Alfalfa Responses to Gypsum Application Measured Using Undisturbed Soil Columns
Abstract
:1. Introduction
2. Results and Discussion
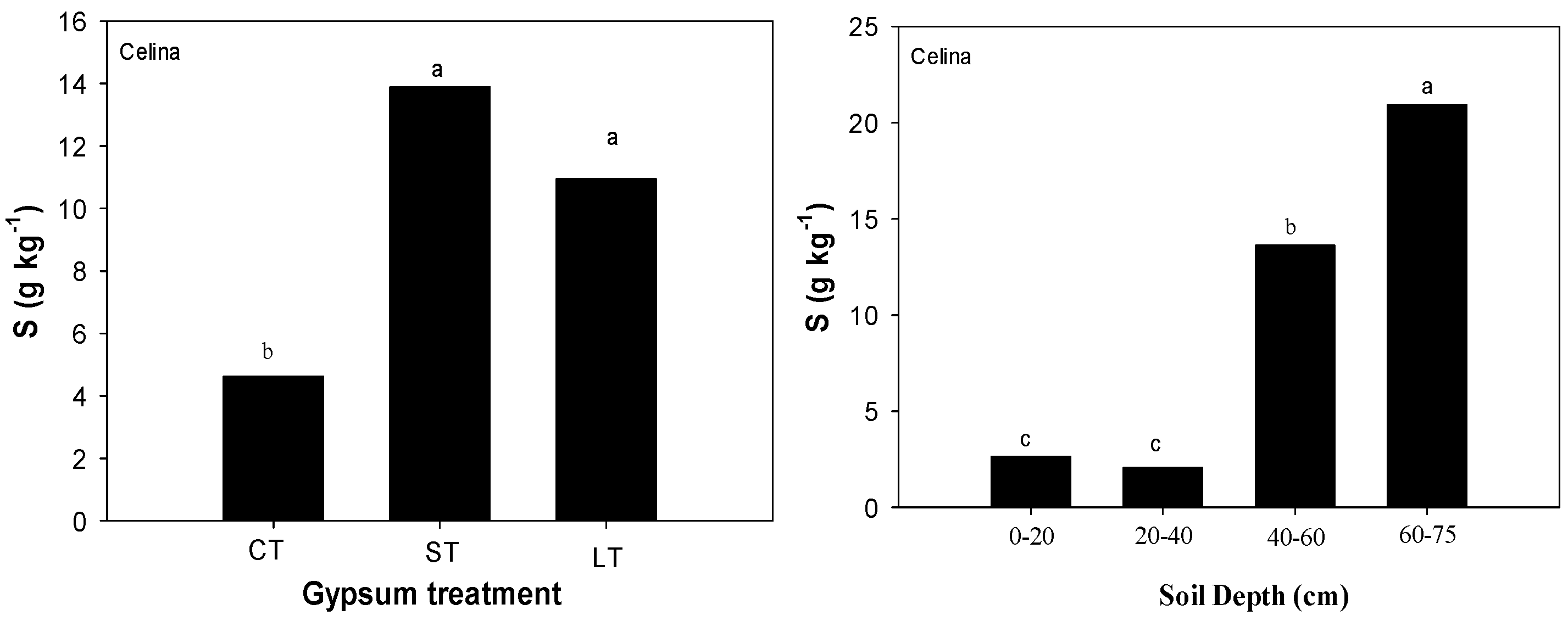
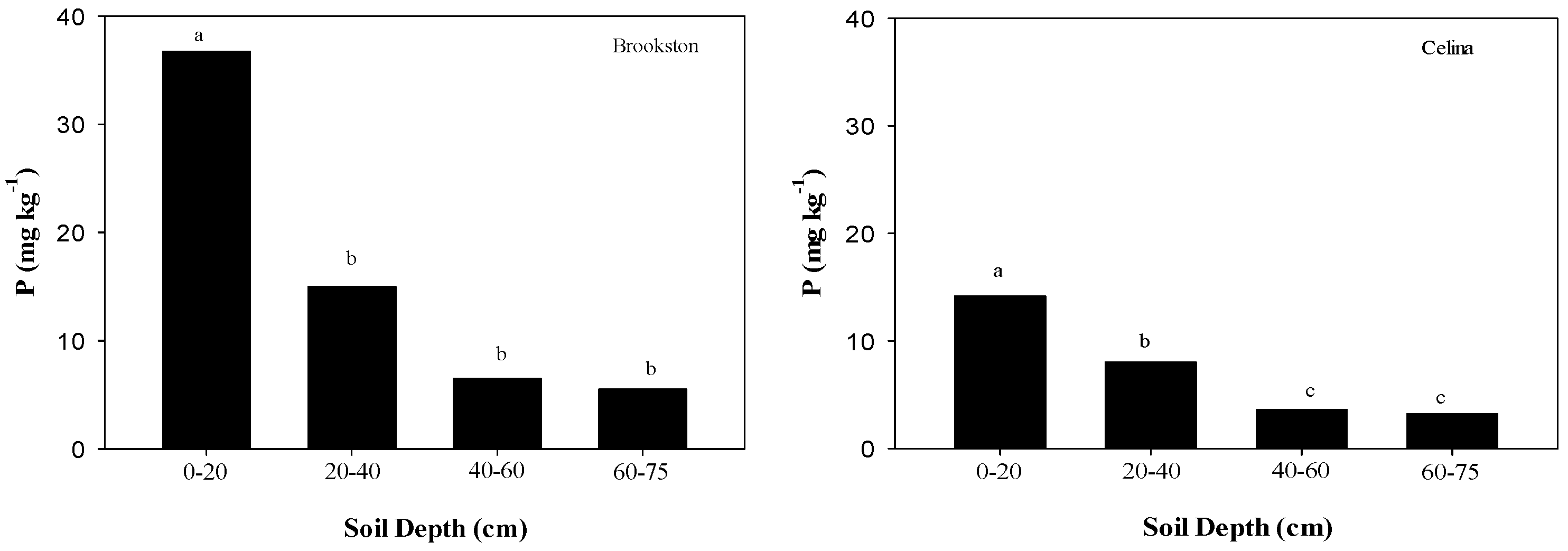
2.1. Soil Chemical Properties
2.2. Plant and Root Responses
2.3. Alfalfa Nutrient and Trace Element Concentrations
2.3.1. Major Nutrients
2.3.2. Selected Elements
3. Materials and Methods
3.1. Study Site
3.2. Field Sampling Prior Soil Columns Collection
3.3. Soil Column Collection
3.4. Alfalfa Greenhouse Experiment Using Collected Soil Columns
3.5. Alfalfa Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casby-Horton, S.; Herrero, J.; Rolong, N.A. Gypsum soils: Their morphology, classification, function, and landscapes. Adv. Agron. 2015, 130, 231–290. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Halvin, J.H. Soil Fertility and Fertilizers, 5th ed.; Macmillan Publishing Company: New York, NY, USA, 1993. [Google Scholar]
- Chen, L.; Dick, W.A. Gypsum as an agricultural amendment: General use guidelines. Bulletin 945. The Ohio State University Extension Service: Columbus, OH, USA, 2011. Available online: http://ohioline.osu.edu/b945/b945.pdf (accessed on 26 March 2012).
- American Coal Ash Association (ACAA). Coal Combustion Products Production and Use Statistics; ACAA: Farmington Hills, MI, USA, 2016. [Google Scholar]
- Payette, R.M.; Wolfe, W.E.; Beeghly, J. Use of clean coal combustion by-products in highway repairs. Fuel 1997, 76, 749–753. [Google Scholar] [CrossRef]
- Kairies, C.L.; Schroeder, K.T.; Cardone, C.R. Mercury in gypsum produced from flue gas desulfurization. Fuel 2006, 85, 2530–2536. [Google Scholar] [CrossRef]
- Chen, L.; Dick, W.A.; Nelson, S. Flue gas desulfurization by-products as sulphur sources for alfalfa and soybean. Agron. J. 2005, 97, 265–271. [Google Scholar] [CrossRef]
- Dick, W.A.; Hao, Y.; Stehouwer, R.C.; Bigham, J.M.; Wolfe, W.E.; Adriano, D.; Beeghly, J.H.; Haefner, R.J. Beneficial uses of flue gas desulfurization by products: examples and case studies of land application. In Land Application of Agricultural, Industrial, and Municipal by Products; Dick, W.A., Ed.; Soil Science Society of America: Madison, WI, USA, 2006; pp. 505–536. [Google Scholar] [CrossRef]
- Favaretto, N.; Norton, L.D.; Joern, B.C.; Brouder, S.M. Gypsum amendment and exchangeable Calcium and Magnesium affecting Phosphorous and Nitrogen Runoff. Soil Sci. Soc. Am. J. 2006, 70, 1788–1796. [Google Scholar] [CrossRef]
