The Polyketide Components of Waxes and the Cer-cqu Gene Cluster Encoding a Novel Polyketide Synthase, the β-Diketone Synthase, DKS
Abstract
:1. Introduction
2. Discussion
2.1. Identifying the Cer-c, -q and -u Genes
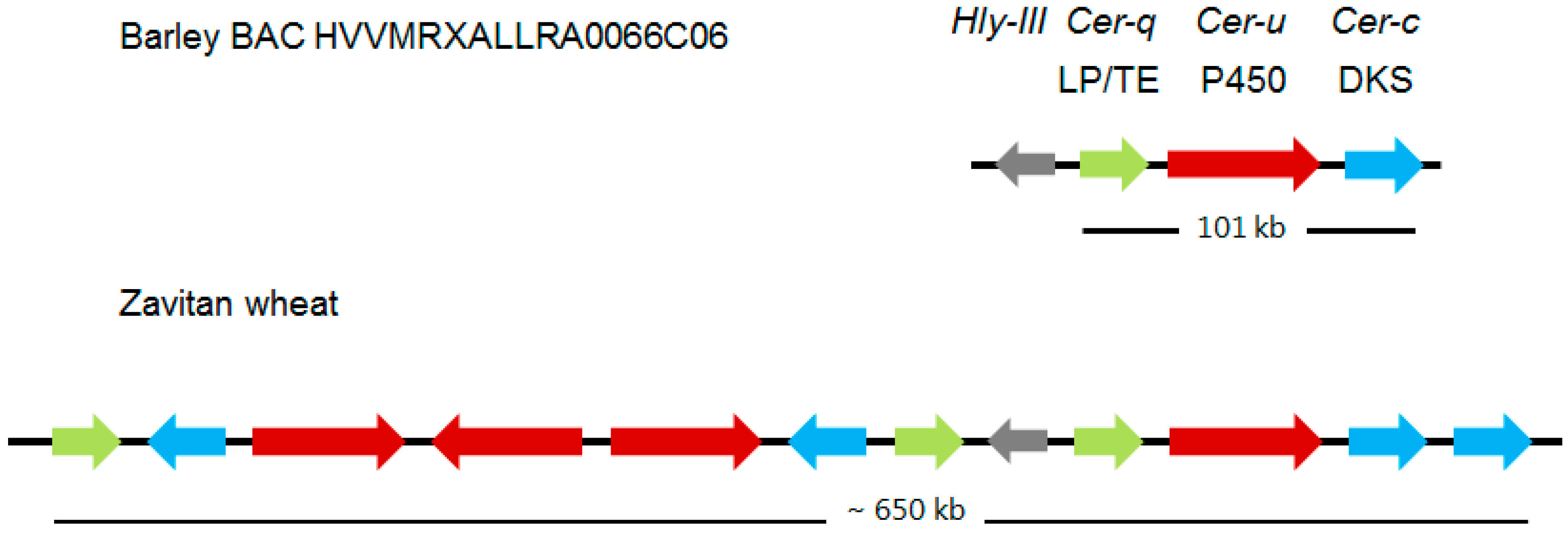
2.2. The Cer-cqu Gene Cluster in Barley
2.3. A Much Larger Cer-cqu Gene Cluster Occurs in Wheat
2.4. Establishing the Barley Wax Polyketide Biosynthetic Pathway
2.5. Esterified Alkan-2-ols Originate from a Branch Near the Origin of the β-Diketone Biosynthetic Pathway
2.6. Deducing the Functions of CER-U and -C in the Wax Polyketide Pathway
2.7. Toward the Function of CER-Q
2.8. Why a Cer-cqu Cluster?
2.9. Besides Cer-c, -q and -u do Other Barley Cer Loci Function in the DKS Polyketide Pathway?
2.10. What Enzyme(s) Extend the Tetraketide Formed by DKS?
2.11. The Third Type of Polyketide in Waxes, the Alkylresorcinols
3. Conclusions
- (1)
- Are there Cer-cqu gene clusters in other species besides barley and wheat, for example, Eucalyptus, a dicot?
- (2)
- What is the contribution of each member of the Cer-cqu cluster in wheat to synthesis of the polyketide aliphatics?
- (3)
- What is the substrate for CER-Q?
- (4)
- What is(are) the subcellular localizations of CER-Q, CER-C/DKS, and CER-U? If occurring in different compartments how are the substrates/products transferred from one to the other?
- (5)
- How are the polyketide aliphatics transported to the cuticle surface?
- (6)
- Does CER-C/DKS carry out additional polyketide partial reactions besides substrate recognition and condensation, such as cleavage of CoA from the final elongated carbon skeleton and its decarboxylation?
- (7)
- In which direction are the carbon skeletons of the β-diketones synthesized in additional species, especially one of those with 2,4-oxo groups, for example, vanilla?
- (8)
- How many condensations does CER-C/DKS carry out; that is, only the two initial ones resulting in retention of the two oxygens, or also all the subsequent six that are accompanied by the three accessory reactions removing the β-oxygens (Figure 4)?
- (9)
- What genes determine the deduced thioesterase, decarboxylase, methylketone reductase, and ester synthase enzymes in the alkan-2-ol ester branch pathway?
- (10)
- Why are AtCER2 orthologs, that are required for the final KCS elongation steps of ubiquitous wax aliphatics [57], apparently not required for the final elongations in synthesis of the β-diketone aliphatics?
- (11)
- What are the roles of the barley Cer-a, -b, -x and -yl loci products in eliminating almost all or all synthesis of polyketide wax aliphatics, and simultaneously modifying synthesis of ubiquitous wax aliphatics? Likewise, for the barley Cer-YY gene, a dominant inhibitor of spike polyketide wax aliphatics, whose mutants simultaneously change the spike ubiquitous wax aliphatics to resemble those found on wild type leaves [63].
- (12)
- What genes regulate synthesis of polyketide aliphatics in addition to wheat Iw1 and its potential homologues in other species, and how do they do so? Are they the same or different to those regulating the ubiquitous aliphatics?
