Selecting Lentil Accessions for Global Selenium Biofortification
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Germination Study
2.3. ICARDA Study
2.4. Se Analysis
2.5. Antioxidant Activity
2.6. Statistical Analysis
3. Results
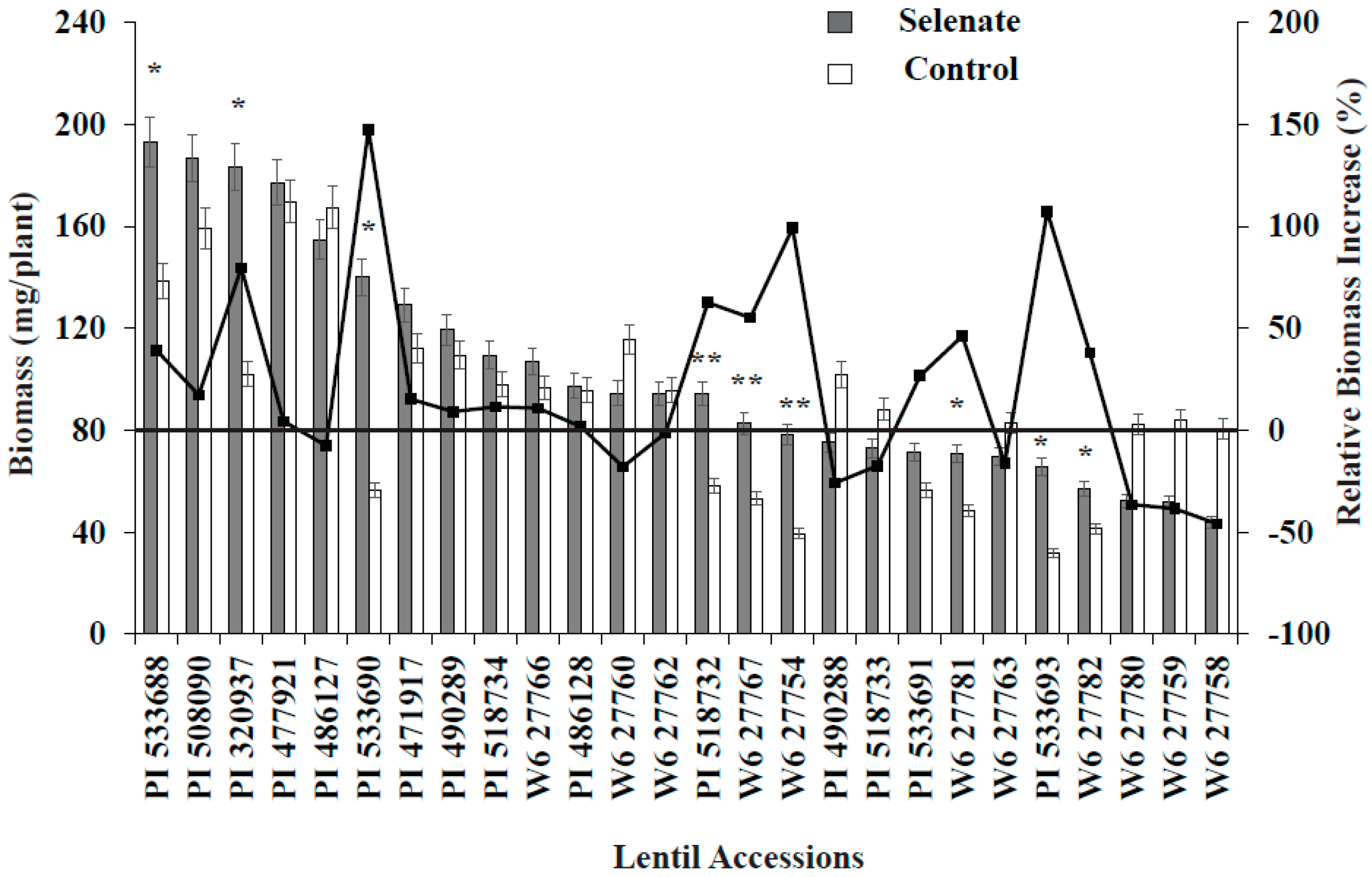
3.1. Germination Study
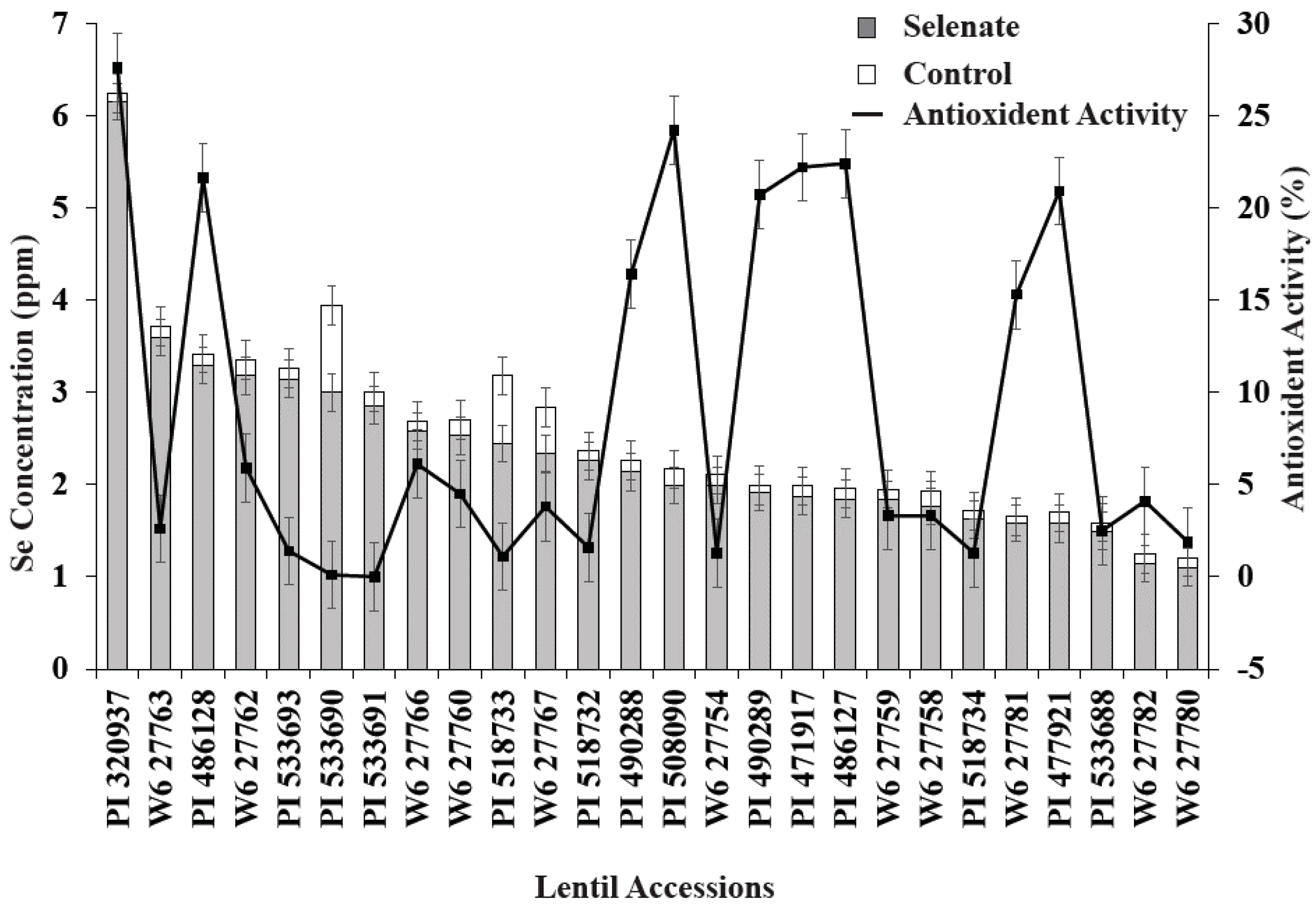