- Miller, W.P. Infiltration and soil loss of three gypsum amended Ultisols under simulated rainfall. Soil Sci. Soc. Am. J. 1987, 55, 783–787. [Google Scholar]
- Norton, L.D.; Dontsova, K.M. Use of soil amendments to prevent soil surface sealing and control erosion. Adv. Geoecol. 1998, 31, 581–587. [Google Scholar]
- Norton, L.D.; Zhang, X.C. Liming to improve chemical and physical properties of soil. In Handbook of Soil Conditioners: Substances that Enhance Physical Properties of Soil; Wallace, A., Terry, R.E., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 309–331. [Google Scholar]
- Shainberg, I.; Sumner, M.E.; Miller, W.P.; Farina, M.P.W.; Pavan, M.A.; Fey, M.V. Use of gypsum on soils: A review. In Advance Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1989; pp. 2–111. [Google Scholar] [CrossRef]
- Dontsova, K.M.; Norton, L.D. Clay dispersion, infiltration and erosion as influenced by exchangeable Ca and Mg. Soil Sci. 2002, 163, 184–193. [Google Scholar] [CrossRef]
- Hammel, J.E.; Sumner, M.E.; Shahandeh, H. Effect of physical and chemical profile modification on soybean and corn production. Soil Sci. Soc. Am. J. 1985, 49, 1508–1511. [Google Scholar] [CrossRef]
- Sumner, M.E. Gypsum and acid soils: The world scene. Adv. Agron. 1993, 51, 1–32. [Google Scholar] [CrossRef]
- Toma, M.; Sumner, M.E.; Weeks, G.; Saigusa, M. Long-term Effects of Gypsum on Crop Yield and Subsoil Chemical Properties. Soil Sci. Soc. Am. J. 1999, 63, 891–895. [Google Scholar] [CrossRef]
- Ritchey, K.D.; Souza, D.M.G.; Costa, U.F. Calcium leaching to increase rooting depth in a Brazilian savanna Oxisol. Agron. J. 1980, 72, 40–44. [Google Scholar] [CrossRef]
- Dontsova, K.; Lee, Y.B.; Slater, B.K.; Bigham, J.M. Gypsum for agricultural use in Ohio-sources and quality of available products. The Ohio State University Extension: Columbus, OH, USA, 2005. Available online: http://ohioline.osu.edu/factsheet/anr-20 (accessed on 10 July 2017).
- Sloan, J.J.; Dowdy, R.H.; Dolan, M.S.; Rehm, G.W. Plant and soil responses to field flue gas desulfurization residue. Fuel 1999, 78, 169–174. [Google Scholar] [CrossRef]
- Electric Power Research Institute (EPRI). Composition and Leaching of FGD Gypsum and Mined Gypsum; EPRI Technical. Rep. 1022146; EPRI: Palo Alto, CA, USA, 2011. [Google Scholar]