Acknowledgments
Conflicts of Interest
References
- Abe, I.; Morita, H. Structure and function of chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 2010, 27, 809–838. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.M.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ogata, H.; Goto, S. Type III synthases: Functional classification and phylogenomics. ChemBioChem 2017, 18, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.H.S.; Lamberton, J.A. Long-chain β-diketones from plant waxes. Chem. Ind. 1962, 48, 2036–2037. [Google Scholar]
- Mikkelsen, J.D. Structure and biosynthesis of β-diketones in barley spike epicuticular wax. Carlsberg Res. Commun. 1979, 44, 133–147. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Baum, B.R.; Hoffman, L.L. A survey of epicuticular waxes among genera of Triticeae. 2. Chemistry. Can. J. Bot. 1980, 58, 2602–2615. [Google Scholar] [CrossRef]
- Dierickx, P.J. New β-diketones from Buxus sempervirens. Phytochemistry 1973, 12, 1498–1499. [Google Scholar] [CrossRef]
- Evans, D.; Knights, B.A.; Math, V.B.; Ritchie, A.L. β-diketones in Rhododendron waxes. Phytochemistry 1975, 14, 2247–2451. [Google Scholar] [CrossRef]
- Jenks, M.A.; Gaston, C.H.; Goodwin, M.S.; Keith, J.A.; Teusink, R.S.; Wood, K.V. Seasonal variation in cuticular waxes on Hosta genotypes differing in leaf surface glaucousness. Hort. Sci. 2002, 37, 673–677. [Google Scholar]
- Schulz, S.; Arsene, C.; Trauber, M.; McNeil, J.N. Composition of lipids from sunflower pollen (Helianthus annuus). Phytochemistry 2000, 54, 325–336. [Google Scholar] [CrossRef]
- van Smeerdijk, D.G. Characterisation of subfossil Sphagnum leaves, rootlets of Ericaceae and their peat by pyrolysis--high-resolution gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis 1987, 11, 377–402. [Google Scholar] [CrossRef]
- Ramaroson-Raonizafinimanana, B.; Gsaydou, E.M.; Bombarda, I. Long-chain aliphatic β-diketones from epicuticular wax of Vanilla bean species. Synthesis of nervonoylacetone. J. Agric. Food Chem. 2000, 48, 4739–4743. [Google Scholar] [CrossRef] [PubMed]
- Racovita, R.C.; Jetter, R. Identification of polyketides in the cuticular waxes of Triticum aestivum cv, Bethlehem. Lipids 2016, 51, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.H.S.; Kranz, Z.H.; Lamberton, J.A. The composition of Eucalyptus and some other leaf waxes. Aust. J. Chem. 1964, 17, 464–476. [Google Scholar] [CrossRef]
- von Wettstein-Knowles, P.; Netting, A.G. Esterified alkan-1-ols and alkan-2-ols in barley epicuticular wax. Lipids 1976, 11, 478–484. [Google Scholar] [CrossRef]
- von Wettstein-Knowles, P. Biosynthetic relationships between β-diketones and esterified alkan-2-ols deduced from epicuticular was of barley mutants. Mol. Gen. Genet. 1976, 144, 43–48. [Google Scholar] [CrossRef]
- von Wettstin-Knowles, P.; Mikkelsen, J.D.; Madsen, J.Ø. Nonan-2-ol esters in sorghum leaf epicuticular wax and their collection by preparative gas chromatography. Carlsberg Res. Commun. 1984, 49, 611–618. [Google Scholar] [CrossRef]
- Tulloch, A.P. Epicuticular waxes from Agropyron dasysachyum, Agropyron Riparium and Agropyron elongatum. Phytochemistry 1984, 22, 1605–1613. [Google Scholar] [CrossRef]
- Racovita, R.C.; Hen-Avivi, S.; Fernandez-Moreno, J.-P.; Granell, A.; Aharoni, A.; Jetter, R. Composition of the cuticular waxes coating flag leaf blades and peduncles of Tritcum aestivum cv. Bethlehem. Phytochemistry 2016, 130, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Baerson, S.R.; Schröder, J.; Cook, D. Alkylresorcinol biosynthesis in plants. New insights from an ancient enzyme family. Plant Sig. Behav. 2010, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Haslam, T.M.; Kunst, L. Extending the story of very-long-chain fatty acid elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Kunst, L.; Samuels, L. Composition of plant cuticular waxes. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; John Wiley & Sons Blackwell: Oxford, UK, 2006; pp. 145–181. [Google Scholar]
- von Wettstein-Knowles, P. The molecular phenotypes of the Eceriferum mutants. In Barley Genetics II; Nilan, R.A., Ed.; Washington State University Press: Pullman, WA, USA, 1971; pp. 146–193. [Google Scholar]
- Netting, A.G.; von Wettstein-Knowles, P. The physico-chemical basis of leaf wettability in wheat. Planta 1973, 114, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Korzun, V.; Malyshev, S.; Voylokov, A.; Börner, A. RFLP-based mapping of three mutant loci in rye (Secale cereale L.) and their relation to homoeologous loci within the Gramineae. Theor. Appl. Genet. 1997, 95, 468–473. [Google Scholar] [CrossRef]
- Schneider, L.M.; Adamski, N.M.; Christensen, C.E.; Stuart, D.B.; Vautrin, S.; Hansson, M.; Uauy, C.; von Wettstein-Knowles, P. The Cer-cqu gene cluster determines three key players in a β-diketone synthase polyketide pathway synthesizing aliphatics in epicuticular waxes. J. Exp. Bot. 2016, 67, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Hen-Avivi, S.; Savin, O.; Racovita, R.C.; Lee, W.-S.; Adamski, N.M.; Malitsky, S.; Almekias-Siegl, E.; Levy, M.; Vautrin, S.; Bergès, H.; et al. A metabolic gene cluster in the wheat W1 and the barley Cer-cqu loci determines β-diketone biosynthesis and glaucousness. Plant Cell 2016, 28, 1440–1460. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ranathunge, K.; Huang, H.; Pei, Z.; Franke, R.; Schreiber, L.; He, C. Wax Crystal-Sparse Leaf1 encodes a β-ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, U.; Lundqvist, A. Mutagen specificity in barley for 1580 eceriferum mutants localized to 79 loci. Hereditas 1988, 108, 1–12. [Google Scholar] [CrossRef]
- von Wettstein-Knowles, P. Genetic control of β-diketone and hydroxy-β-diketone synthesis in epicuticular waxes of barley. Planta 1972, 106, 113–130. [Google Scholar] [CrossRef] [PubMed]
- von Wettstein-Knowles, P.; Søgaard, S. The cer-cqu region in barley:gene cluster or multifunctional gene. Carlsberg Res. Commun. 1980, 45, 123–141. [Google Scholar] [CrossRef]
- Schondelmaier, G.; Fischbeck, A.; Jahoor, A. Linkage studies between morphological and RFLP markers in the barley genome. Barley Genet. Newslett. 1992, 22, 57–62. [Google Scholar]
- Lundqvist, U.; von Wettstein, D. Stock list for the eceriferum mutants III. Barley Genet. Newslett. 1975, 5, 88–90. [Google Scholar]
- Schneider, L.; University of Copenhagen, Copenhagen, Denmark and University of Lund, Lund, Sweden. Personal communication, 2017.
