Relationships between Nutrient Heterogeneity, Root Growth, and Hormones: Evidence for Interspecific Variation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Nitrogen Treatment
2.3. Harvest
2.4. Root Architecture (RA) Measurement and Analysis
2.5. Hormone Extraction and Purification
2.6. Quantification of Hormones by ELISA
2.7. Statistical Analysis
3. Results
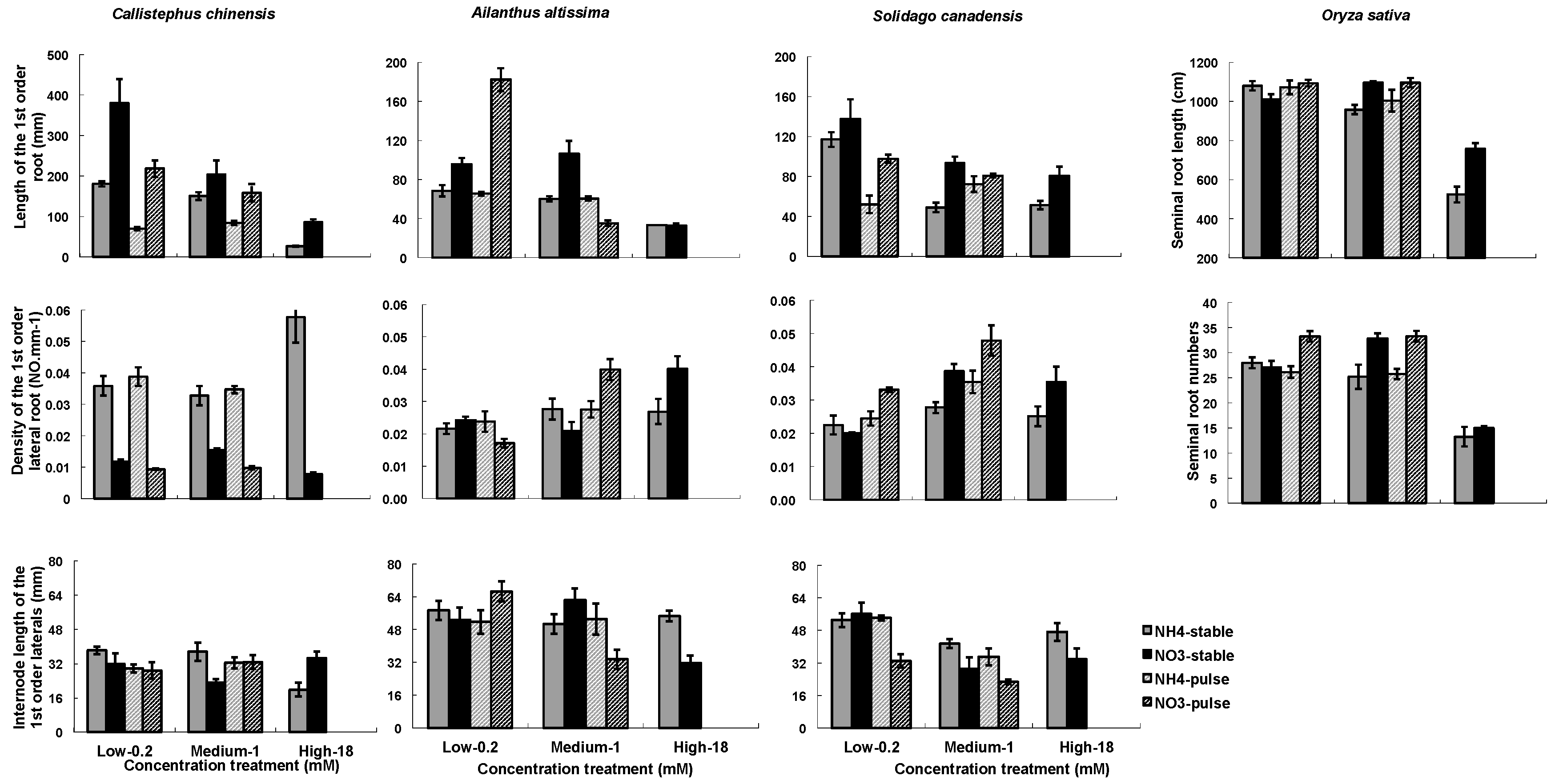
3.1. RA Responses to N Treatments
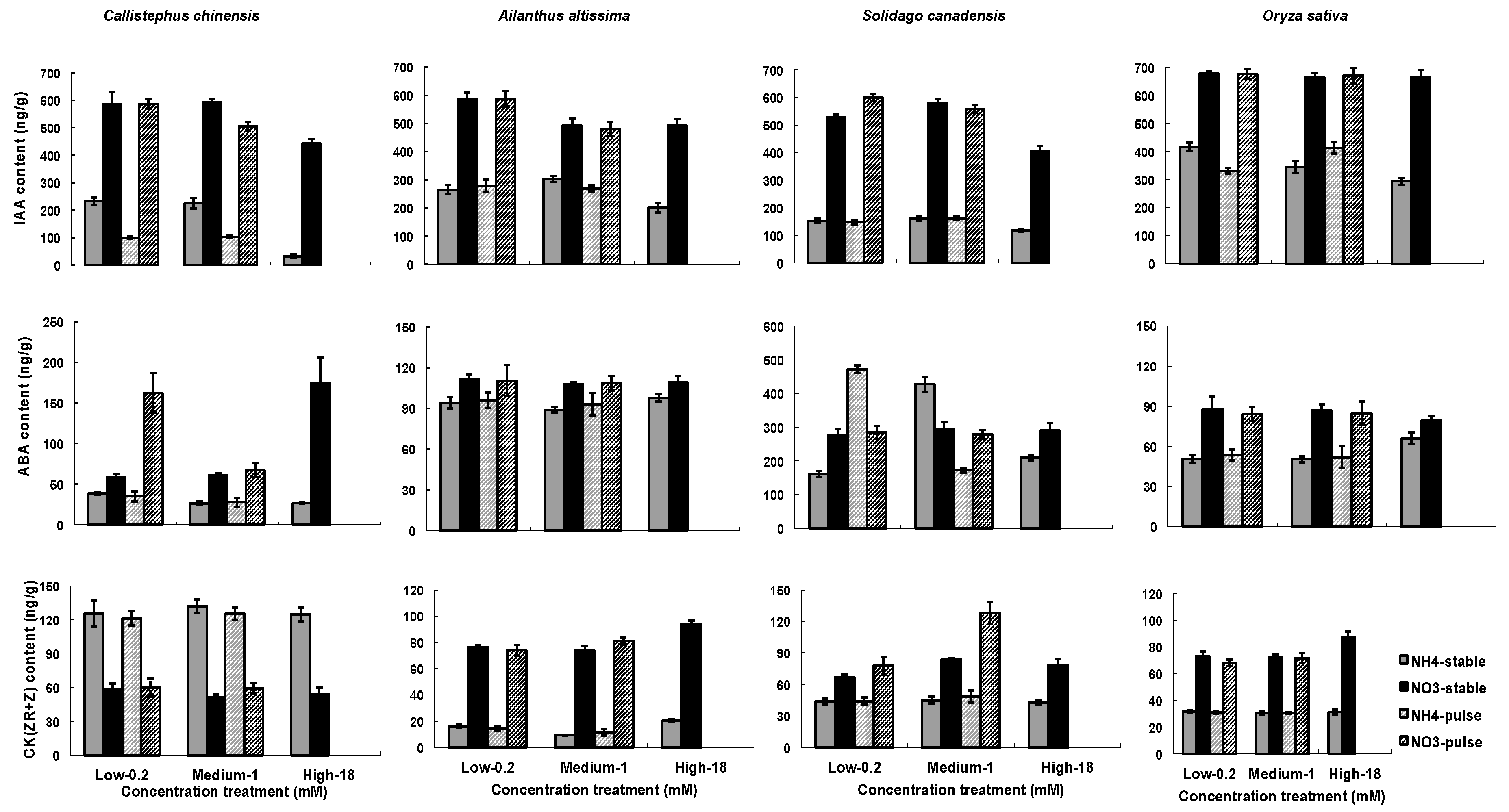
3.2. Hormone Responses of Fine Roots to N Treatment
3.3. Relationships between RA Variables and Root Hormones
4. Discussion
4.1. Effects of Nitrogen Treatment on Root Growth and Architecture
4.2. N Treatment Influences on Hormones
4.3. Relating Root Hormones to RA Features and Root Mass
4.4. Limitations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jackson, R.B.; Caldwell, M.M. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 1993, 74, 612–614. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Fitter, A.H. Architecture and biomass allocation as components of the plastic response of root systems to soil heterogeneity. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above-And Belowground; Academic Press: Cambridge, MA, USA, 1994; pp. 305–323. [Google Scholar]
- Campbell, B.D.; Grime, J.P. A new method of exposing developing root systems to controlled patchiness in mineral nutrient supply. Ann. Bot. 1989, 63, 395–400. [Google Scholar] [CrossRef]
- Robinson, D. The responses of plants to non-uniform supplies of nutrients. New Phytol. 1994, 127, 635–674. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Drew, M.C. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Drew, M.C.; Saker, L.R. Nutrient supply and the growth of the seminal root system in Barley II. Localized, compensatory increases in lateral root growth and rates op nitrate uptake when nitrate supply is restricted to only part of the root system. J. Exp. Bot. 1975, 26, 79–90. [Google Scholar] [CrossRef]