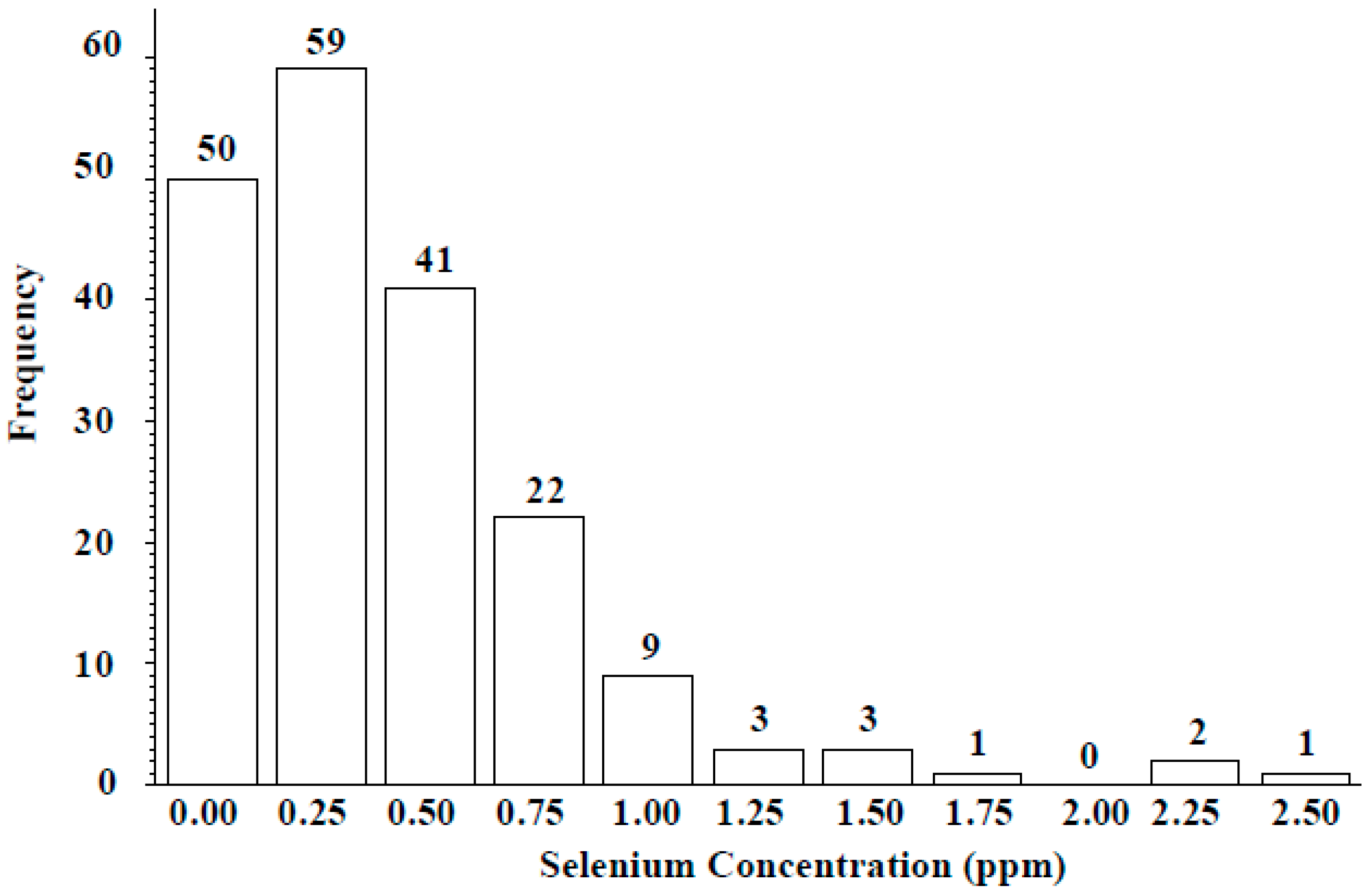
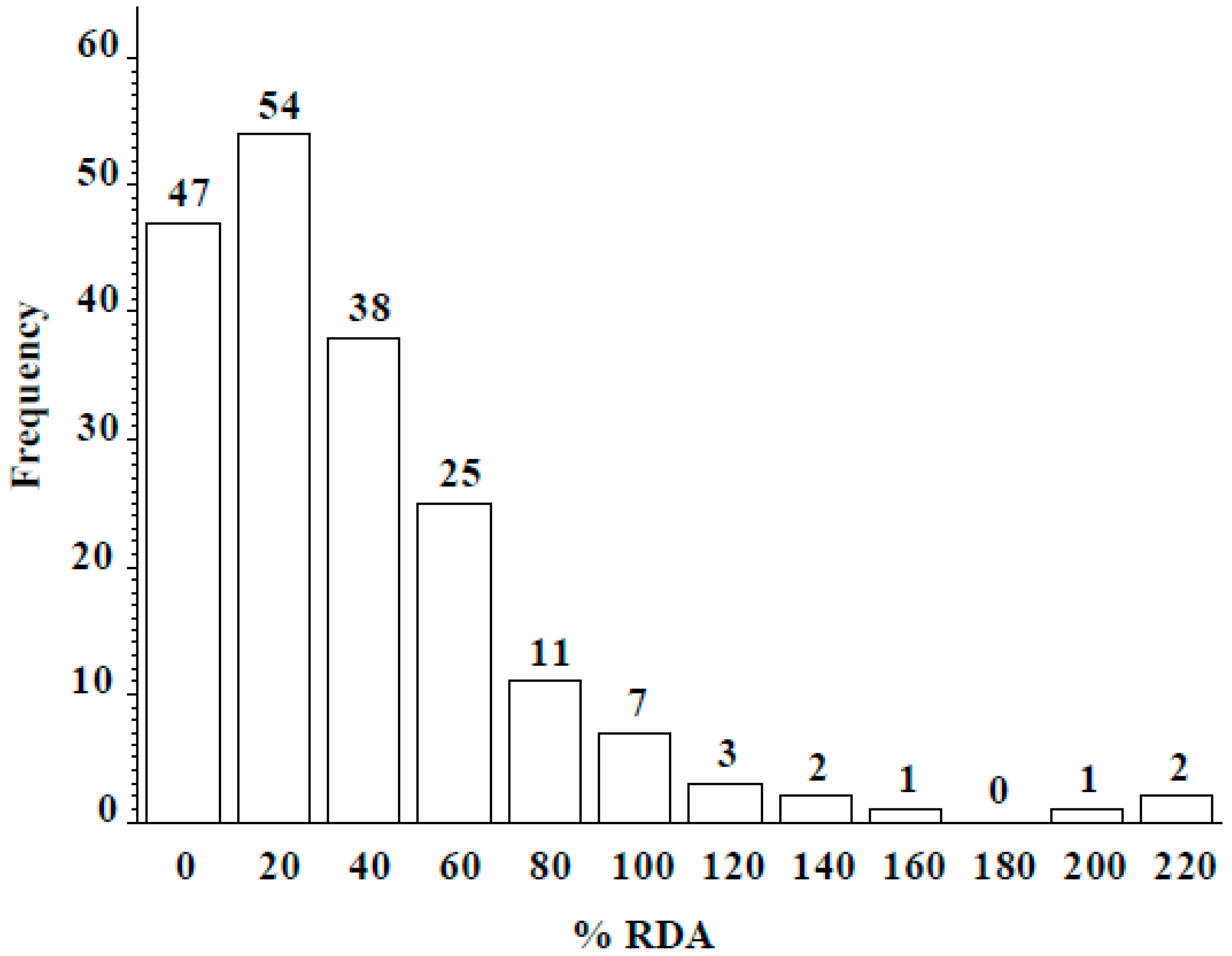
3.2. ICARDA Study
4. Discussion
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- CDC. Micronutrient facts, International Micronutrient Malnutrition Prevention and Control (IMMPaCt). Available online: http://www.cdc.gov/immpact/micronutrients/ (accessed on 5 May 2016).