- Huang, D.; Feurtado, J.A.; Smith, M.A.; Flatman, L.K.; Koh, C.; Cutler, A.J. Long noncoding miRNA gene represses wheat β-diketone waxes. Proc. Natl. Acad. Sci. USA 2017, 114. [Google Scholar] [CrossRef] [PubMed]
- Netting, A.G.; von Wettstein-Knowles, P. Biosynthesis of β-diketones of barley spike epicuticular wax. Arch. Biochem. Biophys. 1978, 174, 613–621. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; von Wettstein-Knowles, P. Biosynthesis of β-diketones and hydrocarbons in barley spike epicuticular wax. Arch. Biochem. Biophys. 1978, 188, 172–181. [Google Scholar] [CrossRef]
- Mikkelsen, J.D. Biosynthesis of esterified alkan-2-ols and β-diketones in barley spike epicuticular wax: Synthesis of radioactive precursors. Carlsberg Res. Commun. 1984, 49, 391–416. [Google Scholar] [CrossRef]
- von Wettstein-Knowles, P.; Madsen, J.Ø. 7-oxopentadecan-2-ol esters—A new epicuticular wax lipid class. Carlsberg Res. Commun. 1984, 49, 55–67. [Google Scholar] [CrossRef]
- Yu, G.; Nguyen, T.T.H.; Guo, Y.; Schauvinhold, I.; Auldridge, M.E.; Bhuiyan, N.; Ben-Israel, I.; Iijima, Y.; Fridman, E.; Noel, J.P.; Pichersky, E. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol. 2010, 154, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Takahashi, Y.; Morita, H.; Noguchi, H. Benzalacetone synthase. A novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum. Eur. J. Biochem. 2001, 268, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- von Wettstein-Knowles, P. Elongases and epicuticular wax biosynthesis. Physiol. Vég. 1982, 20, 797–809. [Google Scholar]
- von Wettstein-Knowles, P. Waxes, cutin, and suberin. In Lipid Metabolism in Plants; Moore, T.S.J., Ed.; CRC Press: London, UK, 1993; pp. 127–166. [Google Scholar]