- Einsmann, J.C.; Jones, R.H.; Pu, M.; Mitchell, A.J. Nutrient foraging traits in 10 co-occurring plant species of contrasting life forms. J. Ecol. 1999, 87, 609–619. [Google Scholar] [CrossRef]
- Hodge, A. Root decisions. Plant Cell Environ. 2009, 32, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mou, P.P.; Jones, R.H. Nutrient foraging via physiological and morphological plasticity in three plant species. Can. J. For. Res. 2006, 36, 164–173. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Hendrick, R.L.; Fogel, R. The demography of fine roots in response to patches of water and nitrogen. New Phytol. 1993, 125, 575–580. [Google Scholar] [CrossRef]
- Fitter, A.H. Costs and benefits of mycorrhizas: Implications for functioning under natural conditions. Experientia 1991, 47, 350–355. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramı́rez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1; 3-dependent manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Forde, B.G. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 1998, 279, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Forde, B.G. Nitrate signalling mediated by the NRT1. 1 Nitrate transporter antagonises l-glutamate-induced changes in root architecture. Plant J. 2008, 54, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef] [PubMed]
- Ganmore-Neumann, R.; Kafkafi, U. Root Temperature and Percentage NO3−/NH4+ Effect on Tomato Plant Development I. Morphology and Growth. Agron. J. 1980, 72, 758–761. [Google Scholar] [CrossRef]
- Anderson, D.S.; Teyker, R.H.; Rayburn, A.L. Nitrogen form effects on early corn root morphological and anatomical development. J. Plant Nutr. 1991, 14, 1255–1266. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Boil. 2009, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Kerk, N.M.; Jiang, K.; Feldman, L.J. Auxin metabolism in the root apical meristem. Plant Physiol. 