Phylogeny of Cirsium spp. in North America: Host Specificity Does Not Follow Phylogeny
Abstract
:1. Introduction
2. Results
| Genus | Species | Source | Voucher at NA | ITS | ETS |
|---|---|---|---|---|---|
| Cirsium | arvense IN1.1 | TBS2004-13 | 48765 | JX867618 | JX867646 |
| Cirsium | arvense Itasca 1 | TBS2004-70 | 48793 | JX867619 | JX867647 |
| Cirsium | arvense Itasca 6 | TBS2004-71 | 48792 | JX867620 | JX867648 |
| Cirsium | arvense MN3 | TBS2004-33 | 48790 | JX867621 | JX867649 |
| Cirsium | arvense ND25s8 | TBS2004-72 | 48842 | JX867622 | JX867650 |
| Cirsium | arvense ND26s35 | TBS2004-58 | 48834 | JX867623 | JX867651 |
| Cirsium | arvense TS | TBS2004-30 | 48817 | JX867624 | JX867652 |
| Cirsium | canescens SD7 | TBS | ---- | JX867625 | JX867653 |
| Cirsium | canovirens | TBS2005-36 | 48883 | JX867626 | JX867654 |
| Cirsium | flodmanii 64 | TBS2004-64 | 48867 | JX867627 | JX867655 |
| Cirsium | flodmanii 905 | TBS2004-62 | 48861 | JX867628 | JX867656 |
| Cirsium | flodmanii SD7 | TBS2005-25 | 48870 | JX867629 | JX867657 |
| Cirsium | flodmanii TS | TBS2004-60 | 48872 | JX867630 | JX867658 |
| Cirsium | foliosum | TBS2005-33 | 48878 | JX867631 | JX867659 |
| Cirsium | hillii | Jeremie Fant, Chicago Bot Gard | JX867632 | JX867660 | |
| Cirsium | muticum | TBS2005-17 | 48873 | JX867633 | JX867661 |
| Cirsium | pitcheri | Jeremie Fant, Chicago Bot Gard | JX867634 | JX867662 | |
| Cirsium | undulatum 903.1 | TBS2004-59 | 48877 | JX867635 | JX867663 |
| Cirsium | undulatum 904.1 | TBS2004-59 | 48877 | JX867636 | JX867664 |
| Cirsium | undulatum SD | TBS sn. 27 July 2005 | 48876 | JX867637 | JX867665 |
| Cirsium | vulgare 1.1 | MF2 | 48794 | JX867638 | JX867666 |
| Cirsium | vulgare 2.1 | MF2 | 48794 | JX867639 | JX867667 |
| Cirsium | vulgare SD7 | TBS2005-26 | 48812 | JX867640 | JX867668 |
| Cirsium | andersonii | GenBank | AF443683 | AF443735 | |
| Cirsium | andrewsii | GenBank | AF443684 | AF443736 | |
| Cirsium | arvense clone 1 | GenBank | AF443680 | AF443734 | |
| Cirsium | arvense clone 2 | GenBank | AF443681 | AF443734 | |
| Cirsium | arvense clone 3 | GenBank | AF443682 | AF443734 | |
| Cirsium | brevistylum | GenBank | AF443685 | AF443737 | |
| Cirsium | calcareum | GenBank | AF443687 | AF443739 | |
| Cirsium | canovirens | GenBank | AF443688 | AF443740 | |
| Cirsium | canum | GenBank | AF443689 | AF443741 | |
| Cirsium | congdonii | GenBank | AF443690 | AF443742 | |
| Cirsium | cymosum | GenBank | AF443691 | AF443743 | |
| Cirsium | discolor | GenBank | AF443692 | AF443744 | |
