1. Introduction
The evolutionary and ecological factors that have promoted the diversity of plant sexual reproductive strategies have been a long-standing area of inquiry among evolutionary biologists. The evolutionary transition between outcrossing and selfing, in particular, has received considerable attention, in part because mating system shifts are widespread and have occurred independently within and across numerous taxa [
1,
2,
3]. Interest in the evolutionary association between floral traits and mating system dates back to Darwin [
4], who noted that self-fertilizing species often produce smaller and less conspicuous flowers than outcrossing congeners. Subsequent investigations have expanded upon Darwin’s work and found consistent differences between selfers and outcrossers in flower size [
5,
6,
7,
8], floral development rates [
9,
10], and pollen:ovule ratios [
11,
12,
13,
14]. The extent to which a species’ mating system influences its pollen performance, however, is not well understood (but see [
15,
16,
17,
18]). There are, however, several predictions concerning how plant mating systems should affect the strength, direction, and outcome of natural selection on pollen performance ([
19] and described here).
Pollen performance traits are often genetically based [
20,
21,
22,
23] and have been predicted to evolve in response to pollen competition if the pollen grains deposited onto stigmas are genetically variable with respect to pollen performance and their number routinely exceeds the number of ovules available for fertilization [
24,
25,
26,
27,
28,
29,
30,
31]. Because the haploid genotypes of pollen grains are expressed during the gametophyte stage, pollen competition may occur among different pollen donors as well as among the pollen grains of a single donor. Pollen competitive ability may be influenced by a variety of pollen characters, including pollen size and pollen grain volume [
32]. Post-pollination processes, including the speed of pollen germination and pollen tube growth through the style, have also been shown to play a major role in determining the siring success of individual male gametophytes [
33,
34,
35]. For example, when multiple pollen donors compete for access to ovules, rapid pollen tube growth through the style is positively correlated with siring success. Moreover, there is often strong overlap in gene expression between gametophytic and sporophytic life stages [
36,
37,
38], suggesting that genes contributing to the success of high-quality gametophytes also enhance the fitness of the subsequent sporophyte generation.
Evidence from studies conducted in natural populations suggests that pollen competition may regularly result in conditions favoring rapid pollen tube growth. First, although pollen limitation has been detected experimentally in many wild plant populations (reviewed by [
39,
40]), there are numerous plant species whose reproductive output is not limited by insufficient pollination (e.g., [
41,
42,
43,
44,
45]). Even in populations in which mean seed production per fruit is pollen-limited, many individual flowers may receive more pollen grains than the number of ovules available for fertilization, and in these flowers selection favoring rapid pollen tube growth may be intense. Moreover, many wild plant species receive more pollen than they require to achieve full seed set [
25,
30,
31,
46], although intra-population and within-plant variation in pollen loads may reduce the consistency of pollen competition [
47,
48]. Second, multiple paternity, wherein the seeds produced by a single fruit are sired by multiple donors, has been reported in several outcrossing species [
49,
50,
51,
52,
53,
54,
55], suggesting that selection among potential sires may occur when multiple pollen genotypes are deposited on stigmas if reproduction is not pollen-limited.
Recently, we proposed three predictions regarding the evolution of pollen performance in predominantly self-fertilizing and outcrossing taxa [
19]. Our predictions were based on the expectation that selfing and outcrossing taxa will differ in both the mean number of pollen genotypes deposited per stigma and the mean number of maternal plants to which individual sires are likely transfer their pollen. First, assuming that the stigmas of individual plants in highly outcrossing populations each receive on average a greater diversity of pollen genotypes than the stigmas of self-fertilizing individuals and that seed production per flower is not pollen-limited, there is a greater opportunity for selection among pollen genotypes in the styles of outcrossing taxa relative to closely related selfing taxa. Consequently, due to sustained selection in outcrossers over multiple generations for traits promoting the fertilization success of individual pollen grains, we predicted that pollen produced by outcrossing taxa should have evolved to germinate and/or to grow more rapidly through styles than the pollen of selfing sister taxa. Therefore, pollen from outcrossing taxa would exhibit faster germination and/or pollen tube growth than pollen from sister selfing taxa because of relatively relaxed selection on pollen performance in the latter since the time of evolutionary divergence. Moreover, if there are any physiological costs associated with rapid pollen tube growth, selfing taxa might even experience selection favoring slower growth. This prediction should also be considered in light of differences between outcrossers and selfers in floral size. Because outcrossers often produce larger flowers than their selfing relatives, their styles are also likely to be longer (e.g., [
9,
56]). In order to achieve fertilization success, the pollen tubes of outcrossers may travel a greater distance than their selfing counterparts, meaning that differences between selfers and outcrossers in pollen tube growth rates might not correspond to differences in how quickly pollen tubes reach the style base, particularly in cases where outcrossers have much longer styles than their selfing sister taxa. If pollen performance has evolved to be sufficiently accelerated in outcrossers and selection on pollen performance traits has relaxed in selfers, however, then we would also predict that the pollen tubes of outcrossers to travel a greater proportion of the style length per unit time than the pollen tubes of their selfing counterparts.