2000, 122, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Feldman, L.J. Regulation of root apical meristem development. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Ponce, G.; Barlow, P.W.; Feldman, L.J.; Cassab, G.I. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant Cell Environ. 2005, 28, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Sachs, T. The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Reed, R.C.; Brady, S.R.; Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Nordström, A.; Tarkowski, P.; Tarkowska, D.; Norbaek, R.; Åstot, C.; Dolezal, K.; Sandberg, G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin–cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 2004, 101, 8039–8044. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the arabidopsis root system. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Müller, A.; Hennig, P.; Kaiser, W.M.; Piotrowski, M.; Weiler, E.W. A role for nitrilase 3 in the regulation of root morphology in sulphur-starving Arabidopsis thaliana. Plant J. 2002, 30, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Ruffel, S.; Gutierrez, R.A.; Gojon, A.; Crawford, N.M.; Coruzzi, G.M.; Lacombe, B. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011, 16, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Signora, L.; de Smet, I.; Foyer, C.H.; Zhang, H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 2001, 28, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.-R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.G.; Romanov, G.A.; Köllmer, I.; Bürkle, L.; Schmülling, T. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J. 2005, 44, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Naitou, T.; Koizumi, N.; Yamashino, T.; Sakakibara, H.; Mizuno, T. Combinatorial microarray analysis revealing Arabidopsis genes implicated in cytokinin responses through the His → Asp phosphorelay circuitry. Plant Cell Physiol. 2005, 46, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Hejátko, J. Hormone interactions at the root apical meristem. Plant Mol. Biol. 2009, 69, 383–396. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Zhang, H.; Inzé, D.; Beeckman, T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.M.; Sarkar, S.F.; Bonetta, D.; McCourt, P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 2003, 34, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Kotur, Z.; Glass, A.D. Characterization of an intact two-component high-affinity nitrate transporter from Arabidopsis roots. Plant J. 2010, 63, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kosola, K.R.; Workmaster, B.A.A.; Spada, P.A. Inoculation of cranberry (Vaccinium macrocarpon) with the ericoid mycorrhizal fungus Rhizoscyphus ericae increases nitrate influx. New Phytol. 2007, 176, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Bollmark, M.; Kubát, B.; Eliasson, L. Variation in endogenous cytokinin content during adventitious root formation in pea cuttings. J. Plant Physiol. 1988, 132, 262–265. [Google Scholar] [CrossRef]
- He, Z. Guidance to experiment on chemical control in crop plants. In Guidance to Experiment on Chemical Control in Crop Plants; Beijing Agricultural University Publishers: Beijing, China, 1993; pp. 60–68. [Google Scholar]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.W.; Jourdan, P.S.; Conrad, W. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 1981, 153, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.M. MANOVA: Multiple response variables and multispecies interactions. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 94–112. [Google Scholar]
- Scheiner, S.M. Multiple response variables and multi-species interactions. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevitch, J., Eds.; Chapman & Hall: New York, NY, USA, 2001; pp. 99–115. [Google Scholar]
- Sattelmacher, B.; Gerendas, J.; Thoms, K.; Brück, H.; Bagdady, N.H. Interaction between root growth and mineral nutrition. Environ. Exp. Bot. 1993, 33, 63–73. [Google Scholar] [CrossRef]