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 18, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Materne, M.; Vandemark, G.; Shrestha, R.; Idrissi, O.; Hacikamiloglu, O.; Bucak, B.; Vandenberg, A. A global survey of effects of genotype and environment on selenium concentration in lentils (Lens culinaris L.): Implications for nutritional fortification strategies. Food Chem. 2011, 125, 72–76. [Google Scholar] [CrossRef]
- Sen Gupta, D.; Thavarajah, D.; Knutson, P.; Thavarajah, P.; McGee, R.J.; Coyne, C.J.; Kumar, S. Lentils (Lens culinaris L.), a Rich Source of Folates. J. Agric. Food Chem. 2013, 61, 7794–7799. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens culinaris L.): A prebiotic-rich whole food legume. Food Res. Int. 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients Plant Cell Monographs; Hell, R., Mendel, R.R., Eds.; Springer: Berlin, Germany, 2010; pp. 225–241. [Google Scholar]
- Yokota, A.; Shigeoka, S.; Onishi, T.; Kitaoka, S. Selenium as inducer of glutathione peroxidase in low-CO2-grown Chlamydomonas reinhardtii. Plant Physiol. 1988, 86, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta 2009, 1790, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, D.; Schlautman, B.; Steffan, S.; Polashock, J.; Vorsa, N.; Zalapa, J. The American cranberry mitochondrial genome reveals the presence of selenocysteine (tRNA-Sec and SECIS) insertion machinery in land plants. Gene 2014, 536, 336–343. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, L.J.; Thavarajah, D.; Vial, E.; Schatz, B.; McGee, R.; Thavarajah, P. The effect of selenium fertilization on lentil (Lens culinaris Medikus) grain yield, seed selenium concentration, and antioxidant activity. Field Crops Res. 2015, 177, 9–14. [Google Scholar] [CrossRef]
- Thavarajah, D.; Ruszkowski, J.; Vandenberg, A. High potential for selenium biofortification of lentils (Lens culinaris L.). J. Agric. Food Chem. 2008, 56, 10747–10753. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, H.; Caron, C.T.; Fedoruk, M.; Diapari, M.; Vandenberg, A.; Coyne, C.J.; McGee, R.; Bett, K.E. Genetic Diversity of Cultivated Lentil (Lens culinaris Medik.) and Its Relation to the World’s Agro-ecological Zones. Front. Plant Sci. 2016, 7, 1093. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Vial, E.; Gebhardt, M.; Lacher, C.; Kumar, S.; Combs, G.F. Will selenium increase lentil (Lens culinaris Medik) yield and seed quality? Front. Plant Sci. 2015, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Ates, D.; Sever, T.; Aldemir, S.; Yagmur, B.; Temel, H.Y.; Kaya, H.B.; Alsaleh, A.; Kahraman, A.; Ozkan, H.; Vandenberg, A.; et al. Identification QTLs Controlling Genes for Se Uptake in Lentil Seeds. PLoS ONE 2016, 11, e0149210. [Google Scholar]
- US National Plant Germplasm System. Available online: https://npgsweb.ars-grin.gov/gringlobal/search.aspx (accessed on 5 May 2016).
- Wakim, R.; Bashour, I.; Nimah, M.; Sidahmed, M.; Toufeili, I. Selenium levels in Lebanese environment. J. Geochem. Explor. 2010, 107, 94–99. [Google Scholar] [CrossRef]
- Oldfield, J.E. Selenium World Atlas. Selenium—Tellurium Development Association, Belgium. Available online: http://www.369.com.cn/En/Se%20Atlas%202002.pdf (accessed on 5 May 2016).