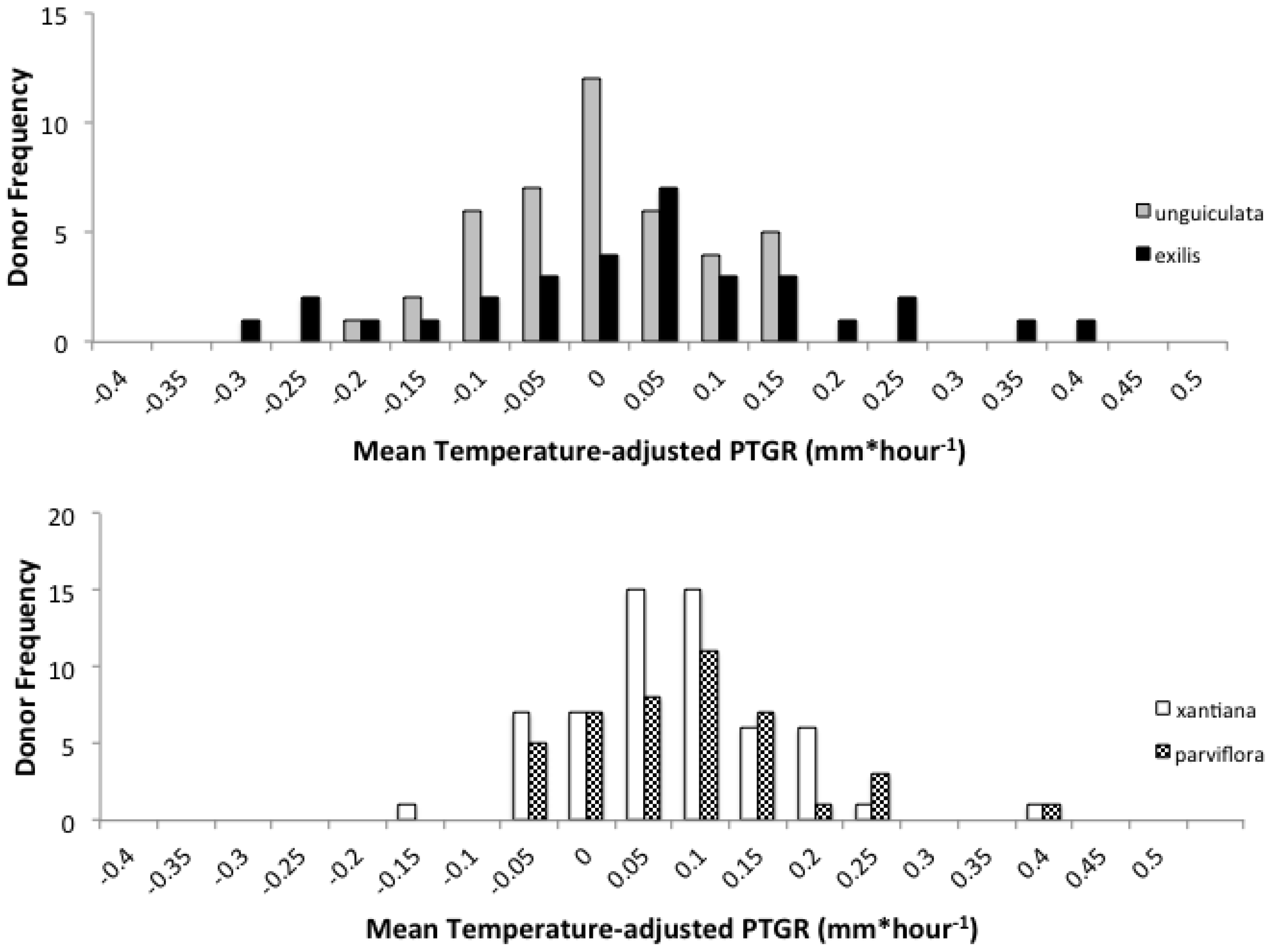
Second, because selfing reduces the number of distinct pollen genotypes deposited on individual stigmas, the comparatively stronger opportunity for selection on pollen performance traits in outcrossing taxa should have purged variation in these traits more effectively in outcrossers relative to selfers. Accordingly, we predicted that outcrossing taxa would harbor less genetically based variation among individual pollen donors in pollen performance traits than closely related selfing taxa. This prediction is based on the expectation that intense pollen competition among pollen genotypes will result in selection favoring rapid pollen germination and/or growth in outcrossing taxa. It should also be noted that maternal plant identity and environmental factors such as temperature and resource availability may also contribute to among-donor variation in pollen tube growth and germination in outcrossing taxa [
57,
58,
59,
60,
61,
62,
63,
64,
65] and that the intensity of pollen competition may vary within and among flowering seasons [
40]. Consequently, evidence of past purging of genetic variation may be difficult to detect. See [
19] for a detailed discussion of mechanisms that may contribute to the maintenance, or to the purging, of genetic variation in pollen performance.
Third, we predicted that in selfing taxa, potentially beneficial epistatic interactions (
i.e., combinations of alleles at different loci that result in particularly high performance) that influence pollen performance are likely to be reliably and consistently inherited over multiple generations and thus accumulate in populations. In other words, because the pollen of selfers experiences successive generations of pollen germination and pollen tube growth in genetically consistent maternal plant tissue, their pollen should evolve to be particularly well adapted to grow within its parental genotype. By contrast, because outcrossers typically mate with