- Gerendás, J.; Zhu, Z.; Bendixen, R.; Ratcliffe, R.G.; Sattelmacher, B. Physiological and biochemical processes related to ammonium toxicity in higher plants. J. Plant Nutr. Soil Sci. 1997, 160, 239–251. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Mou, P.; Jones, R.H.; Tan, Z.; Bao, Z.; Chen, H. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil 2013, 364, 373–384. [Google Scholar] [CrossRef]
- Jia, D.; Pu, M.O.U. Root nutrient foraging of morphological plasticity and physiological mechanism in Callistephus chinensis. Chin. J. Plant Ecol. 2012, 11, 008. [Google Scholar]
- Zhang, H.; Forde, B.G. Regulation of Arabidopsis root development by nitrate availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.A.; Moyano, T.C.; Riveras, E.; Contreras-López, O.; Gutiérrez, R.A. Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proc. Natl. Acad. Sci. USA 2013, 110, 12840–12845. [Google Scholar] [CrossRef] [PubMed]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
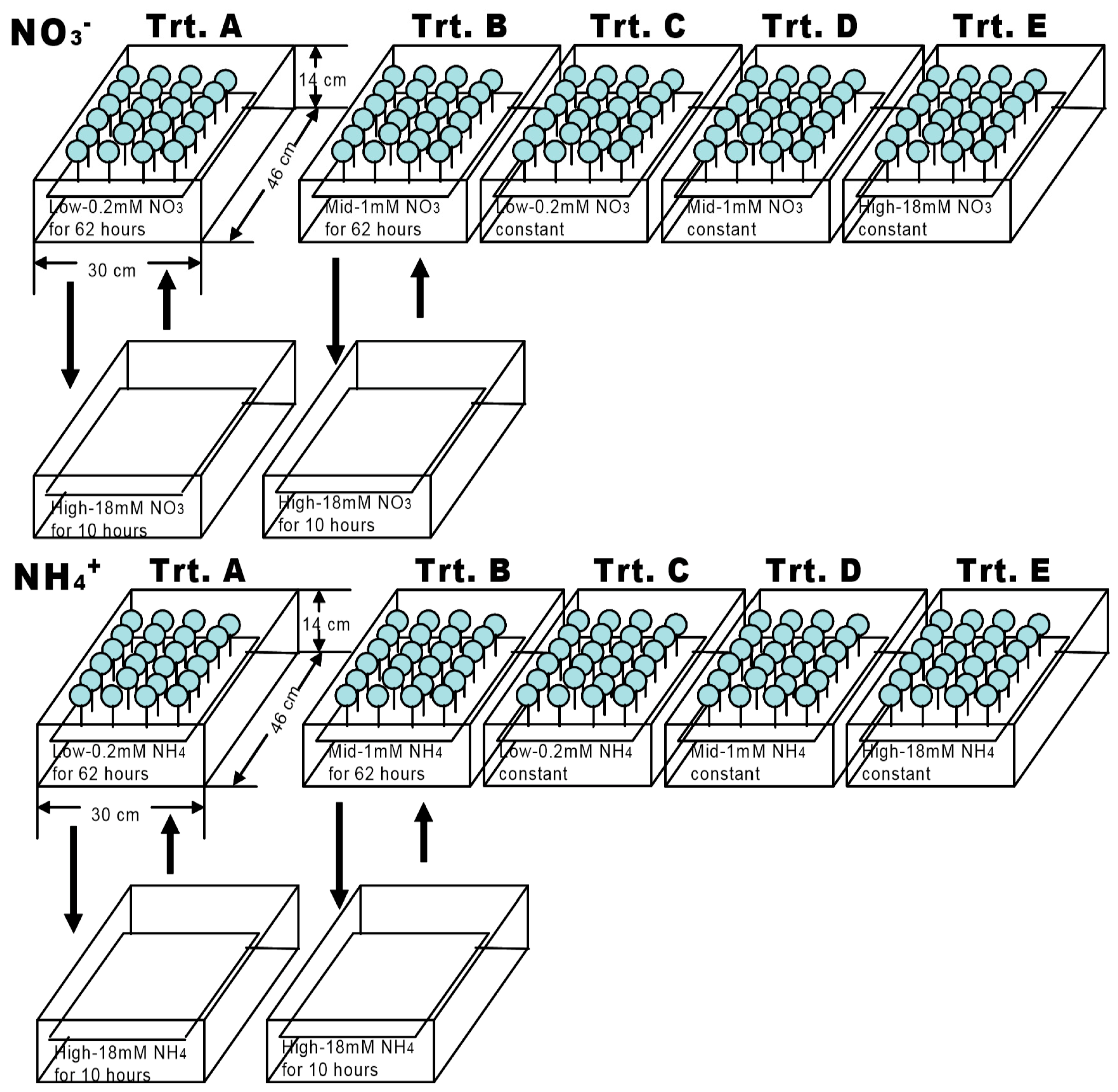

| N Type | N Treatment | N Concentration (mM) | N Applications | Treatment Time Per Period (h) |
|---|---|---|---|---|
| NO3− | A | 0.2/18 | Variable | 62/10 |
| B | 1/18 | Variable | 62/10 | |
| C | 0.2 | Stable | 72 | |
| D | 1 | Stable | 72 | |
| E | 18 | Stable | 72 | |
| NH4+ | A | 0.2/18 | Variable | 62/10 |
| B | 1/18 | Variable | 62/10 | |
| C | 0.2 | Stable | 72 | |
| D | 1 | Stable | 72 | |
| E | 18 | Stable | 72 |
| Nutrient Element | Chemical Formula | Concentration (mM) |
|---|---|---|
| Ferric salt | FeSO4·7H2O | 0.1 |
| EDTA·2Na | 0.1 | |
| Microelement | H3BO4 | 0.04 |
| MnSO4·H2O | 0.002 | |
| ZnSO4·7H2O | 0.001 | |
| CuSO4·5H2O | 0.0003 | |
| KI | 0.005 | |
| Na2Mo4·2H2O | 0.0001 | |
| CoCl2·6H2O | 0.0002 | |
| Macroelement | KH2PO4 | 1.2 |
| MgSO4·7H2O | 1.5 | |
| CaCl2·2H2O | 2 |
| Source | df | FR Mass (g) | 1st ORL (mm) | IBLLR | 1st LRD | df | IAA (ng/g) | ABA (ng/g) | CK(ZR+R) (ng/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | F/sig. | MS | F/sig. | MS | F/sig. | MS | F/sig. | MS | F/sig. | MS | F/sig. | MS | F/sig. | |||
