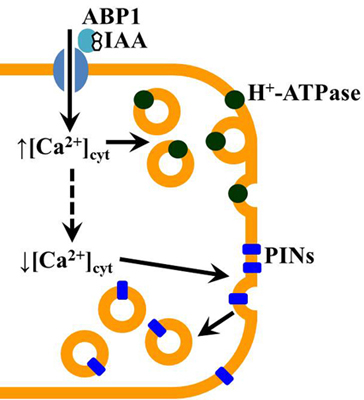
Ca2+-Transport through Plasma Membrane as a Test of Auxin Sensitivity
Abstract
:1. Introduction
2. Results and Discussion





3. Experimental
3.1. Plant Material
3.2. Measurement of Calcium Transport Rate across Plasma Membrane Vesicles from Maize Coleoptile Cells
4. Conclusions
Abbreviations
| ABP1 | auxin binding protein 1 |
| AFB | auxin F-box protein |
| AU | arbitrary units |
| [Ca2+]cyt | concentration of Ca2+ in cytoplasm |
| cyt | cytosol |
| diS-C3-(5) | 3,3'-dipropylthiodicarbocyanine iodide |
| ER | endoplasmic reticulum |
| IAA | indole-3-acetic acid |
| MP | membrane potential |
| 1-NAA | naphthalene-1-acetic acid |
| PLA2 | phospholipase A2 |
| PM | plasma membrane |
| SEM | standard error of the mean |
| TIR1 | transport inhibitor resistant 1 |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef]
- Badescu, G.O.; Napier, R.M. Receptors for auxin: Will it all end in TIRs? Trends Plant Sci. 2006, 11, 217–223. [Google Scholar] [CrossRef]
- Scherer, G.F.; Zahn, M.; Callis, J.; Jones, A.M. A role for phospholipase A in auxin-regulated gene expression. FEBS Lett. 2007, 581, 4205–4211. [Google Scholar] [CrossRef]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Scherer, G.F. Auxin-binding-protein1, the second auxin receptor: What is the significance of a two-receptor concept in plant signal transduction? J. Exp. Bot. 2011, 62, 3339–3357. [Google Scholar] [CrossRef]
- Napier, R.M.V.; Venis, M.A. Tansley review No-79—Auxin action and auxin-binding proteins. New Phytol. 1995, 129, 167–201. [Google Scholar] [CrossRef]
- Shishova, M.; Lindberg, S. A new perspective on auxin perception. J. Plant Physiol. 2010, 167, 417–422. [Google Scholar] [CrossRef]
- Perrot-Rechenmann, C. Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Sauer, M.; Kleine-Vehn, J. Auxin binding protein1: The outsider. Plant Cell 2011, 23, 2033–2043. [Google Scholar] [CrossRef]
- Venis, M.A. Solubilisation and partial purification of auxin-binding sites of corn membranes. Nature 1977, 266, 268–269. [Google Scholar] [CrossRef]
- Shimomura, S.; Sotobayashi, T.; Futai, M.; Fukui, T. Purification and properties of an auxin-binding protein from maize shoot membranes. J. Biochem. 1986, 99, 1513–1524. [Google Scholar]
- Napier, R.M.; Venis, M.A.; Bolton, M.A.; Richardson, L.I.; Butcher, D.W. Preparation and characterisation of monoclonal and polyclonal antibodies to maize membrane auxin-binding protein. Planta 1988, 176, 519–526. [Google Scholar] [CrossRef]
- Palme, K. Molecular analysis of plant signaling elements: Relevance of eukaryotic signal transduction models. Int. Rev. Cytol. 1992, 132, 223–283. [Google Scholar] [CrossRef]
- Shimomura, S.; Liu, W.; Inohara, N.; Watanabe, S.; Futai, M. Structure of the gene for an auxin-binding protein and a gene for 7SL RNA from Arabidopsis thaliana. Plant Cell Physiol. 1993, 34, 633–637. [Google Scholar]
- Shimomura, S.; Watanabe, S.; Ichikawa, H. Characterization of auxin-binding protein 1 from tobacco: Content, localization and auxin-binding activity. Planta 1999, 209, 118–125. [Google Scholar] [CrossRef]
- Lazarus, C.M.; Macdonald, H. Characterization of a strawberry gene for auxin-binding protein, and its expression in insect cells. Plant Mol. Biol. 1996, 31, 267–277. [Google Scholar] [CrossRef]
- Watanabe, S.; Shimomura, S. Cloning and expression of two genes encoding auxin-binding proteins from tobacco. Plant Mol. Biol. 1998, 36, 63–74. [Google Scholar] [CrossRef]
- Löbler, M.S.K.; Hesse, T.; Klämbt, D. Auxin receptors in target tissues. In Molecular Biology of Plant Growth Control; Fox, J.E.J.M., Alan, R., Eds.; Liss: New York, NY, USA, 1987; pp. 279–288. [Google Scholar]