- von Wettstein-Knowles, P. Plant Waxes. In eLS; John Wiley & Sons: Chichester, UK, 2012; pp. 1–11. [Google Scholar]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C., Jr.; Vickery, C.R.; Burkart, M.D.; Noel, J.P. Confluence of structural and chemical biology: Plant polyketide synthases as biocatalysts for a bio-based future. Curr. Opin. Plant Biol. 2013, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- von Wettstein-Knowles, P. Plant Waxes. In eLS; John Wiley & Sons: Chichester, UK, 2016; pp. 1–13. [Google Scholar]
- Mayer, K.M.; Shanklin, J. Identification of amino acid residues involved in substrate specificity of plant acyl-ACP thioesterases using a bioinformatics-guided approach. BMC Plant Biol. 2007, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Kim, H.J.; Shim, D.; Suh, M.C. Molecular and biochemical characterization of the monoacylglycerol lipase gene family of Arabidopsis thaliana. Plant J. 2016, 85, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Pulsifier, I.P.; Lowe, C.; Narayaran, S.A.; Busuttil, A.S.; Vishwanath, S.J.; Domerque, F.; Rowland, O. Acyl-lipid thioesterase 1-4 from Arabidopsis thaliana form a novel family of fatty acyl-acyl carrier protein thioesterases with divergent expression patterns and substrate specificities. Plant Mol. Biol. 2014, 84, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Voelker, T.A.; Worrell, A.C.; Anderson, L.; Bleibaum, J.; Fan, C.; Hawkins, D.J.; Radke, S.E.; Davies, H.M. Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science 1992, 257, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Quilichini, T.D.; Grienenberger, E.; Douglas, C.J. the biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry 2015, 113, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Huang, A.; Osbourne, A. Plant metabolic clusters—From genetics to genomics. New Phytol. 2016, 211, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Osbourne, A. Gene clustering in plant specialized metabolism. Curr. Opion. Biotech. 2014, 26, 91–99. [Google Scholar] [CrossRef] [PubMed]