| Callistephus chinensis | ||||||||||||||||
| Model correction | 9 | 0.0035 | 38.10 *** | 45,354.5 | 74.32 *** | 144.06 | 3.12 ** | 0.0013 | 21.90 *** | 9 | 206,037 | 104.8 *** | 12,879 | 15.5 *** | 5781.5 | 30.5 *** |
| N-type (A) | 1 | 0.011 | 105.73 *** | 108,514 | 177.8 *** | 26.19 | 0.57 | 0.0099 | 162.1 *** | 1 | 1,584,583 | 806.3 *** | 67,765 | 81.5 *** | 49,216 | 260 *** |
| Appl. trt. (B) | 1 | 0.008 | 78.12 *** | 79,321.4 | 78.12 *** | 5.89 | 0.13 | 7.69 × 10−6 | 0.54 | 1 | 63,582.8 | 32.35 *** | 5548.3 | 6.67 * | 71.4 | 0.38 |
| Conc. (C) | 2 | 0.0085 | 85.38 *** | 98,897.4 | 162.1 *** | 94.82 | 2.05 | 2.73 × 10−4 | 4.49 * | 2 | 83,319.9 | 42.4 *** | 10,477 | 12.6 *** | 15.90 | 0.084 |
| AB | 1 | 0.0005 | 4.85 * | 391.55 | 0.642 | 282.02 | 6.10 * | 7.65 × 10−5 | 1.26 | 1 | 17,541.8 | 8.93 ** | 6419 | 7.72 ** | 20.57 | 0.11 |
| AC | 2 | 0.0005 | 4.853 * | 16,213 | 26.57 *** | 385.70 | 8.35 ** | 6.88 × 10−4 | 11.31 *** | 2 | 3150.7 | 1.60 | 10,485 | 12.6 *** | 151.40 | 0.80 |
| BC | 1 | 0.0003 | 3.11 | 5792.3 | 9.491 ** | 85.29 | 1.85 | 1.17 × 10−5 | 0.19 | 1 | 2890.8 | 1.47 | 4138.6 | 4.98 * | 141.40 | 0.75 |
| ABC | 1 | 0.0004 | 4.252 * | 750.4 | 1.23 | 21.12 | 0.46 | 1.23 × 10−6 | 0.02 | 1 | 5204.7 | 2.65 | 5303.7 | 6.38 * | 457.50 | 2.42 |
| Error | 32 | 0.00001 | 610.278 | 46.22 | 6.09 × 10−5 | 31 | 1965.3 | 831.4 | 189.30 | |||||||
| Total | 41 | 40 | ||||||||||||||
| Solidago canadensis | ||||||||||||||||
| Model correction | 9 | 0.081 | 13.54 *** | 14,263.3 | 9.62 *** | 362.4 | 7.94 *** | 0.0002 | 6.50 *** | 9 | 86,667.4 | 55.81 *** | 34,484 | 35.1 *** | 2336.3 | 16.5 *** |
| N-type (A) | 1 | 0.185 | 31.02 *** | 4563.56 | 27.71 *** | 1155.3 | 25.33 | 0.001 | 17.2 *** | 1 | 746,394 | 480.6 *** | 969.8 | 0.99 | 14,487.2 | 102.1 *** |