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- SAS Institute. User’s Guide: Statistics SAS Institute (Version 9.4); SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Hartikainen, H.; Xue, T. The promotive effect of selenium on plant growth as triggered by ultraviolet irradiation. J. Environ. Qual. 1999, 28, 1372–1375. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Petruzzelli, G.; Petacco, F.; Malorgio, F.; Ferri, T. Absorption of selenium by Lactuca sativa as affected by carboxymethylcellulose. Chemosphere 2007, 67, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Combs, G.F. Selenium in lentils (Lens culinaris L.) and theoretical fortification strategies. In Handbook of Food Fortification and Health: From Concepts to Public Health Applications; Preedy, V.R., Srirajaskanthan, R., Patel, V.B., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Thavarajah, D.; Vandenberg, A.; George, G.N.; Pickering, I.J. Chemical form of selenium in naturally selenium-rich lentils (Lens culinaris L.) from Saskatchewan. J. Agric. Food Chem. 2007, 55, 7337–7341. [Google Scholar] [CrossRef] [PubMed]
- Allaway, W.H.; Moore, D.P.; Oldfield, J.E.; Muth, O.H. Movement of physiological levels of selenium from soils through plants to animals. J. Nutr. 1966, 88, 411–418. [Google Scholar] [PubMed]
- Turakainen, M.; Hartikainen, H.; Seppänen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef] [PubMed]
| Origin/Source | n | Genotype (Plant Name) |
|---|---|---|
| Brazil | 3 | PI 518732 (CNPH 84–122) |
| PI 518733 (CNPH 84–123) | ||
| PI 518734 (CNPH 84–125) | ||
| Canada | 1 | PI 471917 (Eston) |
| France | 3 | PI 486128 (Dupuy) |
| PI 490288 (Anicia) | ||
| PI 490289 (Mariette) | ||
| Germany | 1 | PI 320937 (ILL505) |
| Spain | 4 | PI 533688 (870523-13) |
| PI 533690 (Pardina) | ||
| PI 533691 (Lenteja Verdina) | ||
| PI 533693 (Verdina) | ||
| USA | 14 | PI 477921 (Red Chief), PI 486127 (unknown), PI 508090 (Brewer) |
| W6 27754 (Parent of 1048-8R), W6 27758 (Parent of CDC Robin) | ||
| W6 27759 (Parent of Eston), W6 27760 (Parent of Giza-9) | ||
| W6 27762 (Parent of ILL 4605), W6 27763 (Parent of ILL 5588) | ||
| W6 27766 (Parent of ILL 7537), W6 27767 (Parent of ILL 8006 BM4) | ||
| W6 27780 (Parent of Milestone), W6 27781 (Parent of Pardina), | ||
| W6 27782 (Parent of Pennell) | ||
| Total | 26 |
| Origin | Species/Subspecies | n | Accession Number |
|---|---|---|---|
| Armenia | L. culinaris subsp orientalis | 1 | 126939 |
| Cyprus | L. culinaris subsp orientalis | 2 | 72849, 72595 |
| Czech Republic | L. culinaris subsp unknown | 1 | 136657 |
| Iran | L. culinaris subsp unknown | 2 | 72593, 72594 |
| Jordan | L. culinaris subsp orientalis | 5 | 72847, 72848, 72858, 72864, 72865 |
| Lebanon | L. culinaris subsp odemensis | 1 | 110846 |
| L. culinaris subsp unknown | 2 | 72925, 110824 | |
| Poland | L. culinaris subsp orientalis | 1 | 72600 |
| L. culinaris subsp unknown | 5 | 72597, 72598, 136652, 136653, 136658 | |
| Syria | L. culinaris subsp odemensis | 15 | 72640, 72648, 72690, 72697, 72703, 72704 72706, 72758, 72759, 72760, 72893, 107449 119390, 126219, 126220 |
