1. Introduction
The
Moringa oleifera plant, which is also known as “Marengue” in Jamaica, is one of the 13 species in the
Moringa genus. Other species include
Moringa stenopetala and
Moringa ovalifolia.
Moringa oleifera is also called the Horseradish tree, and like horseradish it possesses a taproot system that supports the umbrella-like canopy of the trunk, leaves, and branches [
1,
2]. The leaves are tripinnate and grow with a fragile feather-like drooping crown. Fragrant flowers grow as spreading auxillary panicles and are yellowish white, and when fertilized produce pods resembling that of the common bean (
Phaseolus vulgaris), commonly referred to as string beans or snap beans. Similar to stringbeans, the pods are initially green; however, as
Moringa pods mature, they become brown and thicker [
2]. In Jamaica, flowers and consequently pods are produced at an increased rate during the rainy season, although flowering and fruiting occurs throughout the year. The plant seems to prefer the drier climate of the southern areas in Jamaica but also grows in the north [
3]. Several non-governmental organizations including the Food and Agricultural Organization (FAO), Educational Concerns for Hunger Organization (ECHO), Church World Service, and Trees for Life have endorsed
Moringa as a nutritional gold mine for tropical areas due to its nutritional content and its ability to grow in tropical and drought affected areas [
4,
5].
Moringa oleifera is native to the Himalayas and grows well in sub-tropical and tropical regions of the world. It is widely used in ethnobotany and is thought to cure a variety of diseases [
4].
Moringa has long been a part of Ayurvedic medicine in India and is understandably referred to as the “Miracle Tree”. All parts of the
Moringa plant are edible, with leaves and pods used most frequently. The leaves of the plant are utilized as a nutritional supplement, are thought to boost the immune system as well as energy levels, and are known to have anti-inflammatory as well as antioxidant properties. In Jamaica,
Moringa is used in the preparation of hot and cold beverages (teas and juices) and as meals in many households. The interest in
Moringa started in the 2000s, and this interest continues today. Various investigations have shown that
Moringa contains antioxidants [
4,
6,
7,
8]. Antioxidants are useful in the management of oxidative stress in the body [
9].
Moringa could potentially be used to improve the clinical condition of persons with oxidative stress conditions such as sickle cell anemia (SCA). Oxidative stress is the result of an imbalance between reactive oxidative species (ROS) and antioxidant components in the body. ROS can potentially damage cells in the body, destabilizing the cell integrity by reacting with cellular components [
9]. Antioxidant components are designed to reduce ROS [
9]. The body produces ROS as a part of its normal metabolic processes and consequently has biological mechanisms to counteract oxidation [
10].
In healthy people, the antioxidant system usually restores balance easily by reducing ROS when formed [
9]. People with SCA experience a relatively higher oxidant load due to factors including the increased frequency of erythrocyte destruction (which results in excessive free heme, a potential oxidant). People with SCA also seem to have an increased metabolic rate, which in turn increases the production of oxidants. This increased oxidant production results in an imbalance of oxidants compared to antioxidants causing oxidative stress [
10,
11]. Sickle cell anemics therefore have higher levels of ROS due to the inability of their antioxidant system to compensate for the abnormal free heme plasma levels. This results in inflammation and chronic organ damage [
9,
10].
In Jamaica, 1 in 300 persons are estimated to have SCA in Jamaica. People with SCA experience a variety of symptoms, the consequences of which could be serious and expensive for Jamaicans. Sickle cell anemics often experience sickling crises, for which the disease is named. Leg ulcers, splenomegaly, stroke, pulmonary hypertension, and other conditions may also occur. Inflammation is an underlying symptom associated with many conditions.
People with SCA are treated symptomatically. Symptoms include pain and infections, which are treated with analgesics and antibiotics, respectively. SCA is prevalent in African and Caribbean nations, developing countries with limited resources.
Moringa is a tropical plant that grows easily in these countries and is therefore readily accessible [
4]. The use of the plant would be a cost-effective way of combating SCA by reducing oxidative stress. There appears to be no evidence in the literature of antioxidant activity testing of the Jamaican grown
Moringa plant. This study was initiated to determine the antioxidant activity of
Moringa plants grown in Jamaica.