- Hesse, T.; Feldwisch, J.; Balshusemann, D.; Bauw, G.; Puype, M.; Vandekerckhove, J.; Lobler, M.; Klambt, D.; Schell, J.; Palme, K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989, 8, 2453–2461. [Google Scholar]
- Pelham, H.R. Control of protein exit from the endoplasmic reticulum. Annu. Rev. Cell Biol. 1989, 5, 1–23. [Google Scholar] [CrossRef]
- Jones, A.M. Auxin-binding proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 393–420. [Google Scholar] [CrossRef]
- Diekmann, W.; Venis, M.A.; Robinson, D.G. Auxins induce clustering of the auxin-binding protein at the surface of maize coleoptile protoplasts. Proc. Natl. Acad. Sci. USA 1995, 92, 3425–3429. [Google Scholar] [CrossRef]
- Klambt, D. A view about the function of auxin-binding proteins at plasma membranes. Plant Mol. Biol. 1990, 14, 1045–1050. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Ephritikhine, G.; Klambt, D.; Maurel, C.; Palme, K.; Schell, J.; Guern, J. Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J. 1991, 1, 83–93. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H. Tracking auxin receptors using functional approaches. Crit. Rev. Plant Sci. 1995, 14, 1–25. [Google Scholar] [CrossRef]
- MacDonald, H. Auxin perception and signal transduction. Physiol. Plant 1997, 100, 423–430. [Google Scholar] [CrossRef]
- Shishova, M. Membrane Mechanism of Auxin Action on Plant Cell. Ph.D. Thesis, St. Petersburg University, Moscow, Russia, 1999. [Google Scholar]
- Chen, J.G.; Shimomura, S.; Sitbon, F.; Sandberg, G.; Jones, A.M. The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J. 2001, 28, 607–617. [Google Scholar]
- Chen, J.-G.; Ullah, H.; Young, J.C.; Sussman, M.R.; Jones, A.M. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001, 15, 902–911. [Google Scholar] [CrossRef]
- Effendi, Y.; Rietz, S.; Fischer, U.; Scherer, G.F. The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J. 2011, 65, 282–294. [Google Scholar] [CrossRef]
- Effendi, Y.; Scherer, G.F. Auxin binding-protein1 (ABP1), a receptor to regulate auxin transport and early auxin genes in an interlocking system with PIN proteins and the receptor TIR1. Plant Signal. Behav. 2011, 6, 1101–1103. [Google Scholar] [CrossRef]
- Steffens, B.; Feckler, C.; Palme, K.; Christian, M.; Bottger, M.; Luthen, H. The auxin signal for protoplast swelling is perceived by extracellular ABP1. Plant J. 2001, 27, 591–599. [Google Scholar] [CrossRef]
- Christian, M.S.B.; Schenck, D.; Burmester, S.; Böttger, M.; Lüthen, H. How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol. 2006, 8, 346–352. [Google Scholar] [CrossRef]
- Yamagami, M.; Haga, K.; Napier, R.M.; Iino, M. Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol. 2004, 134, 735–747. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Zimmermann, S.; Thomine, S.; White, I.R.; Millner, P.; Guern, J. Elementary response chains at the plasma membrane involve external ABP1 and multiple electrogenic ion transport proteins. Plant Growth Regul. 1996, 18, 23–28. [Google Scholar] [CrossRef]
- Dahlke, R.I.; Luethen, H.; Steffens, B. ABP1: An auxin receptor for fast responses at the plasma membrane. Plant Signal. Behav. 2010, 5, 1–3. [Google Scholar] [CrossRef]
- Philippar, K.; Fuchs, I.; Luthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Thiel, G.; Ljung, K.; Sandberg, G.; Bottger, M.; et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef]
- Bauly, J.M.; Sealy, I.M.; Macdonald, H.; Brearley, J.; Droge, S.; Hillmer, S.; Robinson, D.G.; Venis, M.A.; Blatt, M.R.; Lazarus, C.M.; et al. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol. 2000, 124, 1229–1238. [Google Scholar] [CrossRef]
- Monshausen, G.D.; Miller, N.D.; Murphy, A.S.; Gilroy, S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011, 65, 309–318. [Google Scholar] [CrossRef]