- Chen, L.; Dick, W.A.; Nelson, S. Flue gas desulfurization by-products additions to acid soil: Alfalfa productivity and environmental quality. Environ. Pollut. 2001, 114, 2. [Google Scholar] [CrossRef]
- Tirado-Corbalá, R.; Slater, B.K.; Dick, W.A.; Bigham, J.; McCoy, E. Hydrologic Properties and Leachate Nutrient Responses of Soil Columns Collected from Gypsum Treated Fields. Soil Tillage Res. 2013, 134, 232–240. [Google Scholar] [CrossRef]
- Farina, M.P.W.; Channon, P. Acid-subsoil ameloriation: II. Gypsum effects on growth and subsoil chemical properties. Soil Sci. Soc. Am. J. 1988, 49, 175–180. [Google Scholar] [CrossRef]
- Caires, E.F.; Fonseca, A.F.; Mendes, J.; Chueri, W.A.; Madruga, E.F. Corn, wheat and soybean yields as a function of the changes in soil chemical characteristics due to surface application of lime and gypsum under no-tillage system. Rev. Bras. Cienc. Solo. 1999, 23, 315–327. [Google Scholar] [CrossRef]
- Wang, D.; Anderson, D.W. Stable carbon isotopes of carbonate pendants from Chernozemic soils of Saskatchewan, Canada. Geoderma 1998, 84, 309–322. [Google Scholar] [CrossRef]
- Syed-Omar, R.; Sumner, M.E. Effect of gypsum on soil potassium and magnesium status and growth of alfalfa. Commun. Soil Sci. Plant Anal. 1991, 22, 2017–2028. [Google Scholar] [CrossRef]
- O’Leary, M.J.; Rehm, G.W. Effect of sulfur on forage yield and quality of alfalfa. J. Fert. Issues 1989, 6, 6–11. [Google Scholar]
- Mullen, R.W.; Lentz, E.M.; Watson, M.E. Soil Fertility Chapter in the Ohio Agronomy Guide; Ohio State University Extension: Columbus, OH, USA, 2005. [Google Scholar]
- Wendell, R.R.; Ritchey, K.D. High-calcium flue gas desulfurization products reduce aluminum toxicity in an Appalachian soil. J. Environ. Qual. 1996, 25, 1401–1410. [Google Scholar] [CrossRef]
- Kost, D.; Chen, L.; Guo, X.; Tian, Y.; Ladwig, K.; Dick, W.A. Effects of flue gas desulfurization and mined gypsums on soil properties and on hay corn growth in eastern Ohio. J. Environ. Qual. 2014, 43, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.B.; Dick, W.A. Sustainable Uses of FGD Gypsum in Agricultural Systems: Introduction. J. Environ. Qual. 2014, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Pickerton, A.; Smith, F.W.; Chewis, D. Pasture Species. In Plant Analysis: An Interpretation Manual, 2nd ed.; Reuter, D.J., Robinson, J.B., Eds.; CSIRO: Canberra, Australia, 1997; pp. 287–342. [Google Scholar]
