, J.H. Classification of Object Size in Retinotectal Microcircuits. Curr. Biol. 2014, 24, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Roeser, T.; Baier, H. Visuomotor Behaviors in Larval Zebrafish after Gfp-Guided Laser Ablation of the Optic Tectum. J. Neurosci. 2003, 23, 3726–3734. [Google Scholar] [PubMed]
- Severi, K.E.; Portugues, R.; Marques, J.C.; O’Malley, D.M.; Orger, M.B.; Engert, F. Neural Control and Modulation of Swimming Speed in the Larval Zebrafish. Neuron 2014, 83, 692–707. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.W.; Vanwalleghem, G.C.; Heap, L.A.; Scott, E.K. Functional Profiles of Visual-, Auditory-, and Water Flow-Responsive Neurons in the Zebrafish Tectum. Curr. Biol. 2016, 26, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Strahle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments-a Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Ek, F.; Olsson, R. Using Visual Lateralization to Model Learning and Memory in Zebrafish Larvae. Sci. Rep. 2015, 5, 8667. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Lopes, G.; Kampff, A.R.; Wilson, S.W. Development of Social Behavior in Young Zebrafish. Front. Neural Circuits 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Huang, K.H.; Portugues, R.; Engert, F. Ontogeny of Classical and Operant Learning Behaviors in Zebrafish. Learn. Mem. 2012, 19, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.K.; Miri, A.; Leung, L.C.; Berndt, A.; Mourrain, P.; Tank, D.W.; Burdine, R.D. Prolonged, Brain-Wide Expression of Nuclear-Localized Gcamp3 for Functional Circuit Mapping. Front. Neural Circuits 2014, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Kassing, V.; Engelmann, J.; Kurtz, R. Monitoring of Single-Cell Responses in the Optic Tectum of Adult Zebrafish with Dextran-Coupled Calcium Dyes Delivered Via Local Electroporation. PLoS ONE 2013, 8, e62846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaev, A.; Leung, K.M.; Odermatt, B.; Lagnado, L. Synaptic Mechanisms of Adaptation and Sensitization in the Retina. Nat. Neurosci. 2013, 16, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, N.; Lowe, A.S.; Walker, A.S.; Abbas, F.; Hunter, P.R.; Thompson, I.D.; Meyer, M.P. Parametric Functional Maps of Visual Inputs to the Tectum. Neuron 2012, 76, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odermatt, B.; Nikolaev, A.; Lagnado, L. Encoding of Luminance and Contrast by Linear and Nonlinear Synapses in the Retina. Neuron 2012, 73, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Jetti, S.K.; Vendrell-Llopis, N.; Yaksi, E. Spontaneous Activity Governs Olfactory Representations in Spatially Organized Habenular Microcircuits. Curr. Biol. 2014, 24, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Yoshida, M.; Kawakami, K.; Hibi, M.; Shimizu, T. Granule Cells Control Recovery from Classical Conditioned Fear Responses in the Zebrafish Cerebellum. Sci. Rep. 2017, 7, 11865. [Google Scholar] [CrossRef] [PubMed]
- Vendrell-Llopis, N.; Yaksi, E. Evolutionary Conserved Brainstem Circuits Encode Category, Concentration and Mixtures of Taste. Sci. Rep. 2015, 5, 17825. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Schoch, H.; Granato, M. Distinct Retinal Pathways Drive Spatial Orientation Behaviors in Zebrafish Navigation. Curr. Biol. 2010, 20, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Engert, F. Visuomotor Transformations Underlying Hunting Behavior in Zebrafish. Curr. Biol. 2015, 25, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Ohkura, M.; Abe, G.; Nakai, J.; Kawakami, K. Real-Time Visualization of Neuronal Activity During Perception. Curr. Biol. 2013, 23, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; del Bene, F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Temizer, I.; Donovan, J.C.; Baier, H.; Semmelhack, J.L. A Visual Pathway for Looming-Evoked Escape in Larval Zebrafish. Curr. Biol. 2015, 25, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Lowe, A.S.; Thompson, I.D.; Meyer, M.P. Emergent Properties of the Optic Tectum Revealed by Population Analysis of Direction and Orientation Selectivity. J. Neurosci. 2013, 33, 13940–13945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronchain, O.J.; Hartley, K.O.; Amaya, E. A Gene Trap Approach in Xenopus. Curr. Biol. 1999, 9, 1195–1198. [Google Scholar] [CrossRef]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging Neural Activity in Worms, Flies and Mice with Improved Gcamp Calcium Indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Transposon Tools and Methods in Zebrafish. Dev. Dyn. 2005, 234, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Tol2: A Versatile Gene Transfer Vector in Vertebrates. Genome Biol. 2007, 8, S7. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Sumiyama, K.; Kawakami, K. Transposon-Mediated Bac Transgenesis in Zebrafish and Mice. BMC Genom. 2009, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Balciunas, D.; Wangensteen, K.J.; Wilber, A.; Bell, J.; Geurts, A.; Sivasubbu, S.; Wang, X.; Hackett, P.B.; Largaespada, D.A.; McIvor, R.S.; et al. Harnessing a High Cargo-Capacity Transposon for Genetic Applications in Vertebrates. PLoS Genet. 2006, 2, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.A.; Robertson, C.P.; Lepage, T.; Johnson, S.L.; Raible, D.W. Nacre Encodes a Zebrafish Microphthalmia-Related Protein That Regulates Neural-Crest-Derived Pigment Cell Fate. Development 1999, 126, 3757–3767. [Google Scholar] [PubMed]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Brainard, D.; Pelli, D. What’s New in Psychtoolbox-3? Perception 2007, 36, 1–16. [Google Scholar]
- Pelli, D.G. The Videotoolbox Software for Visual Psychophysics: Transforming Numbers into Movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Dorostkar, M.M.; Dreosti, E.; Odermatt, B.; Lagnado, L. Computational Processing of Optical Measurements of Neuronal and Synaptic Activity in Networks. J. Neurosci. Methods 2010, 188, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Thevenaz, P.; Ruttimann, U.E.; Unser, M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Niell, C.M.; Smith, S.J. Functional Imaging Reveals Rapid Development of Visual Response Properties in the Zebrafish Tectum. Neuron 2005, 45, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sajovic, P.; Levinthal, C. Visual Cells of Zebrafish Optic Tectum: Mapping with Small Spots. Neuroscience 1982, 7, 2407–2426. [Google Scholar] [CrossRef]
- Walker, A.S.; Burrone, J.; Meyer, M.P. Functional Imaging in the Zebrafish Retinotectal System Using Rgeco. Front. Neural Circuits 2013, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, R.C.; de Polavieja, G.G. Ontogeny of Collective Behavior Reveals a Simple Attraction Rule. Proc. Natl. Acad. Sci. USA 2017, 114, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Gerlai, R. Shoaling Develops with Age in Zebrafish (Danio rerio). Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Mathuru, A.S.; Kibat, C.; Rahman, M.; Lupton, C.E.; Stewart, J.; Claridge-Chang, A.; Yen, S.C.; Jesuthasan, S. The Right Dorsal Habenula Limits Attraction to an Odor in Zebrafish. Curr. Biol. 2014, 24, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, V.; Lucks, V.; Kurtz, R.; Engelmann, J. Adaptation-Induced Modification of Motion Selectivity Tuning in Visual Tectal Neurons of Adult Zebrafish. J. Neurophysiol. 2015, 114, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Rombough, P.; Drader, H. Hemoglobin Enhances Oxygen Uptake in Larval Zebrafish (Danio rerio) but Only under Conditions of Extreme Hypoxia. J. Exp. Biol. 2009, 212, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Olt, J.; Allen, C.E.; Marcotti, W. In Vivo Physiological Recording from the Lateral Line of Juvenile Zebrafish. J. Physiol. 2016, 594, 5427–5438. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Odermatt, B.; Dorostkar, M.M.; Lagnado, L. A Genetically Encoded Reporter of Synaptic Activity in Vivo. Nat. Methods 2009, 6, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Wang, S.Z.; Zhong, W.; Liu, B.H.; Tao, H.W. Functional Elimination of Excitatory Feedforward Inputs Underlies Developmental Refinement of Visual Receptive Fields in Zebrafish. J. Neurosci. 2011, 31, 5460–5469. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, K.; Meza Santoscoy, P.; Lygdas, K.; Nikolaeva, Y.; MacDonald, R.B.; Cunliffe, V.T.; Nikolaev, A. Imaging Neuronal Activity in the Optic Tectum of Late Stage Larval Zebrafish. J. Dev. Biol. 2018, 6, 6. https://doi.org/10.3390/jdb6010006
Bergmann K, Meza Santoscoy P, Lygdas K, Nikolaeva Y, MacDonald RB, Cunliffe VT, Nikolaev A. Imaging Neuronal Activity in the Optic Tectum of Late Stage Larval Zebrafish. Journal of Developmental Biology. 2018; 6(1):6. https://doi.org/10.3390/jdb6010006
Chicago/Turabian StyleBergmann, Katharina, Paola Meza Santoscoy, Konstantinos Lygdas, Yulia Nikolaeva, Ryan B. MacDonald, Vincent T. Cunliffe, and Anton Nikolaev. 2018. "Imaging Neuronal Activity in the Optic Tectum of Late Stage Larval Zebrafish" Journal of Developmental Biology 6, no. 1: 6. https://doi.org/10.3390/jdb6010006