| Cirsium | douglasii | GenBank | AF443686 | AF443738 | |
| Cirsium | eatonii | GenBank | AF443694 | AF443746 | |
| Cirsium | edule | GenBank | AF443711 | AF443763 | |
| Cirsium | ehrenbergii | GenBank | AF443726 | AF443778 | |
| Cirsium | faucium | GenBank | AF443725 | AF443777 | |
| Cirsium | fontinale var. obispoense | GenBank | AF443696 | AF443748 | |
| Cirsium | henryi | GenBank | AF443697 | AF443749 | |
| Cirsium | hydrophilum | GenBank | AF443698 | AF443750 | |
| Cirsium | jorullense | GenBank | AF443699 | AF443751 | |
| Cirsium | lineare | GenBank | AF443727 | AF443779 | |
| Cirsium | mohavense | GenBank | AF443700 | AF443752 | |
| Cirsium | monocephalum | GenBank | AF443701 | AF443753 | |
| Cirsium | monspessulanum | GenBank | AF443717 | AF443769 | |
| Cirsium | muticum | GenBank | AF443722 | AF443774 | |
| Cirsium | neomexicanum | GenBank | AF443718 | AF443770 | |
| Cirsium | occidentale var. venustum | GenBank | AF443703 | AF443755 | |
| Cirsium | occidentale | GenBank | AF443702 | AF443754 | |
| Cirsium | palustre | GenBank | AF443704 | AF443756 | |
| Cirsium | pitcheri | GenBank | AF443705 | AF443757 | |
| Cirsium | quercetorum | GenBank | AF443706 | AF443758 | |
| Cirsium | remotifolium | GenBank | AF443707 | AF443759 | |
| Cirsium | rhaphilepis | GenBank | AF443708 | AF443760 | |
| Cirsium | rhothophilum | GenBank | AF443709 | AF443761 | |
| Cirsium | rydbergii | GenBank | AF443710 | AF443762 | |
| Cirsium | scariosum | GenBank | AF443693 | AF443745 | |
| Cirsium | spinosissimum | GenBank | AF443720 | AF443772 | |
| Cirsium | subniveum | GenBank | AF443712 | AF443764 | |
| Cirsium | tioganum | GenBank | AF443721 | AF443773 | |
| Cirsium | tymphaeum | GenBank | AF443723 | AF443775 | |
| Cirsium | utahense | GenBank | AF443713 | AF443765 | |
| Cirsium | velatum | GenBank | AF443714 | AF443766 | |
| Cirsium | vulgare clone 1 | GenBank | AF443715 | AF443767 | |
| Cirsium | vulgare clone 2 | GenBank | AF443716 | AF443738 | |
| Cirsium | wheeleri | GenBank | AF443719 | AF443771 | |
| Carduus | acanthoides | MF3 | 48795 | JX867643 | JX867669 |
| Carduus | nutans | GenBank | AF443678 | AF443730 | |
| Carduus | nutans | TBS2005-14 | 48801 | JX867642 | JX867670 |
| Carduus | tenuiflorus | GenBank | AF44679 | AF4433731 | |
| Carthamus | oxyacanthus | GenBank | AJ867986-7 | AJ867985 | |
| Centaurea | rigidi | GenBank | AJ867989 | AJ867988 | |
| Cynara | scolymus | TBS | greenhouse grown | JX867643 | JX867671 |
| Helianthus | anuus | TBS | greenhouse grown | JX867644 | Not sequenced |
| Jurinea | narynensi | GenBank | AJ868001-2 | AJ868000 | |