- Cleland, R.E. The outer epidermis of Avena and maize coleoptiles is not a unique target for auxin in elongation growth. Planta 1991, 186, 75–80. [Google Scholar]
- Rudashevskaya, E.L.; Yemelyanov, V.V.; Kirpichnikova, A.A.; Burova, E.A.; Bobinova, O.A.; Shishova, M.F. The dependence of age changes in maize coleoptile and mesocotyl growth activity on indole-3-acetic acid content. Bull. St. Petersburg Univ. 2002, 3, 99–106. [Google Scholar]
- Shishova, M.; Yemelyanov, V.; Rudashevskaya, E.; Lindberg, S. A shift in sensitivity to auxin within development of maize seedlings. J. Plant Physiol. 2007, 164, 1323–1330. [Google Scholar] [CrossRef]
- Oka, K.; Naitou, S.; Yoshida, M.; Ishikawa, H.; Ohta, E.; Sakata, M. Membrane potential measurement of protoplasdts isolated from Vigna mungo hypocotil using a fluorescent probe, diS-C3-(5). Plant Cell Physiol. 1987, 28, 843–849. [Google Scholar]
- Suzuki, H.; Wang, Z.Y.; Yamakoshi, M.; Kobayashi, M.; Nozawa, T. Probing the transmembrane potential of bacterial cells by voltage-sensitive dyes. Anal. Sci. 2003, 19, 1239–1242. [Google Scholar] [CrossRef]
- Shishova, M.F.; Inge-Vechtomova, N.I.; Vykhvalov, K.A.; Rudashevskaya, E.L.; Polevoi, V.V. Auxin-dependent transport of K+ and Ca2+ across the membrane of plasmalemma vesicles from coleoptile cells. Russ. J. Plant Physiol. 1998, 45, 67–73. [Google Scholar]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Ephritikhine, G.; Klambt, D.; Ghislain, M.; Guern, J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc. Natl. Acad. Sci. USA 1989, 86, 891–895. [Google Scholar] [CrossRef]
- Rück, A.P.K.; Venis, M.A.; Napier, R.M.; Felle, H. Patch-clamp analysis establishes a role for an auxin-binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J. 1993, 4, 41–46. [Google Scholar]
- Thiel, G.; Blatt, M.R.; Fricker, M.D.; White, I.R.; Millner, P. Modulation of K+ channels in Vicia stomatal guard cells by peptide homologs to the auxin-binding protein C-terminus. Proc. Natl. Acad. Sci. USA 1993, 90, 11493–11497. [Google Scholar] [CrossRef]
- Gehring, C.A.; McConchie, R.M.; Venis, M.A.; Parish, R.W. Auxin-binding-protein antibodies and peptides influence stomatal opening and alter cytoplasmic pH. Planta 1998, 205, 581–586. [Google Scholar] [CrossRef]
- Fellner, M.E.G.; Barbier-Brygoo, H.; Guern, J. An antibody raised to a maize auxin-binding protein has inhibitory effects on cell division of tobacco mesophyll protoplasts. Plant Physiol. Biochem. 1996, 34, 133–138. [Google Scholar]
- Jones, A.M.; Im, K.H.; Savka, M.A.; Wu, M.J.; DeWitt, N.G.; Shillito, R.; Binns, A.N. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 1998, 282, 1114–1117. [Google Scholar] [CrossRef]
- Venis, M.A.; Napier, R.M.; Barbier-Brygoo, H.; Maurel, C.; Perrot-Rechenmann, C.; Guern, J. Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc. Natl. Acad. Sci. USA 1992, 89, 7208–7212. [Google Scholar]
- Zimmermann, S.; Thomine, S.; Guern, J.; Barbier-Brygoo, H. An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J. 1994, 6, 707–716. [Google Scholar]
- Leblanc, N.; Perrot-Rechenmann, C.; Barbier-Brygoo, H. The auxin-binding protein Nt-ERabp1 alone activates an auxin-like transduction pathway. FEBS Lett. 1999, 449, 57–60. [Google Scholar] [CrossRef]
- Robert, S.; Kleine-Vehn, J.; Barbez, E.; Sauer, M.; Paciorek, T.; Baster, P.; Vanneste, S.; Zhang, J.; Simon, S.; Covanova, M.; et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 2010, 143, 111–121. [Google Scholar] [CrossRef]
- Murphy, A.S.; Peer, W.A. Vesicle trafficking: ROP-RIC roundabout. Curr. Boil. 2012, 22, R576–R578. [Google Scholar] [CrossRef]
- Bassham, D.C.; Blatt, M.R. SNAREs: Cogs and coordinators in signaling and development. Plant Physiol. 2008, 147, 1504–1515. [Google Scholar] [CrossRef]