multiple, genetically distinct partners over their lifetimes, such beneficial epistatic interactions are more likely to break down due to recombination, and are therefore less likely to remain stable in outcrossing taxa than selfing taxa. Moreover, outcrossers should experience strong selection favoring the ability of pollen to perform well in a wide variety of stigmatic and stylar genotypes, while selection favoring such tolerance may be comparatively weak in autogamous selfers, whose pollen grains rarely germinate on foreign maternal genotypes. Consequently, we hypothesized that in regularly selfing taxa, pollen should have evolved to perform better following self-pollinations than following cross-pollinations. By contrast, in habitually outcrossing taxa, we predicted that pollen would either perform better following cross-pollinations than self-pollinations (e.g., [
66]) or that the pollen source (cross or self) would not significantly influence pollen performance (e.g., [
17,
67,
68]).
Previous research conducted on genotypes sampled from selfing and outcrossing
Clarkia tembloriensis (Onagraceae) populations and cultivated in growth chambers provides preliminary support for our predictions. Smith-Huerta [
17] and Kerwin and Smith-Huerta [
15] compared
in vivo pollen tube growth rates (PTGR) between two
C. tembloriensis populations (one highly selfing and one highly outcrossing). Both investigations found that pollen from the outcrossing population tended to grow more quickly than pollen from the selfing population. This difference between selfing and outcrossing populations was observed following both cross-pollinations and self-pollinations of individual pollen donors. To date, however, very few published studies have compared pollen performance between selfers and outcrossers in multiple populations under natural conditions where ecological factors such as pollinator service and climate may also influence the evolution of pollen performance traits (but see [
18]).