| Onopordum | acaulon | GenBank | AF443676 | AF443728 | |
| Onopordum | illyricum | GenBank | AY78046 | AF4433729 | |
| Saussurea | riederi | GenBank | AJ868070-1 | AJ868069 | |
| Tagetes | spp. | TBS | greenhouse grown | JX867645 | JX867672 |
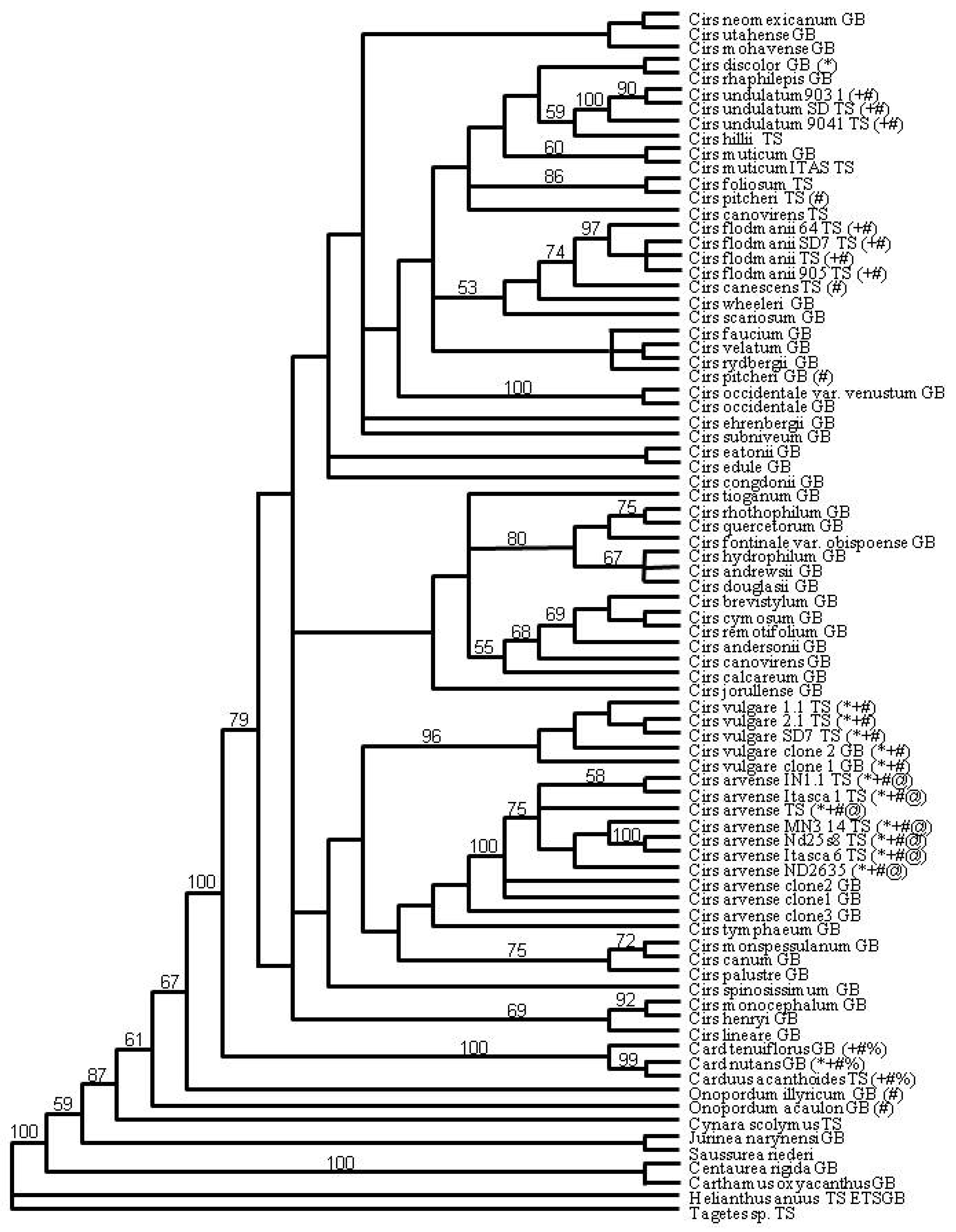
3. Discussion
4. Experimental Section
4.1. Plant Material
4.2. Amplification and Sequencing
4.3. Data Analysis
5. Conclusions
Acknowledgment
References
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Elstrand, N.C.; et al. The population biology of invasive species. Ann. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Simberloff, D. How much information on population biology is needed to manage introduced species? Conserv. Biol. 2003, 17, 83–92. [Google Scholar] [CrossRef]
- Louda, S.M.; Rand, T.A.; Russell, F.L.; Arnett, A.E. Assessment of ecological risks in weed biocontrol: Input from retrospective ecological analyses. Biol. Control 2005, 35, 253–264. [Google Scholar] [CrossRef]
- Hodgson, J.M. The response of Canada thistle ecotypes to 2,4-D, amitrole, and intensive cultivation. Weed Sci. 1970, 18, 253–254. [Google Scholar]
- Donald, W.W. Management and control of Canada thistle (Cirsium arvense). Rev. Weed Sci. 1990, 5, 193–250. [Google Scholar]
- Nissen, S.J.; Masters, R.A.; Lee, D.J.; Rowe, M.L. DNA-based markers systems to determine genetic diversity of weedy species and their application to biocontrol. Weed Sci. 1995, 44, 504–513. [Google Scholar]
- Lavergne, S.; Molofsky, J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Natl. Acad. Sci. USA 2007, 104, 3883–3888. [Google Scholar]
- McClay, A.S. Canada thistle. In Biological Control of Invasive Plants in the Eastern United States; van Driesche, R., Blossey, B., Hoddle, M., Lyon, S., Reardon, R., Eds.; USDA Forest Service Publication FHTET-2002-04: USDA Forest Service, Morgantown, WV., USA, 2002; pp. 217–228. [Google Scholar]
- Tiley, G.E.D. Biological flora of the British Isles: Cirsium arvense (L.) Scop. J. Ecol. 2010, 98, 938–983. [Google Scholar] [CrossRef]
- Guggisberg, A.; Welk, E.; Sforza, R.; Horvath, D.P.; Anderson, J.V.; Foley, M.E.; Rieseberg, L.H. Invasion history of North American Canada thistle, Cirsium arvense. J. Biogeogr. 2012, 39, 1919–1931. [Google Scholar] [CrossRef]
- Voss, E.G. Michigan Flora: Part III Dicots (Pyrolaceae to Compositae); Cranbrook Institute: Bloomfield Hills, MI, USA, 1996; pp. 510–524. [Google Scholar]
- Invaders Database System. Available online: http://invader.dbs.umt.edu/noxious_weeds/noxlist.asp/ (accessed on 18 July 2012).