| L. culinaris subsp orientalis | 49 | 72534, 72638, 72639, 72645, 72647, 72680 72685, 72688, 72689, 72691, 72699, 72715 72719, 72720, 72750, 72751, 72754, 72761 72765, 72767, 72777, 72778, 72824, 72825 72853, 72854, 72866, 72868, 72869, 72872 72878, 72880, 72881, 72882, 72883, 72884 72887, 72888, 72890, 72892, 107448, 116048 116049, 116052, 126223, 135399, 135443 136777, 139285 | |
| L. culinaris subsp tomentosus | 4 | 72686, 72820, 72845, 136814 | |
| L. culinaris subsp unknown | 32 | 72643, 72644, 72646, 72666, 72668, 72669 72672, 72675, 72676, 72692, 72693, 72721 72769, 72770, 72818, 72852, 72870, 72871 72873, 72876, 72877, 107447, 110594, 110803 116043, 116045, 116047, 126221, 126222 135385, 135410, 135415 | |
| Tajikistan | L. culinaris subsp odemensis | 1 | 72899 |
| L. culinaris subsp orientalis | 5 | 72904, 72905, 72907, 136679, 140379 | |
| Turkmenia | L. culinaris subsp orientalis | 1 | 72901 |
| Turkey | L. culinaris subsp odemensis | 2 | 72562, 136673 |
| L. culinaris subsp orientalis | 33 | 72527, 72529, 72530, 72602, 72604, 72606 72608, 72610, 72611, 72612, 72613, 72616 72617, 72618, 72619, 72620, 72621, 72626 72627, 72628, 72629, 72632, 72726, 72746 72748, 72816, 72830, 114416, 116008, 116010 116029, 136677, 72623 | |
| L. culinaris subsp tomentosus | 1 | 72625 | |
| L. culinaris subsp unknown | 21 | 72724, 72742, 72743, 72744, 72800, 72801 72804, 72805, 72831, 72835, 72836, 72855 116015, 116027, 116028, 116034, 136662 136665, 136666, 136669, 136670 | |
| Uzbekistan | L. culinaris subsp odemensis | 1 | 72900 |
| L. culinaris subsp orientalis | 5 | 72895, 72896, 72897, 72908, 72909 | |
| L. culinaris subsp unknown | 1 | 72592 | |
| Total | 191 |
| Source | df | Mean Squares | ||
|---|---|---|---|---|
| Biomass | Antioxidant | Se Uptake | ||
| Genotype | 25 | * | * | * |
| Se treatment | 1 | * | * | * |
| Replication | 2 | NS | NS | NS |
| Genotype × Se treatment | 25 | * | * | * |
| Error | 102 | 0.5 | 3.1 | 0.1 |
| Person correlation coefficient (n = 156) | ||||
| Biomass | 1.00 | 0.41 * | 0.20 * | |
| Antioxidant | 0.41 * | 1.00 | 0.54 * | |
| Se uptake | 0.20 * | 0.54 * | 1.00 | |
| Country of Origin | No of Samples | Seed Se Concentration (µg g−1) | |
|---|---|---|---|
| Range | Mean | ||
| Armenia | 1 | 0.59 | 0.59 |
| Cyprus | 2 | 0.20–0.51 | 0.36 |
| Czech Republic | 1 | 0.91 | 0.91 |
| Iran | 2 | 0.30–0.90 | 0.60 |
| Jordan | 5 | 0.18–0.81 | 0.34 |
| Lebanon | 3 | 0.02–0.49 | 0.23 |
| Poland | 6 | 0.01–0.66 | 0.32 |
| Syria | 100 | 0.00–2.45 | 0.38 |
| Tajikistan | 6 | 0.00–0.53 | 0.16 |
| Turkmenia | 1 | 0.31 | 0.31 |
| Turkey | 57 | 0.0–2.36 | 0.54 |
| Uzbekistan | 7 | 0.0–0.76 | 0.18 |
| Mean ± SD | 0.41 ± 0.22 | ||
| Total | 191 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thavarajah, D.; Abare, A.; Mapa, I.; Coyne, C.J.; Thavarajah, P.; Kumar, S. Selecting Lentil Accessions for Global Selenium Biofortification. Plants 2017, 6, 34. https://doi.org/10.3390/plants6030034
Thavarajah D, Abare A, Mapa I, Coyne CJ, Thavarajah P, Kumar S. Selecting Lentil Accessions for Global Selenium Biofortification. Plants. 2017; 6(3):34. https://doi.org/10.3390/plants6030034
Chicago/Turabian StyleThavarajah, Dil, Alex Abare, Indika Mapa, Clarice J. Coyne, Pushparajah Thavarajah, and Shiv Kumar. 2017. "Selecting Lentil Accessions for Global Selenium Biofortification" Plants 6, no. 3: 34. https://doi.org/10.3390/plants6030034