| Appl. trt. (B) | 1 | 0.04 | 0.67 | 1944.77 | 11.81 ** | 305.4 | 6.69 * | 0.0003 | 9.00 ** | 1 | 98.34 | 0.063 | 780.5 | 0.80 | 1601 | 11.3 ** |
| Conc. (C) | 2 | 0.063 | 10.63 ** | 2759.3 | 16.75 *** | 745.56 | 16.3 *** | 0.0005 | 13.5 *** | 2 | 5557 | 3.58 * | 4853.5 | 4.94 * | 1061.6 | 7.48 ** |
| AB | 1 | 0.044 | 7.34 * | 5.25 | 0.032 | 146.1 | 3.2 | 0.00005 | 1.47 | 1 | 2100 | 8.93 ** | 15.857 | 0.016 | 1310.3 | 9.24 ** |
| AC | 2 | 0.077 | 12.93 *** | 64.88 | 0.39 | 55.43 | 1.22 | 0.00004 | 1.13 | 2 | 4757.8 | 3.06 | 6273.9 | 6.39 ** | 788.9 | 5.56 * |
| BC | 1 | 0.011 | 1.84 | 2441.6 | 14.82 ** | 18.59 | 0.41 | 0.00001 | 0.14 | 1 | 12,482 | 8.04 * | 142,701 | 145 *** | 314.84 | 2.22 |
| ABC | 1 | 0.17 | 28.44 *** | 1356.6 | 8.24 * | 110.8 | 2.43 | 0.00003 | 0.66 | 1 | 5454.79 | 3.51 | 131,337 | 134 *** | 324 | 2.21 |
| Error | 18 | 0.006 | 164.71 | 45.62 | 0.000035 | 22 | 1552.91 | 981.85 | 141.86 | |||||||
| Total | 28 | 31 | ||||||||||||||
| Ailanthus altissima | ||||||||||||||||
| Model correction | 9 | 0.00085 | 12.56 *** | 7849.1 | 42.41 *** | 431.33 | 4.35 ** | 0.0002 | 4.96 * | 9 | 65,961.4 | 35.72 *** | 415.6 | 3.70 ** | 3665.7 | 100.7 *** |
| N-type (A) | 1 | 0.0034 | 50.10 *** | 8408 | 45.45 *** | 399.8 | 4.03 | 0.00013 | 453 *** | 1 | 471,249 | 255.2 *** | 3103.9 | 27.7 *** | 30,343.5 | 833.3 *** |
| Appl. trt. (B) | 1 | 0.00001 | 0.17 | 173.84 | 0.94 | 103.2 | 1.04 | 0.00001 | 3.22 | 1 | 121.08 | 0.066 | 12.29 | 0.11 | 70.83 | 1.95 |
| Conc. (C) | 2 | 0.001 | 14.78 *** | 9233.5 | 49.9 *** | 548.4 | 5.53 * | 0.00039 | 14.7 *** | 2 | 13,051.3 | 7.07 ** | 116.1 | 1.04 | 466.5 | 12.81 *** |
| AB | 1 | 0.00002 | 0.30 | 37.27 | 0.20 | 35.618 | 0.36 | 0.00004 | 1.60 | 1 | 520.26 | 0.28 | 47.11 | 0.42 | 2.423 | 0.067 |
| AC | 2 | 0.00065 | 9.53 ** | 4748.4 | 25.7 *** | 436.8 | 4.40 * | 0.00014 | 5.28 * | 2 | 8631.3 | 4.67 * | 61.76 | 0.55 | 78.99 | 2.17 |
| BC | 1 | 0.00018 | 2.62 | 12,057 | 65.18 *** | 442.97 | 4.46 * | 0.00025 | 9.43 ** | 1 | 3409.6 | 1.85 | 20.67 | 0.18 | 57.11 | 1.57 |