- Lym, R.G.; Duncan, C.L. Canada thistle Cirsium arvense (L.) Scop. In Invasive Plants of Range and Wildlands and Their Environmental, Economic, and Societal Impacts; Duncan, C.L., Clark, J.K., Eds.; Weed Science Society of America: Lawrence, KS, USA, 2005; pp. 69–83. [Google Scholar]
- Hoeft, E.V.; Jordan, N.; Zhang, J.; Wyse, D.L. Integrated cultural and biological control of Canada thistle in conservation tillage soybean. Weed Sci. 2001, 49, 642–646. [Google Scholar] [CrossRef]
- Tichich, R.P.; Doll, J.D. Field-based evaluation of a novel approach for infecting Canada thistle (Cirsium arvense) with Pseudomonas syringae pv. tagetis. Weed Sci. 2006, 54, 166–171. [Google Scholar] [CrossRef]
- Cripps, M.G.; Gassmann, A.; Fowler, S.V.; Bourdôt, G.W.; McClay, A.S.; Edwards, G.R. Classical biological control of Cirsium arvense: Lessons from the past. Biol. Control 2011, 57, 165–174. [Google Scholar] [CrossRef]
- Louda, S.M.; O’Brien, C.W. Unexpected ecological effects of distributing the exotic weevil, Larinus planus (F), for the biological control of Canada thistle. Conserv. Biol. 2002, 16, 717–727. [Google Scholar] [CrossRef]
- Louda, S.M.; Pemberton, R.W.; Johnson, M.T.; Follett, P.A. Nontarget effects—The Achilles’ Heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Ann. Rev. Entomol. 2003, 48, 365–396. [Google Scholar]
- Rand, T.A.; Louda, S.M. Exotic weed invasion increases the susceptibility of native plants to attack by a biocontrol herbivore. Ecology 2004, 85, 1548–1554. [Google Scholar]
- Kelch, D.G.; Baldwin, B.G. Phylogeny and ecological radiation of New World thistles (Cirsium, Cardueae-Compositae) based on ITS and ETS rDNA sequence data. Mol. Ecol. 2003, 12, 141–151. [Google Scholar]
- Slotta, T.A.B.; Rothhouse, J.; Horvath, D.P.; Foley, M.E. Genetic Diversity of Canada thistle (Cirsium arvense) in North Dakota. Weed Sci. 2006, 54, 1080–1085. [Google Scholar] [CrossRef]
- Slotta, T.A.B.; Foley, M.E.; Chao, S.; Hufbauer, R.A.; Horvath, D.P. Assessing genetic diversity of Canada thistle (Cirsium arvense) in North America with microsatellites. Weed Sci. 2010, 58, 387–394. [Google Scholar] [CrossRef]
- Garcia-Jacas, N.; Garnatje, T.; Susanna, A.; Vilatersana, R. Tribal and subtribal delimitation and phylogeny of the Cardueae (Asteraceae): A combined nuclear and chloroplast DNA analysis. Mol. Phylogenet. Evol. 2002, 22, 51–64. [Google Scholar]
- Bruckart, W.L.; Poltis, D.J.; DeFago, G.; Rosenthal, S.S.; Supkoff, D.M. Susceptibility of Carduus, Cirsium and Cynara scolymus species artificially inoculated with Puccinia carduorum from musk thistle. Biol. Control 1996, 6, 215–221. [Google Scholar] [CrossRef]
- Kissinger, D.G. Curculionidae of America North of Mexico, A Key to the Genera; Taxonomic Publications: South Lancaster, MA, USA, 1964; p. 143. [Google Scholar]
- Moore, R.J.; Frankton, C. Cytotaxonomy of Cirsium species of the Eastern United States, with a key to the eastern species. Can. J. Bot. 1969, 47, 1257–1275. [Google Scholar] [CrossRef]
- Lym, R.G.; Christianson, K.M. The Thistles of North Dakota, North Dakota State University Extension Service Bulletin; W-1120; North Dakota State University: Fargo, ND, USA, 1996. [Google Scholar]
- Kartesz, J.T. The Biota of North America Program (BONAP). North American Plant Atlas. Available online: http://www.bonap.org/MapSwitchboard.html/ (accessed on 17 July 2012).