2. Results
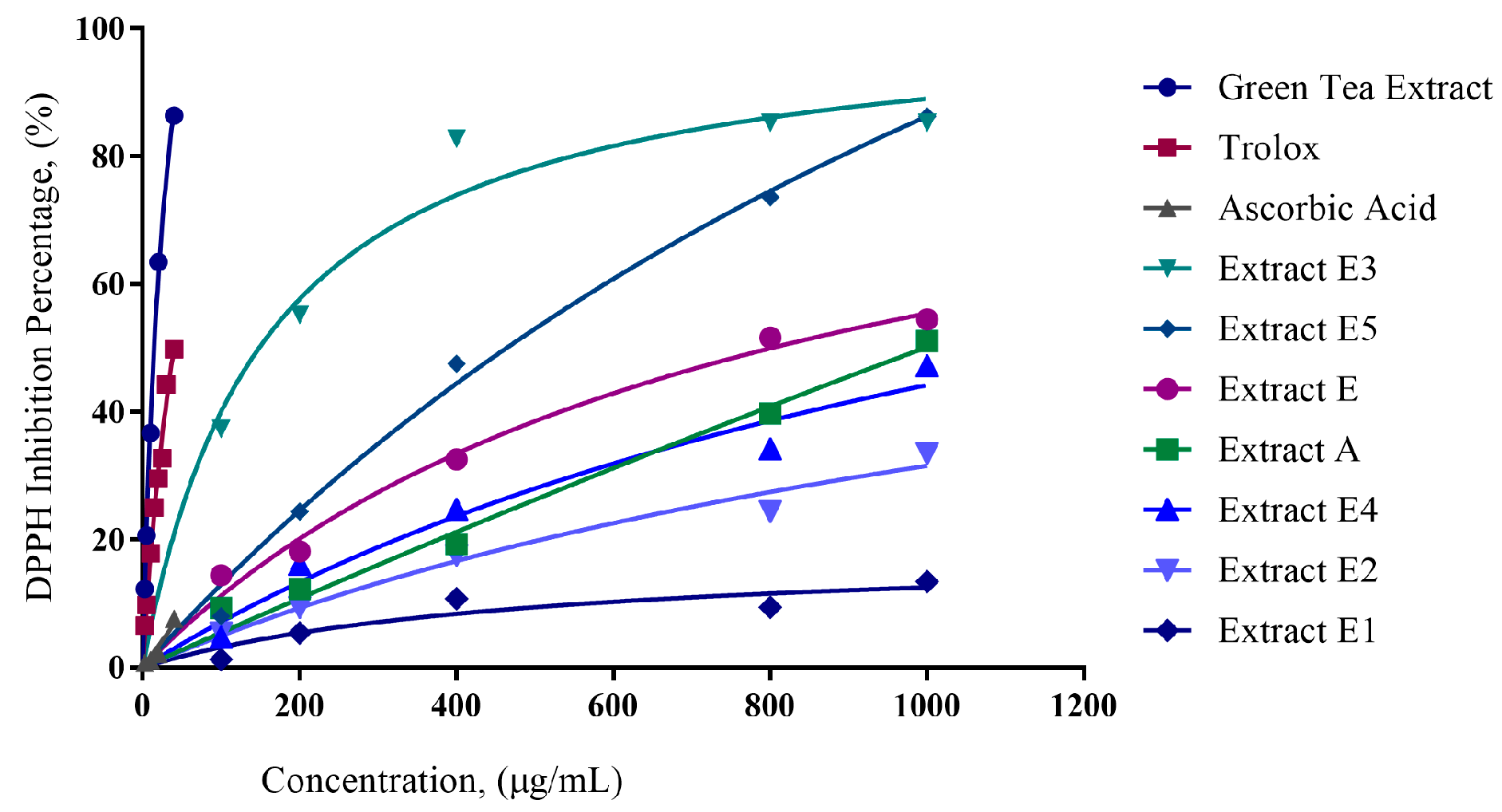
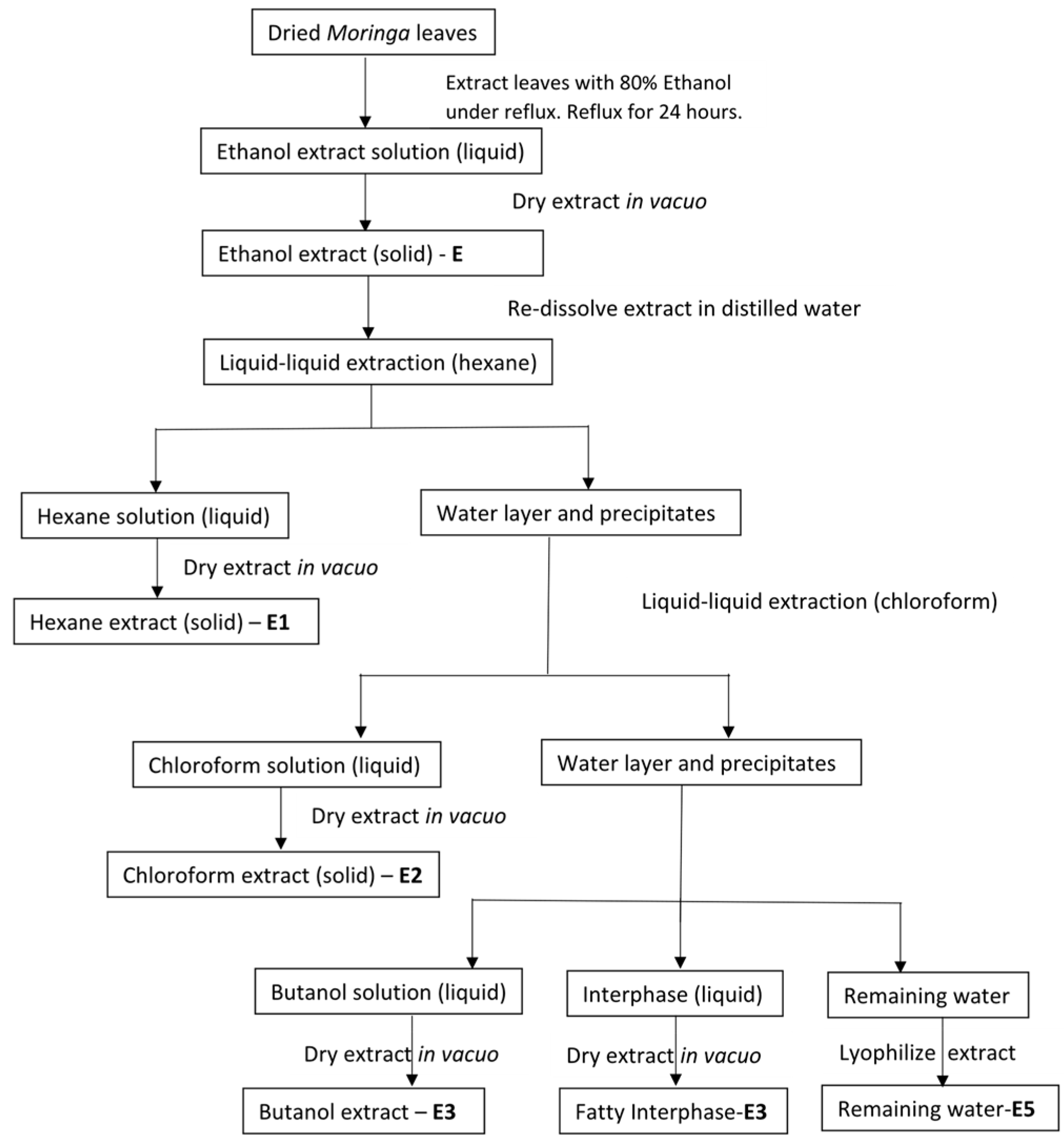
Initial extraction with ethanol (E) resulted in 37% recovery. Further fractionation using various solvents, namely hexane, chloroform, butanol, and water was performed. In relation to the ethanol extract, the percentage recovery for the solvent extracts was as follows: hexane-E1 (48%), chloroform-E2 (1.75%), butanol-E3 (9.46%), and water-E5 (18.26%). DPPH reduction percentages are shown in
Figure 1. The slope of the DPPH reduction percentage plot was used as an indicator of antioxidant capacity.