In recent decades, the annual wildflower genus
Clarkia has been the subject of extensive investigation of the evolution and ecology of mating system transitions (e.g., [
9,
10,
69,
70,
71,
72,
73,
74]). Within the genus, selfing has evolved independently from outcrossing numerous times [
56,
75,
76]. Here we investigate the correlated evolution of pollen performance traits and mating system in two pairs of predominantly outcrossing and selfing sister taxa that occur in the southern Sierra Nevada, California: the pollinator-dependent outcrosser
Clarkia xantiana ssp.
xantiana Gray (hereafter “
xantiana”) and its selfing sister subspecies,
C. xantiana ssp.
parviflora (Eastw.) H. Lewis and Raven (hereafter “
parviflora”), as well as the outcrosser
C. unguiculata Lindley (hereafter “
unguiculata”) and its selfing sister species
C. exilis H. Lewis and Vasek (hereafter “
exilis”). We address the following questions: (1) Does pollen from outcrossing taxa exhibit faster pollen germination and faster pollen tube growth rates (PTGR) than pollen from closely related selfing taxa in field populations? An observation of slower pollen germination or pollen tube growth in selfers than outcrossers would provide evidence in support of the hypothesis that, since the divergence between sister taxa, selection favoring rapid pollen performance traits in selfers has weakened relative to its strength in their outcrossing counterparts. Alternatively, in the absence of strong gametophytic selection (
i.e., in the selfers), if there is a fitness cost associated with rapid pollen germination or pollen tube growth, then selection may have favored lower rates of these traits in selfers relative to their outcrossing progenitors; (2) Do outcrossing taxa exhibit less phenotypic variation among individuals in pollen