- Brauer, D.; Aiken, G.E.; Pote, D.H.; Livingston, S.J.; Norton, L.D.; Way, T.R.; Edwards, J.H. Amendment effects on soil test phosphorus. J. Environ. Qual. 2005, 34, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Dick, W.A. Reclamation of acidic mined lands in humic areas. Adv. Agron. 1987, 41, 377–405. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Wolf, B.; Mills, H.A. Plant Analysis Handbook, 1st ed.; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991. [Google Scholar]
- Robson, A.D.; Reuter, D.J. Diagnosis of copper deficiency and toxicity. In Copper in Soils and Plants; Loneragan, J.F., Robson, A.D., Graham, R.D., Eds.; Academic Press: New York, NY, USA, 1981; pp. 287–312. [Google Scholar]
- Warncke, D.; Brown, J.R. Potassium and other basic cations. In Recommended Chemical Soil Test Procedures for the North Central Region; NCR Publication No. 221; Missouri Agricultural Experiment Station: Columbia, MO, USA, 1988; pp. 31–33. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis, Part 3-Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 894–895. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3-Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Hossne, L.R. Dissolution for total elemental analysis. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 49–64. [Google Scholar]
- Combs, S.M.; Denning, J.L.; Frank, K.D. North Central Regional Research Publication Recommended Chemical Soil Test Procedures No.221; Chapter 8-Sulfate-Sulfur; Missouri Agricultural Experiment Station, University of Missouri--Columbia: Columbia, SC, USA, 1998; pp. 35–40. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Australian laboratory handbook of soil and water chemical methods. In Australian Soil and Land Survey Handbook; Inkata Press: Melourne, Australia, 1992; Volume 3. [Google Scholar]
- International Standard, ISO 10694:1995e. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis); International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Dreimanis, A. Quantitative gasometric determination of calcite and dolomite using Chittick apparatus. J. Sed. Pet. 1962, 32, 520–529. [Google Scholar] [CrossRef]
- Hutton, K.J.; Brown, L.C.; Holmes, G.R.; Krietemeyer, D.R.; Coltman, K.M. Device for collecting large-diameter, undisturbed soil cores. Appl. Eng. Agricult. 1992, 8, 799–806. [Google Scholar] [CrossRef]


| Soil | pH | P-Bray 1 | TC † | EC | Ca | Mg | K | Total-S | SO4-S | Root Dry Weight |
|---|---|---|---|---|---|---|---|---|---|---|