- von Wettstein-Knowles, P. Genetics and biosynthesis of plant epicuticular waxes. In Advances in the Biochemistry and Physiology of Lipids; Appelqvist, L.-Å., Liljenberg, C., Eds.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1979; pp. 1–26. [Google Scholar]
- Lundqvist, U.; von Wettstein-Knowles, P. Phenotypic diversity of barley spike waxes resulting from mutations at locus cer-n. Carlsberg Res. Commum. 1983, 48, 321–344. [Google Scholar] [CrossRef]
- Haslam, T.; Gerelle, W.; Graham, S.W.; Kunst, L. The unique role of the ECERIFERUM2-LIKE clade of the BAHD acyltransferase superfamily in cuticular wax metabolism. Plants 2017, 6, 23. [Google Scholar] [CrossRef] [PubMed]
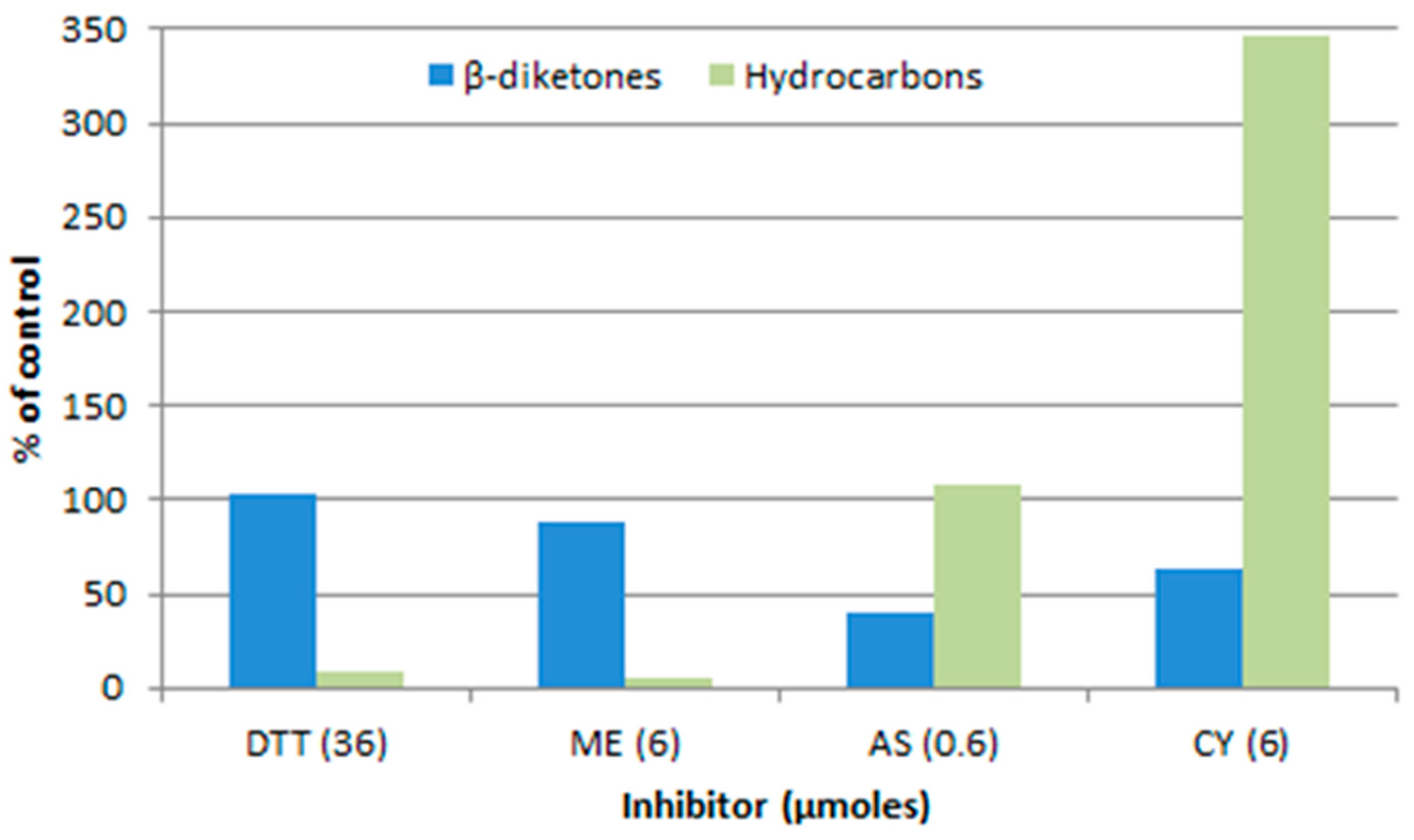
- Mikkelsen, J.D. The effect of inhibitors on the biosynthesis of the long chain lipids with even carbon numbers in barley spike epicuticular wax. Carlsberg Res. Commun. 1978, 43, 15–35. [Google Scholar] [CrossRef]
- Briggs, D.E. Hydrocarbons, phenols and sterols of the testa and pigment strand in the grain of Hordeum distichon. Phytochemistry 1974, 13, 987–996. [Google Scholar] [CrossRef]
- Adamski, N.M.; Bush, M.S.; Simmonds, J.; Turner, A.S.; Mugford, S.G.; Jones, A.; Findlay, K.; Pedentchouk, N.; von Wettstein-Knowles, P.; Uauy, C. The Inhibitor of wax 1 locus (Iw1) prevents formation of β-and OH-β-diketones in wheat cuticular waxes and maps to a sub-cM interval on chromosome arm 2BS. Plant J. 2013, 74, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jetter, R. Very long chain alkylresorcinols accumulate in the intracuticular wax of rye (Secale cereal L.) leaves near the tissue surface. Phytochemistry 2008, 69, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Svelander, C.; Karlsson, G.; Savolainen, O.I. Identification and quantification of even and odd chained 5-n alkylresorcinols, branched chain-alkylresorcinols and methylalkylresorcinols in Quinoa (Chenopodium quinoa). Food Chem. 2017, 220, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, U.; von Wettstein-Knowles, P. Dominant mutations at Cer-YY change barley spike wax into leaf blade wax. Carlsberg Res. Commun. 1982, 47, 29–43. [Google Scholar] [CrossRef]
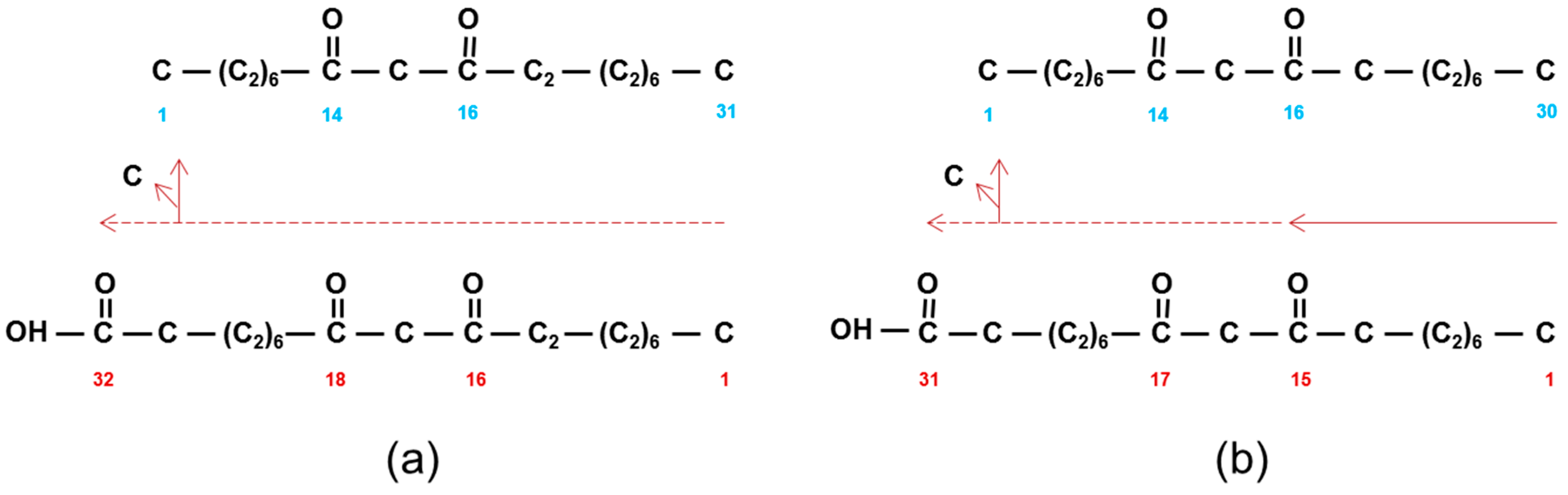
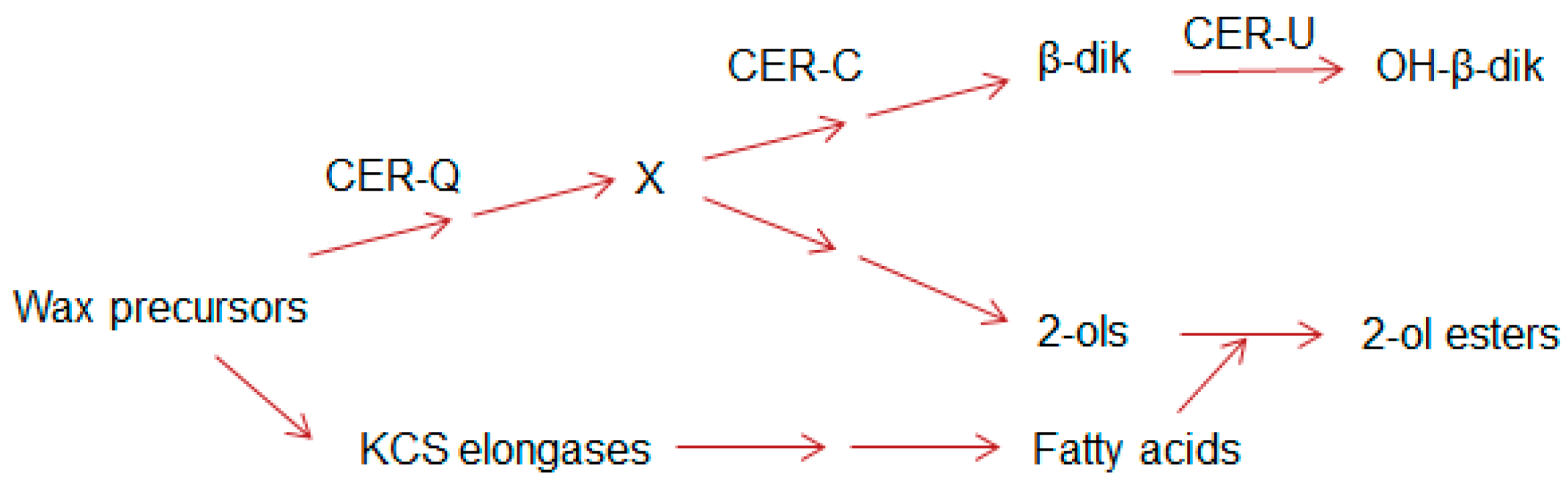
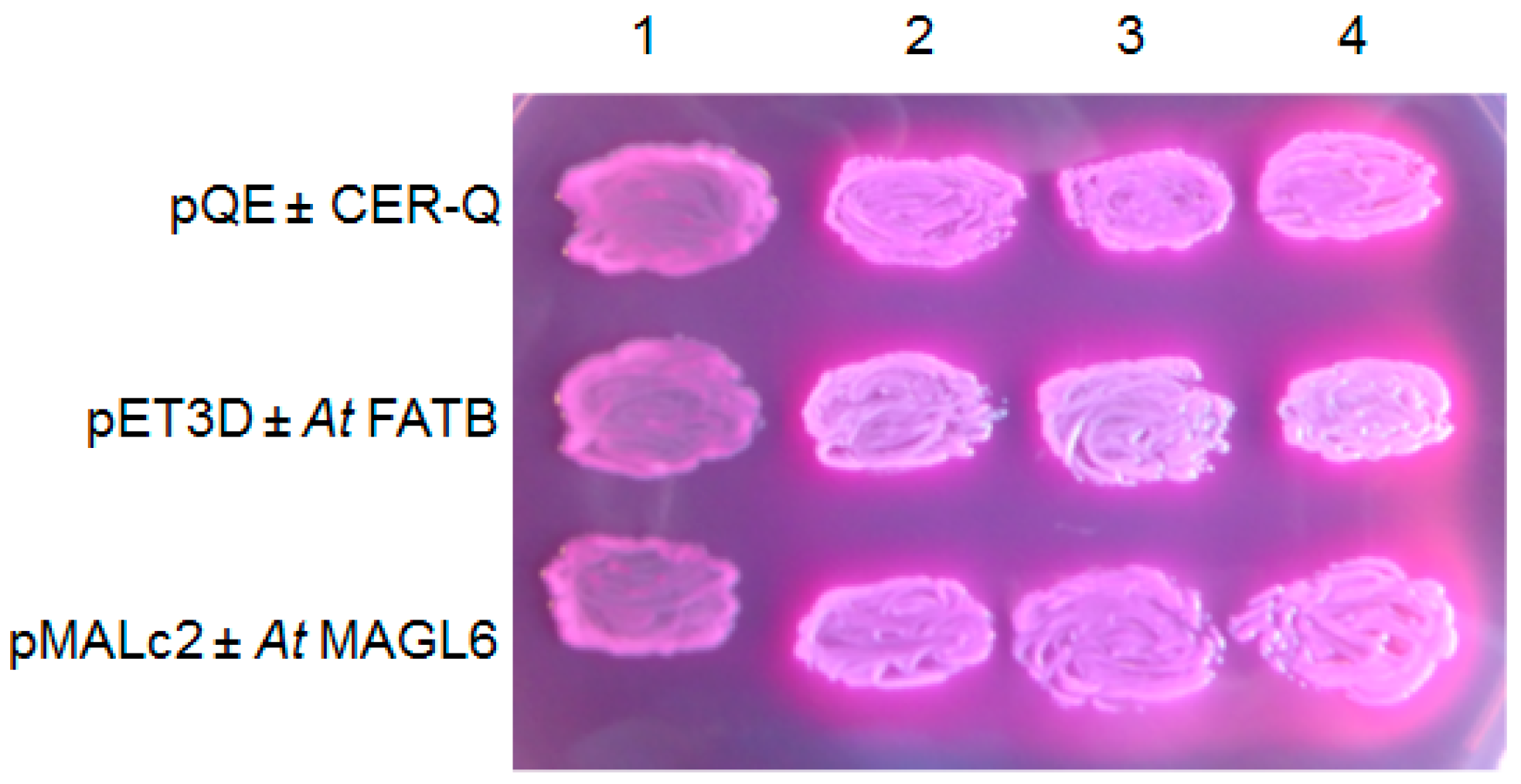
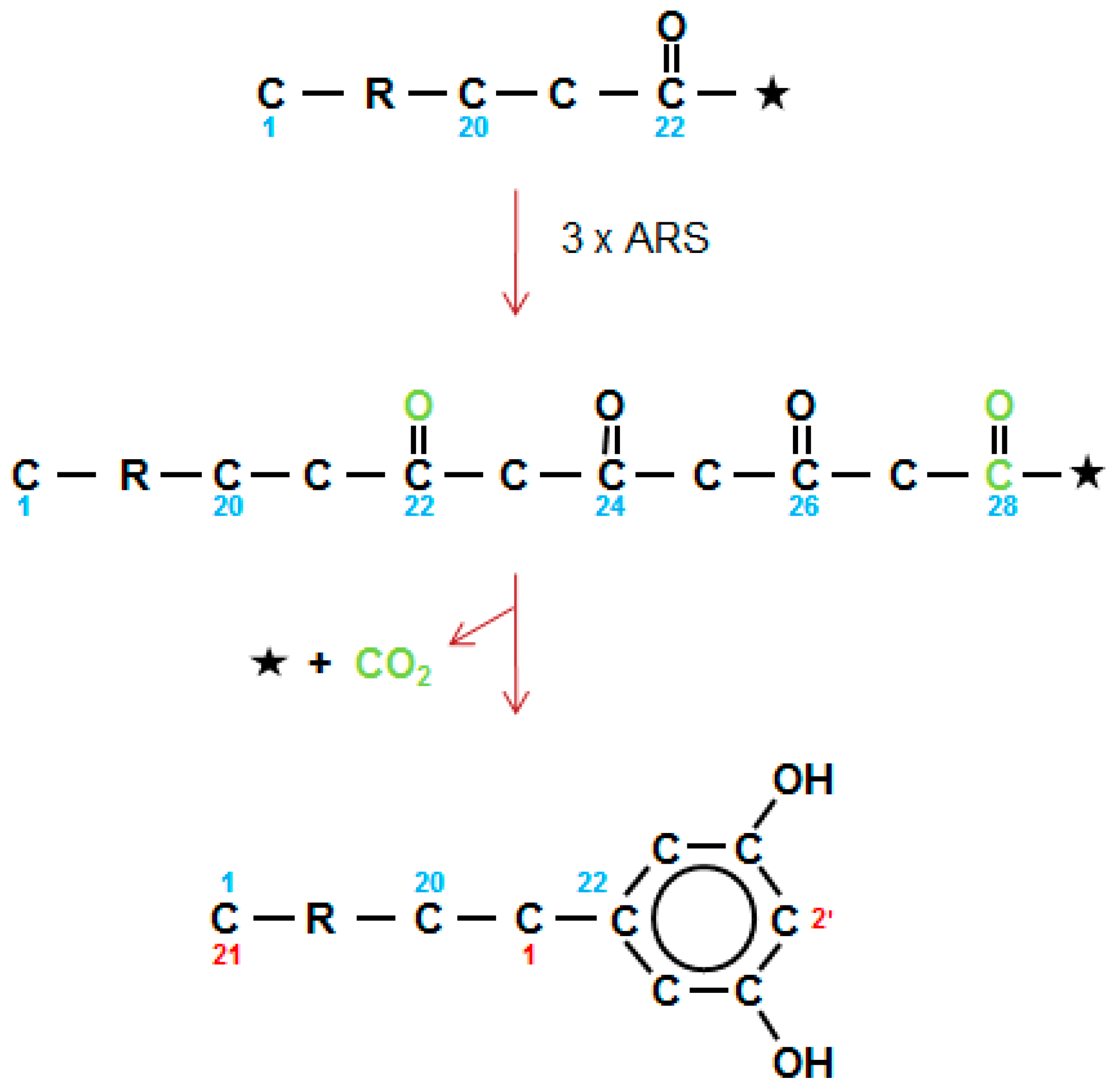
 ). The 3-oxo substrate is then decarboxylated to form methylketones that are converted to alkan-2-ols (top right). Neither methylketones nor free alkan-2-ols occur in the wax, instead the latter are found as the alcohol moiety of esters (not shown). DKS/CER-C is the diketone synthase carrying out two extensions in barley giving a tetraketide intermediate (middle) presumably after activation of the 3-oxo substrate by CoA (★). KCR, β-ketoacyl-CoA reductase; HCD; hydroxyacyl-CoA dehydratase, ECR; enoyl-CoA reductase; “KCS”, condensing enzyme carrying out 5 or 6 extensions (5X, 6X) of the DKS synthesized tetraketides; C, carbon released by unknown mechanism. Numbering in direction of synthesis, except in boxes which give the IUPAC name requiring numbering in opposite direction of synthesis, of the three in vivo synthesized β-diketones [38]. Weight % of the β-diketones is given in Table 4. The homologs of CER-Q and DKS in wheat are named Diketone Metabolism-Hydrolase and Diketone Metabolism-PKS, DMH and DMP, respectively [27].