Moringa extracts E3 and E5 have higher slopes compared to E1 and E2 (
Figure 1); therefore, E3 and E5 have higher antioxidant capacities compared to E1 and E2.
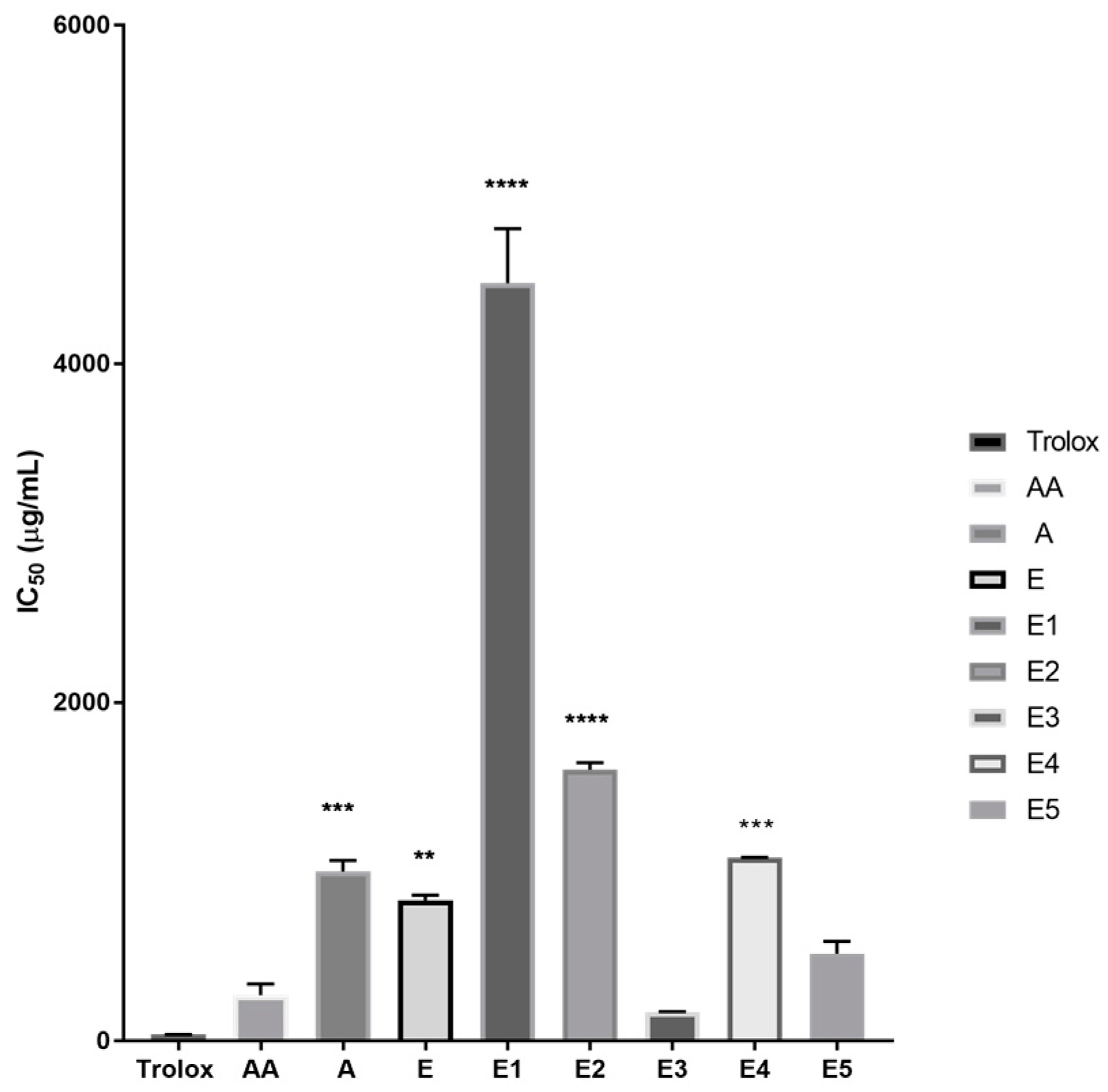
IC
50 values, representing the sample concentration at which 50% of the DPPH radical has been reduced, were calculated from the DPPH reduction percentages. There is an inverse relation between IC
50 values and antioxidant activities; this means that lower IC
50 values indicate a higher antioxidant capacity. Extracts E2 and E1 had IC
50 values of 1604 µg/mL and 4477 µg/mL, respectively, while extracts E, E3, E4, E5, and A had IC
50 values of 832.8 µg/mL, 172.6 µg/mL, 1085 µg/mL, 516.9 µg/mL, and 1003 µg/mL, respectively. Based on the graph (
Figure 1) showing the percentages of DPPH reduction, extracts E3 and E5 possessed more effective antioxidative capacity compared to the other extracts.