- Geldner, N.; Friml, J.; Stierhof, Y.D.; Jurgens, G.; Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413, 425–428. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.D.; Friml, J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Boil. 2007, 17, 520–527. [Google Scholar] [CrossRef]
- Friml, J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur. J. Cell Boil. 2010, 89, 231–235. [Google Scholar] [CrossRef]
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Perez-Henriquez, P.; Raikhel, N.V.; Norambuena, L. Endocytic trafficking towards the vacuole plays a key role in the auxin receptor SCF(TIR)-independent mechanism of lateral root formation in A. thaliana. Mol. Plant 2012, 5, 1195–1209. [Google Scholar]
- Sutter, J.U.; Homann, U.; Thiel, G. Ca2+-stimulated exocytosis in maize coleoptile cells. Plant Cell 2000, 12, 1127–1136. [Google Scholar]
- Sutter, J.U.; Denecke, J.; Thiel, G. Synthesis of vesicle cargo determines amplitude of Ca2+-sensitive exocytosis. Cell Calcium 2012, 52, 283–288. [Google Scholar] [CrossRef]
- Thiel, G.; Sutter, J.U.; Homann, U. Ca2+-sensitive and Ca2+-insensitive exocytosis in maize coleoptile protoplasts. Pflug. Archiv. Eur. J. Physiol. 2000, 439, R152–R153. [Google Scholar] [CrossRef]
- Campanoni, P.; Blatt, M.R. Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 2007, 58, 65–74. [Google Scholar] [CrossRef]
- Quaite, E.; Parker, R.E.; Steer, M.W. Plant cell extension: Structural implications for the origin of the plasma membrane. Plant Cell Environ. 1983, 6, 429–432. [Google Scholar] [CrossRef]
- Felle, H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta 1988, 174, 495–499. [Google Scholar] [CrossRef]
- Shishova, M.; Lindberg, S. Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. J. Plant Physiol. 2004, 161, 937–945. [Google Scholar] [CrossRef]
- Hager, A.; Debus, G.; Edel, H.G.; Stransky, H.; Serrano, R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 1991, 185, 527–537. [Google Scholar]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef]
- Phillips, G.D.; Preshaw, C.; Steer, M.W. Dictyosome vesicle production and plasma membrane turnover in auxin-stimulated outer epidermal cells of coleoptile segments from Avena sativa (L.). Protoplasma 1988, 145, 59–65. [Google Scholar] [CrossRef]
- Yalovsky, S.; Bloch, D.; Sorek, N.; Kost, B. Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 2008, 147, 1527–1543. [Google Scholar] [CrossRef]
- Hwang, J.U.; Gu, Y.; Lee, Y.J.; Yang, Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell 2005, 16, 5385–5399. [Google Scholar] [CrossRef]
- Xu, T.; When, M.; Nagawa, S.; Fu, Y.; Chen, J.-G.; Wu, M.-J.; Perrot-Rechenmann, K.; Friml, J.; Jones, A.; Yang, Z. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2011, 143, 99–110. [Google Scholar]
- Ivkova, M.N.; Pechatnikov, V.A.; Ivkov, V.G.; Pletnev, V.V. Mechanism of the fluorescent response of carbocyanine probe diS-C3-(5) to membrane potential change. Biofizika 1983, 28, 160–170. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kirpichnikova, A.A.; Rudashevskaya, E.L.; Yemelyanov, V.V.; Shishova, M.F. Ca2+-Transport through Plasma Membrane as a Test of Auxin Sensitivity. Plants 2014, 3, 209-222. https://doi.org/10.3390/plants3020209
Kirpichnikova AA, Rudashevskaya EL, Yemelyanov VV, Shishova MF. Ca2+-Transport through Plasma Membrane as a Test of Auxin Sensitivity. Plants. 2014; 3(2):209-222. https://doi.org/10.3390/plants3020209
Chicago/Turabian StyleKirpichnikova, Anastasia A., Elena L. Rudashevskaya, Vladislav V. Yemelyanov, and Maria F. Shishova. 2014. "Ca2+-Transport through Plasma Membrane as a Test of Auxin Sensitivity" Plants 3, no. 2: 209-222. https://doi.org/10.3390/plants3020209