| Brookston | ||||||||||
| Gypsum | 0.629 ‡ | 0.118 | 0.0003 | 0.139 | 0.002 | 0.0004 | 0.173 | 0.0001 | 0.814 | <0.0001 |
| Depth | 0.030 | <0.001 | <0.0001 | 0.959 | 0.005 | <0.0001 | 0.141 | 0.0007 | 0.698 | 0.045 |
| Gypsum Depth | 0.990 | 0.333 | <0.0001 | 0.997 | 0.696 | 0.028 | 0.550 | <0.0001 | 0.955 | <0.0001 |
| Celina | ||||||||||
| Gypsum | 0.239 | 0.246 | <0.0001 | 0.528 | <0.0001 | 0.0009 | 0.060 | 0.012 | 0.680 | <0.0001 |
| Depth | 0.005 | <0.0001 | <0.0001 | 0.954 | <0.0001 | 0.004 | 0.001 | 0.0001 | 0.958 | <0.0001 |
| Gypsum Depth | 0.934 | 0.112 | <0.0001 | 0.904 | 0.035 | 0.002 | 0.018 | 0.082 | 0.979 | <0.0001 |
| Soil/Depth | TC † | Ca | Mg | K | S | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | g·kg−1 | g·kg−1 | g·kg−1 | g·kg−1 | g·kg−1 | ||||||||||
| Brookston | CT a | ST | LT | CT | ST | LT | CT | ST | LT | CT | ST | LT | CT | ST | LT |
| 0–20 | 19.0 aA‡ | 20.5 aA§ | 17.7 aA | 2.37 | 2.66 | 2.61 | 4.64 bC | 6.05 aC | 2.96 cC | 1.49 | 1.53 | 1.30 | 4.47 bA | 5.25 aA | 3.77 cA |
| 20–40 | 13.6 bB | 20.6 aA | 14.9 bA | 3.03 | 3.76 | 3.11 | 8.31 aA | 7.41 bB | 5.06cB | 1.42 | 1.12 | 1.12 | 2.84 cB | 4.60 aB | 3.93 bA |
| 40–60 | 12.0 aB | 6.7 bB | 13.2 aB | 2.56 | 3.39 | 3.18 | 8.31 aA | 8.04 bA | 7.09 cA | 1.23 | 0.88 | 1.24 | 2.07 cC | 3.34 aC | 3.00 bB |
| 60–75 | 7.1 bC | 3.5 cB | 11.8aB | 2.28 | 2.85 | 2.85 | 7.65 aB | 7.19 bB | 7.32 bA | 1.24 | 0.86 | 1.28 | 2.10 bC | 2.54 aD | 2.85 aB |
| Celina | |||||||||||||||
| 0–20 | 12.2 aB | 11.3 aC | 13.0 aC | 1.14 aA | 1.76 aC | 1.52 aC | 2.25 bC | 4.92 aA | 1.87 bC | 0.67 bB | 1.22 aA | 0.70 bA | 2.51 | 2.73 | 2.67 |
| 20–40 | 4.4 cC | 31.9 aB | 6.9 bD | 1.20 cA | 2.04 aBC | 1.59 bC | 4.28 aB | 5.32 aA | 2.51 bB | 0.67 bB | 0.80 aB | 0.65 bA | 1.65 | 2.06 | 2.47 |
| 40–60 | 3.4 cC | 44.1 aA | 22.7 bB | 1.88 cA | 3.07 aAB | 2.10 bB | 7.78 aA | 3.05 bB | 3.38 bA | 0.87 aA | 0.43 cC | 0.52 bA | 5.44 | 23.6 | 11.8 |
| 60–75 | 30.6 bA | 46.0 aA | 47.8 aA | 1.69 bA | 3.19 aA | 3.21aA | 4.19 aB | 1.77 bC | 1.61 bC | 0.52 aC | 0.42 bC | 0.26 cB | 8.86 | 27.1 | 26.8 |
| Soil/Depth | CT † | ST | LT | |
|---|---|---|---|---|
| cm | kg·m−3 | |||
| Brookston | ||||
| 0–10 | 83.3 b‡A | 110 aA§ | 129 aA | |
| 10–20 | 43.3 aB | 62.0 aB | 59.9 aB | |
| 20–40 | 19.1 aC | 14.9 aC | 13.2 aC | |
| 40–60 | 12.1 aC | 9.9 aC | 12.0 aC | |
| 60–75 | 4.7 aC | 4.2 aC | 4.7 aC | |
| Total ¶ | 162 b | 201 a | 219 a | |