Figure 2 also shows that the IC
50 values of extracts E3 and E5 were lower compared to the other extracts.
3. Discussion
Antioxidant activity of
Moringa oleifera leaf extracts from Jamaica was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [
6,
12]. This seems to be the first study of antioxidant activity using
Moringa sourced from Jamaica, as the literature tends to lack any such information. It should be noted that these extracts were produced from a direct ethanolic extraction of the
Moringa leaves. Typically, when antioxidant principles are being extracted from samples or when antioxidant tests are being conducted, an alcoholic solvent, aqueous solvent, or a combination of both is used as the extracting agent [
6,
13,
14,
15].
The crude extract E was sequentially extracted using solvents of different polarities. This allowed for the sequential separation of compounds based on the affinity to the solvent. This further subdivision of extracts provided a clearer picture as to which solvents have the greater capacity to accumulate antioxidants as well as concentrating the antioxidant extracts to smaller fractions. Extracts E1 and E2 were produced using hexane and chloroform, respectively. Extracts E3 and E5 were produced using polar solvents, namely butanol and water, respectively. In
Figure 1, the DPPH reduction percentage was calculated for samples and controls.
The IC
50 values for each sample was calculated to show the concentration of sample needed to reduce 50% of the DPPH in the assay. Low IC
50 values correspond to high antioxidant activity. The IC
50 values show that E3 < E5 < E < A < E4 < E2 < E1 (
Figure 2). Both E1 and E2 showed lower antioxidant activities than other extracts, with E1 being the lowest. This could be explained by the fact that hexane was the most non-polar solvent used in the experiment. Antioxidant compounds are known to accumulate in polar solvents and it is understandable that they would not be present in substantial amounts in an extract produced from hexane. Chloroform is more polar than hexane, so the increased antioxidant activity seen in extract E2, compared with extract E1 (hexane), is expected [
16,
17]. This data also suggests that liquid–liquid extraction of crude extract E with low polarity solvents, hexane and chloroform, was effective in separating fractions with high antioxidant potential, thus producing a more concentrated antioxidant fraction (the residual fraction after extraction with chloroform). This fraction when extracted further produced extracts E3 and E5, which had higher antioxidant capacity compared to the extracts.