- Zwölfer, H. Evolutionary and ecological relationships of the insect fauna of thistles. Ann. Rev. Entomol. 1988, 33, 103–122. [Google Scholar]
- Volovnik, S.V. Distribution and ecology of some species of Cleoninae (Coleoptera, Curculionidae). III. Genus Larinus Germ. Entomol. Rev. 1996, 75, 10–19. [Google Scholar]
- Zwölfer, H.; Harris, P. Biology and host specificity of Rhinocyllus conicus (Froel.) (Col., Curculionidae), a successful agent for biocontrol of the thistle, Carduus nutans L. Z. Angew. Entomol. 1984, 97, 36–62. [Google Scholar]
- Arnett, A.; Louda, S. Re-test of Rhinocyllus conicus host specificity and the prediction of ecological risk in biological control. Biol. Conserv. 2002, 106, 251–257. [Google Scholar] [CrossRef]
- Rees, N.E. Impact of Rhinocyllus conicus on thistles in southwestern Montana. Environ. Entomol. 1977, 6, 839–842. [Google Scholar]
- Louda, S.M.; Kendall, D.; Connor, J.; Simberloff, D. Ecological effects of an insect introduced for the biological control of weeds. Science 1997, 277, 1088–1090. [Google Scholar] [CrossRef]
- Ward, R.H.; Pienkowski, R.L.; Kok, L.T. Host specificity of the first-instar of Ceuthorhynchidius horridus, a weevil for biological control of thistle. J. Econ. Entomol. 1974, 67, 735–737. [Google Scholar]
- Wiggins, G.J.; Grant, J.F.; Lambdin, P.L.; Ranney, J.W.; Wilkerson, J.B.; van Manen, F.T. Spatial prediction of habitat overlap of introduced and native thistles to identify potential areas of nontarget activity of biological control agents. Environ. Entomol. 2010, 39, 1866–1877. [Google Scholar] [CrossRef]
- Anderson, R.S. Weevils and plants: Phylogenetic versus ecological mediation of evolution of host plant associations in Curculioninae (Coleoptera: Curculionidae). Mem. Ent. Soc. Can. 1993, 165, 197–232. [Google Scholar] [CrossRef]
- Russell, F.L.; Louda, S.M. Indirect interaction between two native thistles mediated by an invasive exotic floral herbivore. Oecologia 2005, 146, 373–384. [Google Scholar] [CrossRef]
- Barton, J. Predictability of pathogen host range in classical biological control of weeds: An update. BioControl 2012, 57, 289–305. [Google Scholar] [CrossRef]
- Bruckart, W.L., III. Supplemental risk evaluations and status of Puccinia carduorum for biological control of musk thistle. Biol. Control 2005, 32, 348–355. [Google Scholar] [CrossRef]
- Berner, D.K.; Bruckart, W.L. Comparing predictions from mixed model equations with host range determinations from historical disease evaluation data of two previously released weed biological control pathogens. Biol. Control 2012, 60, 207–215. [Google Scholar]
- Bureš, P.; Wang, Y.-F.; Horová, L.; Suda, J. Genome size variation in central European species of Cirsium (Compositae) and their natural hybrids. Ann. Bot. 2004, 94, 353–363. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Bon, M.C.; Cock, M.J.W.; Cristofaro, M.; Biase, A.D.; de Clerck-Floate, R.; Ellison, C.A.; Hinz, H.L.; Hufbauer, R.A.; Julien, M.H.; et al. Applying molecular-based approaches to classical biological control of weeds. Biol. Control 2011, 58, 1–21. [Google Scholar] [CrossRef]
- Wapshere, A.J. Host specificity of phytophagous organisms and the evolutionary centres of plant genera or sub-genera. Entomophaga 1974, 19, 301–309. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar]
- Swofford, D.L. PAUP* Phylogenetic Analysis using Parsimony, ver. 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Slotta, T.A.B.; Horvath, D.P.; Foley, M.E. Phylogeny of Cirsium spp. in North America: Host Specificity Does Not Follow Phylogeny. Plants 2012, 1, 61-73. https://doi.org/10.3390/plants1020061
Slotta TAB, Horvath DP, Foley ME. Phylogeny of Cirsium spp. in North America: Host Specificity Does Not Follow Phylogeny. Plants. 2012; 1(2):61-73. https://doi.org/10.3390/plants1020061
Chicago/Turabian StyleSlotta, Tracey A. Bodo, David P. Horvath, and Michael E. Foley. 2012. "Phylogeny of Cirsium spp. in North America: Host Specificity Does Not Follow Phylogeny" Plants 1, no. 2: 61-73. https://doi.org/10.3390/plants1020061