| Celina | ||||
| 0–10 | 70.0 bA | 76.0 bA | 125 aA | |
| 10–20 | 41.0 aB | 46.0 aB | 55.5 aB | |
| 20–40 | 10.0 aC | 9.9 aC | 12.0 aC | |
| 40–60 | 12.0 aC | 8.6 aC | 4.9 aC | |
| 60–75 | 4.5 aC | 3.9 aC | 4.7 aC | |
| Total | 138 b | 144 b | 202 a | |
| Soil | Treatment † | Ca | K | Mg | P | S | N |
|---|---|---|---|---|---|---|---|
| g·kg−1 | % | ||||||
| Brookston | CT | 15.3 ‡ | 23.4 ab | 3.60 a | 2.88 b | 4.34 b | 4.76 |
| ST | 15.2 | 27.2 a | 3.63 a | 3.62 a | 4.69 a | 4.82 | |
| LT | 15.4 | 22.2 b | 3.18 b | 2.94 b | 4.78 a | 4.74 | |
| P > F | 0.68 | 0.035 | 0.006 | 0.002 | 0.005 | 0.82 | |
| Celina | CT | 18.0 | 15.8 b | 4.76 a | 2.33 | 4.73 | 4.68 |
| ST | 16.3 | 21.9 a | 4.12 b | 2.49 | 4.68 | 4.66 | |
| LT | 18.4 | 18.6 a | 3.75 c | 2.15 | 5.04 | 4.53 | |
| P > F | 0.17 | 0.007 | 0.005 | 0.42 | 0.17 | 0.08 | |
| Soil | Treatment † | Al | B | Ba | Cd | Cr | Cu | Fe | Mn | Mo | Ni | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·kg−1 | ||||||||||||
| Brookston | CT | 298 ‡ | 27.3 b | 27.1 b | 0.26 b | 0.71 | 1.36 a | 341 | 88.6 a | 1.43 b | 4.10 | 341 |
| ST | 649 | 33.9 a | 30.3 a | 0.48 a | 1.05 | 1.94 a | 445 | 41.4 b | 3.05 a | 3.25 | 445 | |
| LT | 437 | 36.4 a | 21.4 c | 0.19 b | 0.64 | 0.85 b | 451 | 51.4 b | 0.92 b | 3.60 | 451 | |
| P > F | 0.49 | 0.012 | 0.0003 | 0.0013 | 0.55 | 0.004 | 0.72 | 0.003 | 0.0001 | 0.093 | 0.72 | |
| Celina | CT | 206 | 14.3 b | 0.90 | 0.10 | 0.73 | 1.55 | 300 | 76.2 | 1.65 | 2.89 | 300 |
| ST | 411 | 19.6 a | 0.90 | 0.14 | 0.79 | 0.87 | 446 | 77.9 | 0.73 | 3.05 | 446 | |
| LT | 226 | 26.6 a | 0.90 | 0.06 | 0.65 | 0.59 | 367 | 73.8 | 1.26 | 2.31 | 367 | |
| P > F | 0.59 | 0.018 | N/A | 0.07 | 0.89 | 0.32 | 0.74 | 0.79 | 0.11 | 0.19 | 0.74 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado-Corbalá, R.; Slater, B.K.; Dick, W.A.; Barker, D. Alfalfa Responses to Gypsum Application Measured Using Undisturbed Soil Columns. Plants 2017, 6, 29. https://doi.org/10.3390/plants6030029
Tirado-Corbalá R, Slater BK, Dick WA, Barker D. Alfalfa Responses to Gypsum Application Measured Using Undisturbed Soil Columns. Plants. 2017; 6(3):29. https://doi.org/10.3390/plants6030029
Chicago/Turabian StyleTirado-Corbalá, Rebecca, Brian K. Slater, Warren A. Dick, and Dave Barker. 2017. "Alfalfa Responses to Gypsum Application Measured Using Undisturbed Soil Columns" Plants 6, no. 3: 29. https://doi.org/10.3390/plants6030029