performance traits than their selfing sister taxa in field populations, consistent with the prediction that selection has more effectively purged variation in pollen performance in outcrossers due to more intense gametophytic competition among genotypes? and (3) Does pollen from selfing taxa (
exilis and
parviflora) exhibit faster growth following self-pollination than following cross-pollination? To address these questions, we evaluated three pollen performance traits in each taxon: pollen germination (a categorical variable indicating whether pollen tubes had penetrated the style), PTGR (the mean speed by which pollen tubes travel through the style), and the proportion of the entire style length traveled per hour (
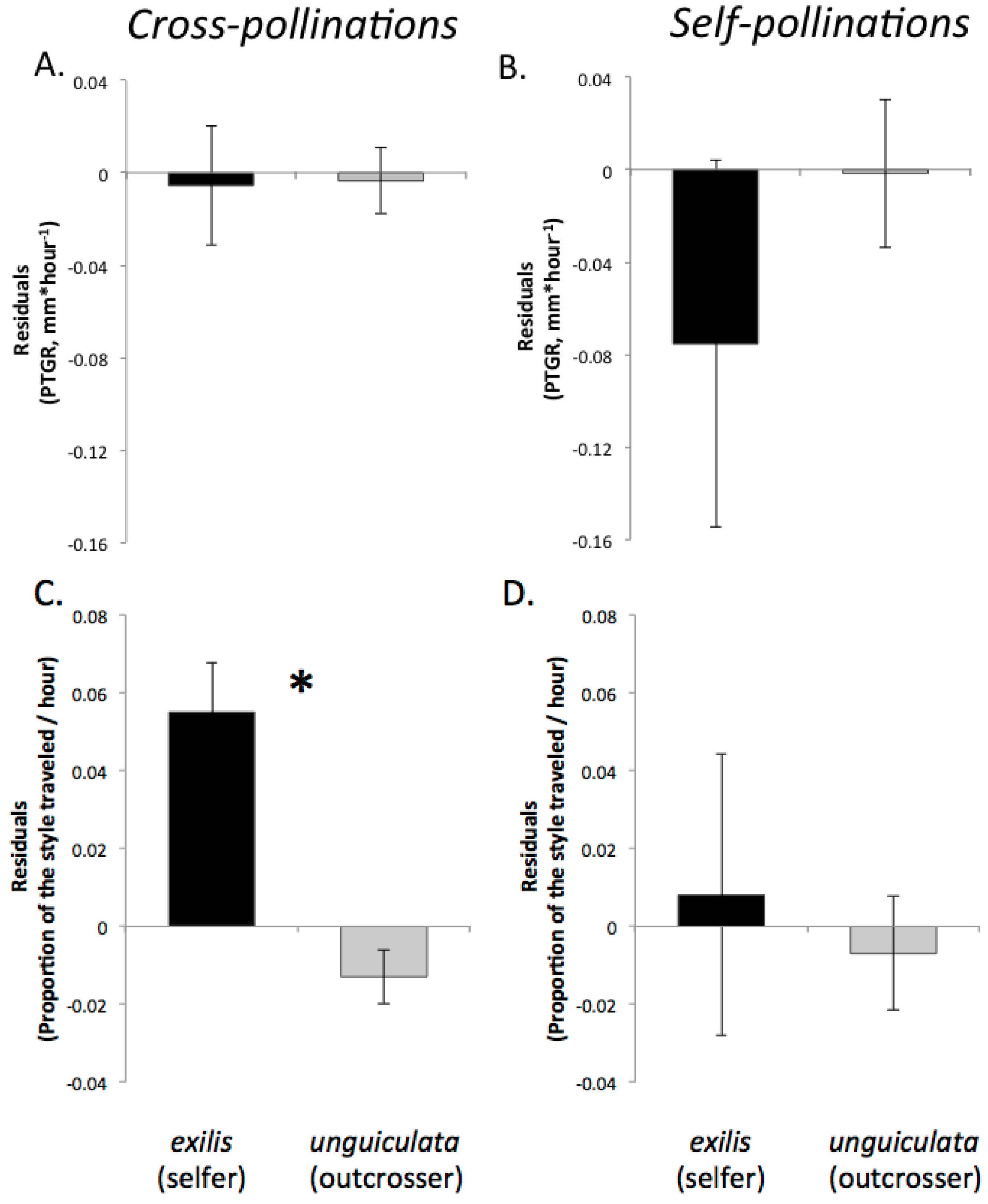
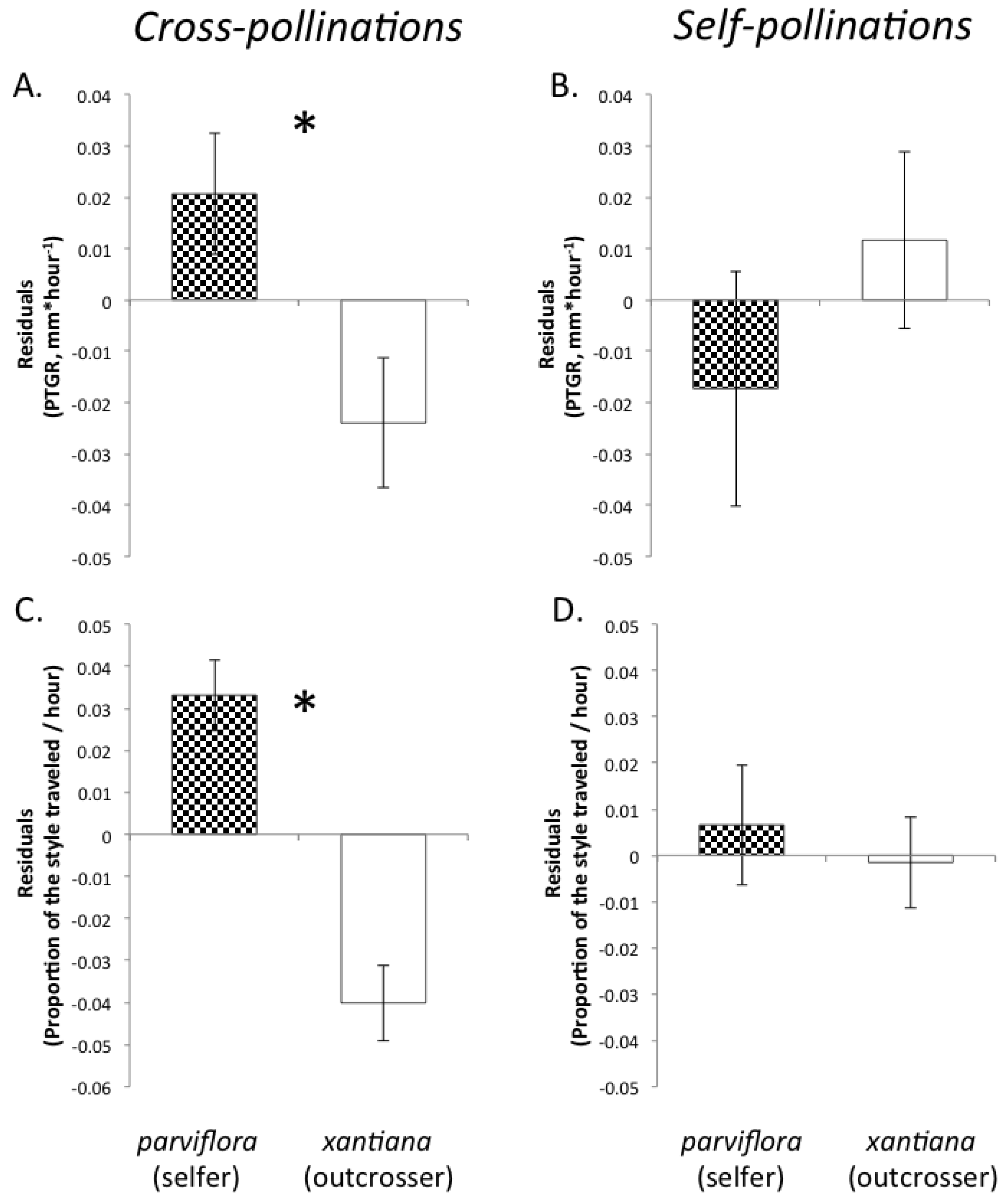
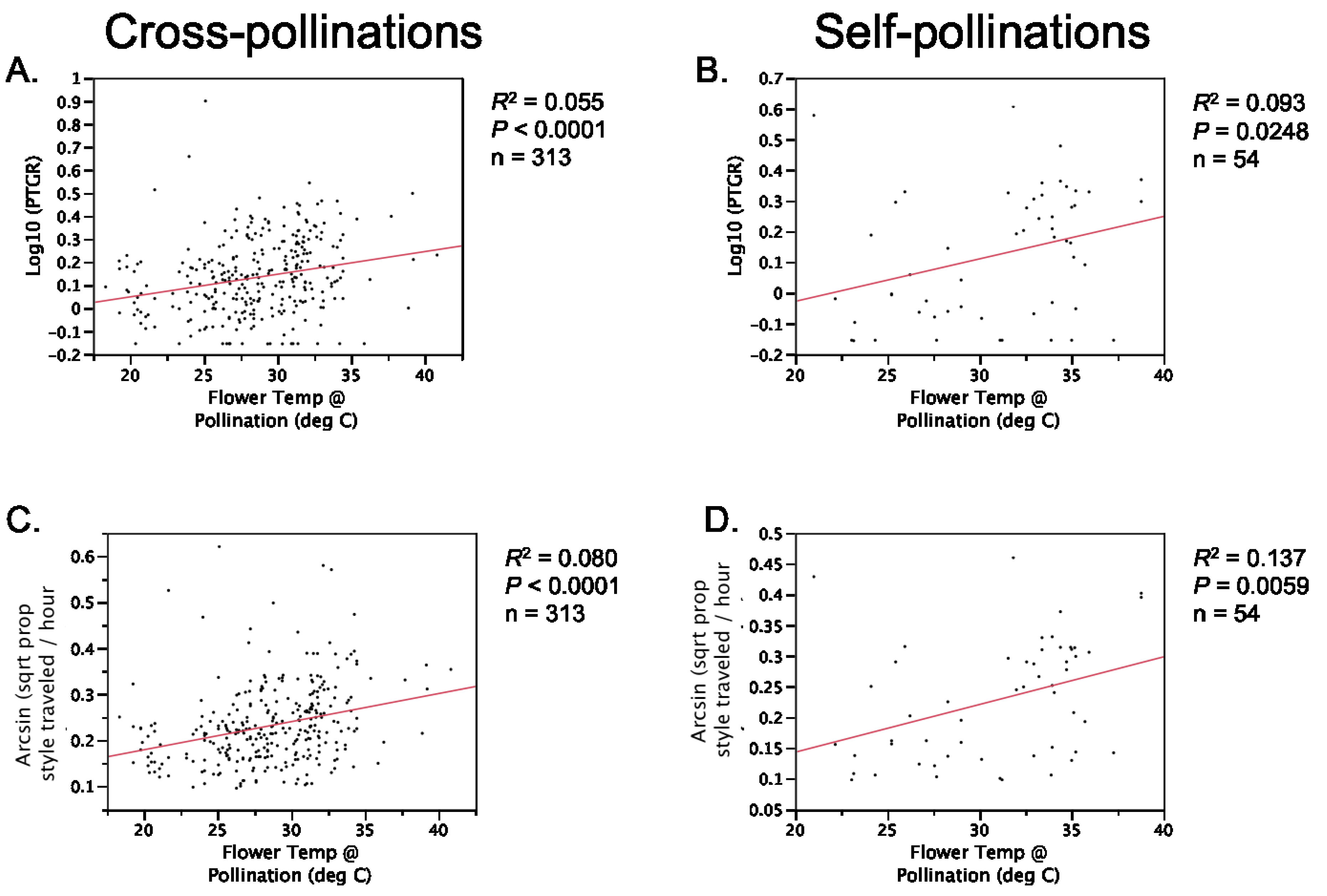
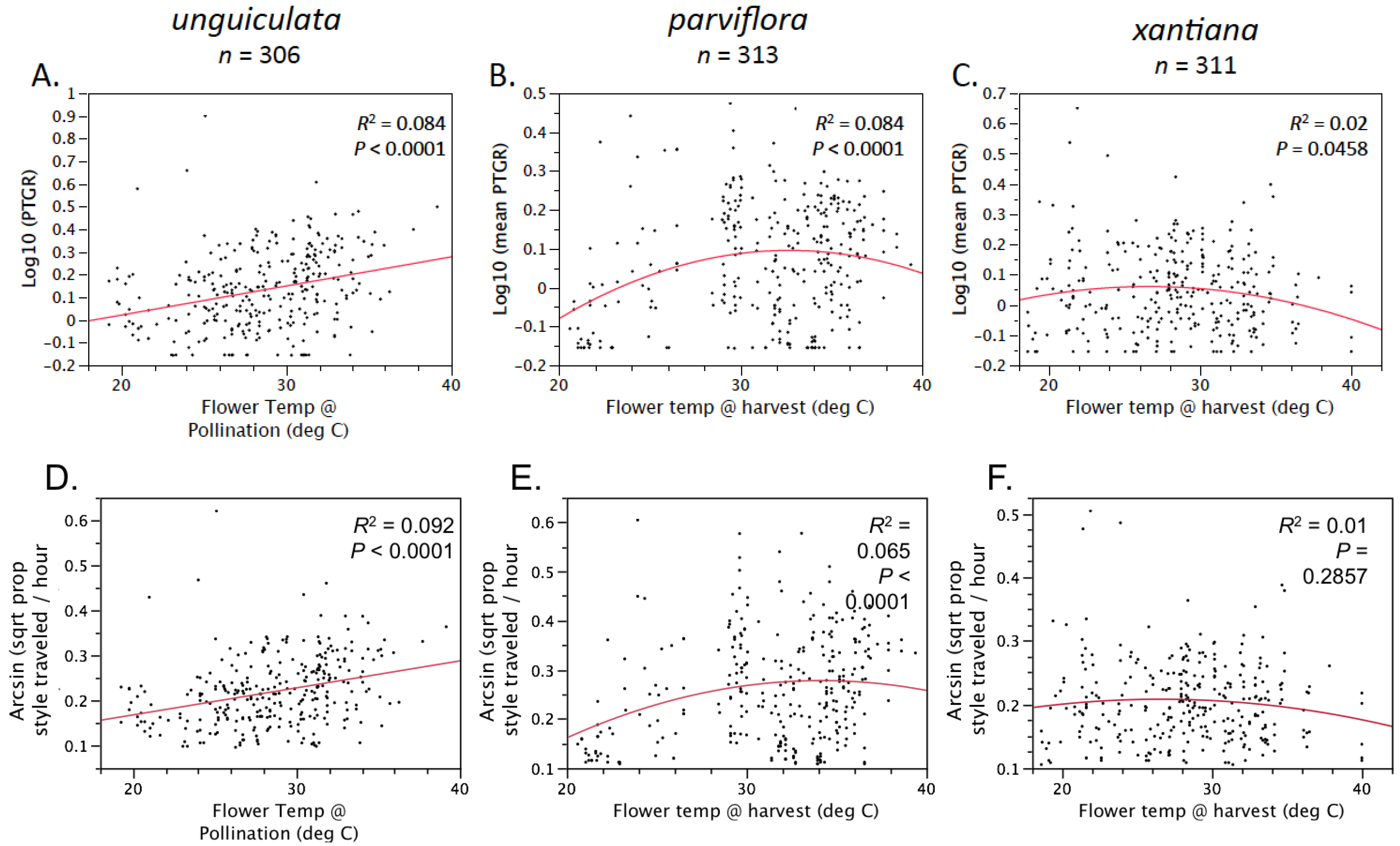
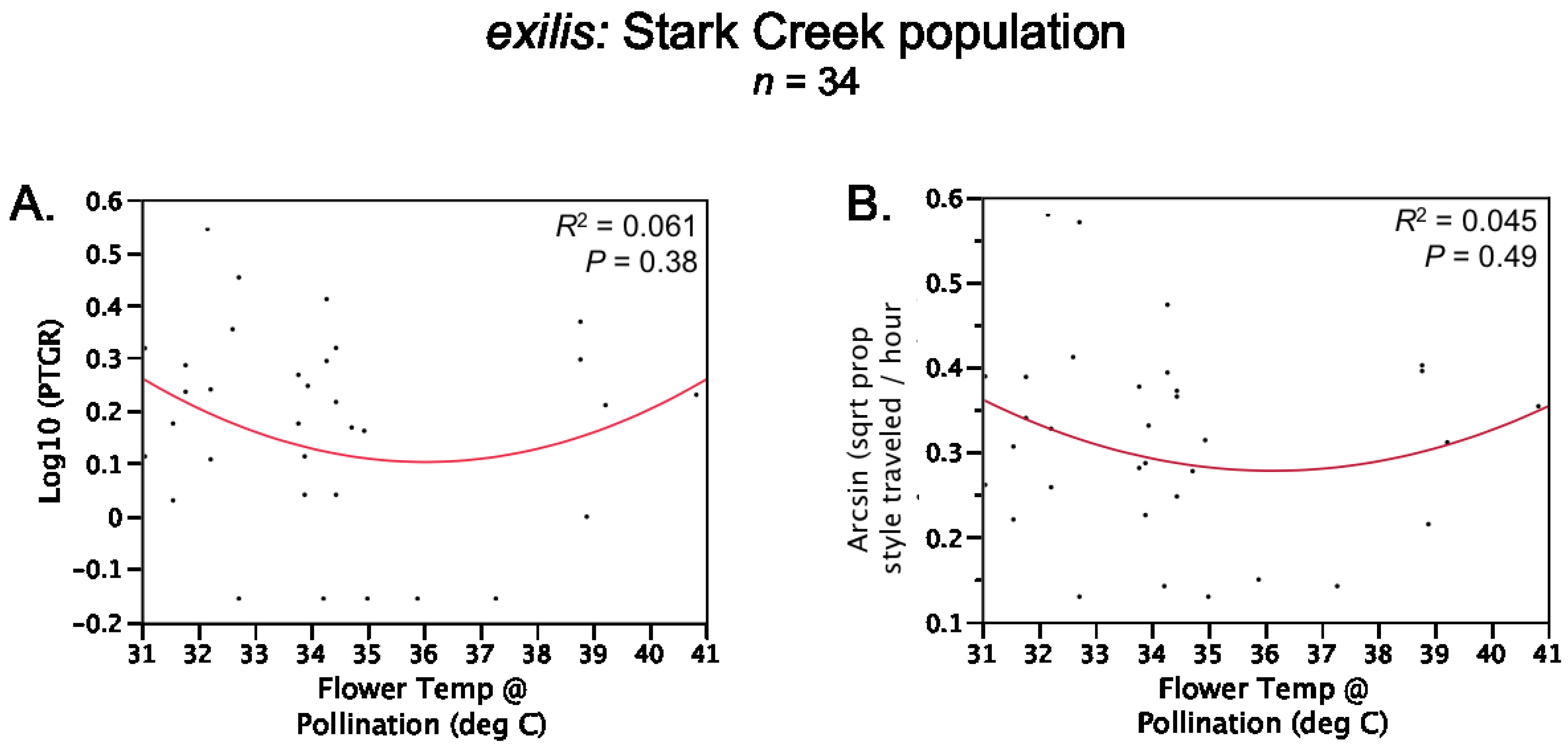
Table 1). Prior to comparing sister taxa with respect to the latter two traits, we controlled statistically for the effect of temperature on pollen tube growth. See the
Experimental Section for a detailed description of how traits were measured and compared between sister taxa.