Extract E3 had the highest antioxidant activity since it had the lowest IC
50 value. It is notable that extract E3 possessed a similar IC
50 value to ascorbic acid (vitamin C), a known antioxidant, which suggests that extract E3 could be a valuable antioxidant. Extract E had a lower antioxidant activity than extracts E3 and E5 based on the IC
50 value; however, it still had a good antioxidant activity compared with ascorbic acid. These results suggest that the components responsible for antioxidant activity in
Moringa leaves are polar compounds. The values for
Moringa compared with the standard Trolox were not an exact match compared with values found in the literature; however, when the values were normalized as a ratio, the values correlated with results obtained by Chumark et al. (2008) [
18]. Chumark et al. also showed that Trolox has a lower IC
50 value compared to the
Moringa extract, with
Moringa being 35 times less potent than Trolox. In fact, it seems that the Jamaican
Moringa had comparatively better antioxidant activity, as this study showed
Moringa was only four times less potent than Trolox. At the time of this study,
Moringa could be found in only a few regions of the island, and the material analyzed was from a single region [
3]. Further studies are needed to test the
Moringa leaves from other regions in Jamaica extracted under the same conditions to determine if the antioxidant activity differs from parish to parish, possibly depending on the nature of the soil. All leaves used in this assay were harvested at the same time. Researchers in Pakistan, Iqbal and Bhanger (2006), assessed the variations in the antioxidant activity of
Moringa leaves in different seasons and sample locations and found that the antioxidant activity varied based on season and locations of the plants from which the samples were obtained [
19]. However, it is important to note that Pakistan has a larger variation of climatic conditions compared to Jamaica. It is possible that the plants were affected by the cooler and/or drier conditions experienced there. In Jamaica, given the low variability in climatic conditions, it is possible that the Jamaican plants would not have significant differences in antioxidant activities based on the location and time of harvest. Additionally, it is also possible that Jamaican
Moringa has adapted over the years to the moderate climate experienced in Jamaica compared with Pakistan’s climate. Although the antioxidant activities of Jamaican
Moringa leaves should be independent of the harvesting locations, this information is not known. This data would be significant if
Moringa were to be exploited commercially. It may also be useful to investigate antioxidant activities of
Moringa plants throughout the Caribbean, where the climatic conditions are generally similar but the nature of the soil may vary.
Siddharaj and Becker (2003) have shown that different agroclimatic conditions can result in different antioxidant capacities [
6]. In his results, it was seen that India had the best antioxidant capacity compared to samples taken in Central America (Nicaragua) and Africa. This study, however, did not focus on the soil quality, which is another factor that could affect the quality of bioactive principles in plants. Forster and colleagues (2015) assessed the impact of sulfur (a soil factor) compared to water availability. In the field, water availability would typically be determined by rainfall, which is a climatic factor [
20]. This study showed that the availability of sulfur had a positive impact on the presence of the beneficial bioactive principles in
Moringa. There seems to be no direct study yet done on the effect of other soil factors such as pH, soil nutrient levels, and soil type on the antioxidant capacity in
Moringa leaves. The studies mentioned above however, seem to allude to the premise that these factors could be responsible for differences between the antioxidant activity seen in different countries.
It should also be noted that the genotype of Moringa in Jamaica has not been elucidated. There is therefore no concrete evidence to indicate whether Jamaican Moringa is a different cultivar compared to those in Asia or Africa. Moringa was initially introduced to Jamaica in 1784; however, Asian and African migrants have entered Jamaica over the years, and it is quite possible that these immigrants may have brought different varieties or cultivars of Moringa oleifera. This means that there could be several cultivars of Moringa oleifera present in Jamaica as well. For example, a variety with completely white flowers and a different flavor profile has been noted in Jamaica.
Ndhlala and colleagues (2014) analyzed 13
Moringa cultivars from four regions (Thailand, Taiwan, United States of America, and South Africa) for antioxidant capacity. The data showed that the samples from Thailand had the best antioxidant capacity, whereas samples from South Africa (Silver Hill) had the lowest activity [
21]. Ndhlala and others (2014) postulated among other things that climatic/environmental differences between the regions could be responsible for the variations seen [
21].
Moringa has been present in Thailand for several decades, and it is possible that the antioxidant profile is due to the plant's adaptation to its environs [
21]. Both Jamaica and Thailand have tropical climates; similarly, the environmental conditions under which the Jamaican
Moringa plant is grown could have a positive effect on its antioxidant capacity. A comparison between samples from different agroclimatic origins would be beneficial to determine if this postulation is a valid claim. Analyses of soil factors would also be beneficial in providing a broader picture of the impact of environmental conditions on antioxidant capacity in
Moringa oleifera.