). The 3-oxo substrate is then decarboxylated to form methylketones that are converted to alkan-2-ols (top right). Neither methylketones nor free alkan-2-ols occur in the wax, instead the latter are found as the alcohol moiety of esters (not shown). DKS/CER-C is the diketone synthase carrying out two extensions in barley giving a tetraketide intermediate (middle) presumably after activation of the 3-oxo substrate by CoA (★). KCR, β-ketoacyl-CoA reductase; HCD; hydroxyacyl-CoA dehydratase, ECR; enoyl-CoA reductase; “KCS”, condensing enzyme carrying out 5 or 6 extensions (5X, 6X) of the DKS synthesized tetraketides; C, carbon released by unknown mechanism. Numbering in direction of synthesis, except in boxes which give the IUPAC name requiring numbering in opposite direction of synthesis, of the three in vivo synthesized β-diketones [38]. Weight % of the β-diketones is given in Table 4. The homologs of CER-Q and DKS in wheat are named Diketone Metabolism-Hydrolase and Diketone Metabolism-PKS, DMH and DMP, respectively [27].
 ). The 3-oxo substrate is then decarboxylated to form methylketones that are converted to alkan-2-ols (top right). Neither methylketones nor free alkan-2-ols occur in the wax, instead the latter are found as the alcohol moiety of esters (not shown). DKS/CER-C is the diketone synthase carrying out two extensions in barley giving a tetraketide intermediate (middle) presumably after activation of the 3-oxo substrate by CoA (★). KCR, β-ketoacyl-CoA reductase; HCD; hydroxyacyl-CoA dehydratase, ECR; enoyl-CoA reductase; “KCS”, condensing enzyme carrying out 5 or 6 extensions (5X, 6X) of the DKS synthesized tetraketides; C, carbon released by unknown mechanism. Numbering in direction of synthesis, except in boxes which give the IUPAC name requiring numbering in opposite direction of synthesis, of the three in vivo synthesized β-diketones [38]. Weight % of the β-diketones is given in Table 4. The homologs of CER-Q and DKS in wheat are named Diketone Metabolism-Hydrolase and Diketone Metabolism-PKS, DMH and DMP, respectively [27].
). The 3-oxo substrate is then decarboxylated to form methylketones that are converted to alkan-2-ols (top right). Neither methylketones nor free alkan-2-ols occur in the wax, instead the latter are found as the alcohol moiety of esters (not shown). DKS/CER-C is the diketone synthase carrying out two extensions in barley giving a tetraketide intermediate (middle) presumably after activation of the 3-oxo substrate by CoA (★). KCR, β-ketoacyl-CoA reductase; HCD; hydroxyacyl-CoA dehydratase, ECR; enoyl-CoA reductase; “KCS”, condensing enzyme carrying out 5 or 6 extensions (5X, 6X) of the DKS synthesized tetraketides; C, carbon released by unknown mechanism. Numbering in direction of synthesis, except in boxes which give the IUPAC name requiring numbering in opposite direction of synthesis, of the three in vivo synthesized β-diketones [38]. Weight % of the β-diketones is given in Table 4. The homologs of CER-Q and DKS in wheat are named Diketone Metabolism-Hydrolase and Diketone Metabolism-PKS, DMH and DMP, respectively [27].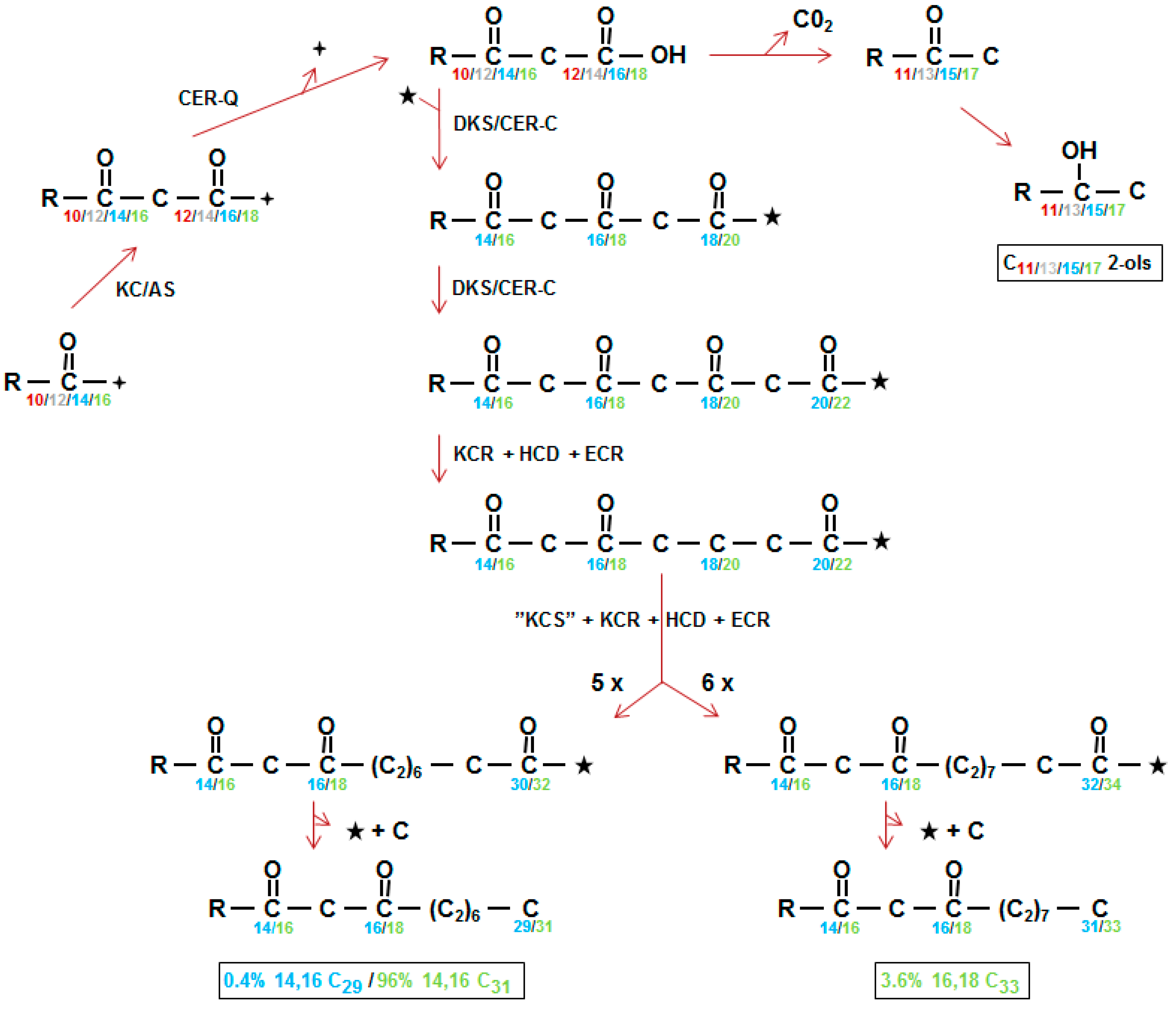

| Plant | Location | Chain Length | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 21 | 23 | 25 | 27 | 29 | 31 | 33 | |||
| Eucalyptus risdoni | Stem and leaf wax | 12,14 * | 14,16 | [4] | ||||||
| Acacia podalyriaefolia + baileyana | Stem and leaf wax | 16,18 * | [4] | |||||||
| Festuca glauca | Stem and leaf wax | 12,14 * | [4] | |||||||
| Dianthus carophyllus | Stem and leaf wax | 10,12 12,14 | 12,14 * | 12,14 14,16 | [4,5] | |||||
| Hordeum vulgare | Spike, leaf sheath and internode wax | 12,14 14,16 | 14,16 * | 16,18 | [5] | |||||
| Triticum species | Spike, peduncle and flag leaf wax | 14,16 * | [6] | |||||||
| Buxus sempervirens | Leaf wax | 6,8 | 8,10 * | 10,12 | [7] | |||||
| Rhodedendron baileyi | Leaf wax, | 8,10 | 10,12 * | [8] | ||||||
| Rhodedendron racemosum + hemitrichotum | Leaf wax | 8,10 | 14,16 * | [8] | ||||||
| Hosta “Krossa regal” | Leaf wax | 10,12 | 10,12 ◊ | [9] | ||||||
| Helianthus annus 1 | Pollen coats | 4,6 | 4,6 6,8 ◊ | 4,6 ◊ 6,8 | 4,6 ◊ 6,8 ◊ 10,12 | 4,6 6,8 10,12 | 6,8 10,12 | 10,12 | 10,12 | [10] |
| Sphagnum section Acutifolia | Subfossil roots and leaflets | 2,4 | 2,4 ◊ | 2,4 ◊ | 2,4 | [11] | ||||
| Vanilla fragrans + tahitensis 2 | Oily gum in pods | 2,4 | 2,4 * | 2,4 | 2,4 | 2,4 | [12] | |||
| Triticum aestivum | Flag leaf and peduncle waxes | 2,4 | [13] | |||||||
| Plant | Location | β-DKs 1 | Chain Length 2 | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| 7 | 9 | 11 | 13 | 15 | 17 | ||||
| Eucalyptus risdoni | Stem and leaf | present | xx | xx | xx | x | [14] | ||
| Eucalyptus globulus | Stem and leaf | present | xx | x | xx | x | [14] | ||
| Hordeum vulgare | See Table 3 | present | x | xx | xxx | x | [15,16] | ||
| Sorghum bicolor | Seedling leaf | absent | x | [17] | |||||
| Agropyron sp | Whole flowering plants | present | x | xx | [18] | ||||
| Triticum aestivum | See Table 3 | present | xx | x | x | xx | xxx | x | [19] |
| Organ | Cuticle Surface | Barley [23] | Wheat [24] | Rye 1 | Rice [28] |
|---|---|---|---|---|---|
| Spikes, panicles | + | + | + | − | |
| Peduncles, leaf sheaths, internodes | Upper Lower | + − | + − | + + | − − |
| Flag leaf | Adaxial Abaxial | − − | − + | − + | − − |
| Vegetative leaves | Adaxial Abaxial | − − | − − | − + | − − |
| C29 | C31 | C33 | |
|---|---|---|---|
| Bonus | 0.39 | 95.97 | 3.64 |
| 32 mutants from 26 Cer loci 1 | 0.51 ± 0.32 | 95.44 ± 1.02 | 4.02 ± 1.11 |
| OD273 2 | Homolog Carbon Number | Alkanes | |||
|---|---|---|---|---|---|
| 21 + 23 + 25 | 27 + 29 | 31 + 33 | % of HC 3 | ||
| Bonus | 0.72 | 2.0 | 16.8 | 76.2 | 95.0 |
| b.64 | 0.03 | 5.4 | 23.2 | 68.2 | 96.8 |
| a.6 | 0.05 | 30.4 | 29.3 | 12.6 | 72.3 |
| a.12 | 0.05 | 36.9 | 32.0 | 12.6 | 81.5 |
| a.33 | 0.04 | 36.3 | 27.3 | 8.4 | 72.0 |
| yl.187 | 0.19 | 43.1 | 28.8 | 9.7 | 81.6 |
| b.4 | 0.08 | 4.8 | 51.8 | 39.8 | 96.4 |
| b.66 | 0.05 | 12.5 | 40.0 | 40.8 | 93.3 |
| b.79 | 0.05 | 6.5 | 43.0 | 44.5 | 94.0 |
| x.60 | 0.12 | 23.1 | 26.5 | 23.7 | 73.3 |
| a.154 | 0.51 | 14.8 | 30.0 | 44.2 | 89.0 |
| b.96 | 0.40 | 4.1 | 20.9 | 71.3 | 96.3 |
| z.113 | 0.11 | 13.2 | 15.0 | 63.5 | 91.7 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Wettstein-Knowles, P. The Polyketide Components of Waxes and the Cer-cqu Gene Cluster Encoding a Novel Polyketide Synthase, the β-Diketone Synthase, DKS. Plants 2017, 6, 28. https://doi.org/10.3390/plants6030028
Von Wettstein-Knowles P. The Polyketide Components of Waxes and the Cer-cqu Gene Cluster Encoding a Novel Polyketide Synthase, the β-Diketone Synthase, DKS. Plants. 2017; 6(3):28. https://doi.org/10.3390/plants6030028
Chicago/Turabian StyleVon Wettstein-Knowles, Penny. 2017. "The Polyketide Components of Waxes and the Cer-cqu Gene Cluster Encoding a Novel Polyketide Synthase, the β-Diketone Synthase, DKS" Plants 6, no. 3: 28. https://doi.org/10.3390/plants6030028