| ABC | 1 | 0.00013 | 1.87 * | 10,548 | 57.02 *** | 1016.8 | 10.30 ** | 0.00036 | 13.29 ** | 1 | 280.19 | 0.15 | 16.65 | 0.15 | 2.86 | 0.078 |
| Error | 25 | 0.000007 | 184.99 | 99.22 | 0.00003 | 21 | 1846.71 | 112.22 | 36.41 | |||||||
| Total | 34 | 30 | ||||||||||||||
| Oryza sativa | ||||||||||||||||
| Model correction | 9 | 0.008 | 22.45 *** | 160,207 | 40.70 *** | 0.36 | 0.46 | 188.97 | 6.06 *** | 9 | 146,533 | 347.3 *** | 987.4 | 8.81 *** | 1845.34 | 95.3 *** |
| N-type (A) | 1 | 0.025 | 70.84 *** | 108,655 | 27.60 *** | 1.02 | 1.30 | 103.56 | 3.32 | 1 | 1,087,922 | 2579 *** | 5678.7 | 50.7 *** | 15,212 | 786 *** |
| Appl. trt. (B) | 1 | 1.08 × 10−5 | 0.031 | 12,518.9 | 3.18 | 0.23 | 0.29 | 98.78 | 3.17 | 1 | 1979.9 | 4.69 * | 5.438 | 0.049 | 41.19 | 2.22 |
| Conc. (C) | 2 | 0.016 | 45.73 *** | 467,408 | 118.74 *** | 0.36 | 0.46 | 438.24 | 14.0 *** | 2 | 11,209.9 | 26.6 *** | 33.89 | 0.30 | 198.4 | 1.75 |
| AB | 1 | 3.59 × 10−5 | 0.10 | 1329.99 | 0.34 | 0.18 | 0.23 | 138.13 | 4.43 * | 1 | 3543.1 | 8.38 ** | 11.66 | 0.10 | 33.87 | 1.75 |
| AC | 2 | 0.001 | 1.78 | 53,198.5 | 13.52 *** | 0.095 | 0.12 | 8.89 | 0.29 | 2 | 2389.5 | 5.66 * | 376.3 | 3.36 * | 201.36 | 10.4 ** |
| BC | 1 | 0.002 | 4.84 * | 2.62 | 0.001 | 0.56 | 0.71 | 17.71 | 0.57 | 1 | 1784.9 | 4.23 * | 10.05 | 0.09 | 56.68 | 2.93 |
| ABC | 1 | 0.001 | 3.03 | 16,682.1 | 4.24 * | 0.53 | 0.67 | 5.73 | 0.18 | 1 | 1785.08 | 4.23 * | 2.03 | 0.018 | 45 | 2.33 |
| Error | 32 | 0.00 | 3936.27 | 0.79 | 31.2 | 23 | 421.881 | 112.03 | 19.35 | |||||||
| Total | 41 | 32 | ||||||||||||||
| Species | N Types | Dependent Variable | Regression | Significance | R2 |
|---|---|---|---|---|---|
| Callistephus chinesis | NO3− | Root Mass (g) | =−0.013 + 0.78 (IAA) | 0.00 | 0.61 |
| 1st ORL (mm) | =0.036 + 0.49 (IAA) | 0.021 | 0.33 | ||
| 1st LRD (#/mm) | =−0.041 − 0.54 (ABA) | 0.009 | 0.50 | ||
| NH4+ | Root Mass (g) | =0.035 + 0.96 (IAA) | 0.00 | 0.90 | |
| 1st ORL (mm) | =0.063 + 0.89 (IAA) | 0.00 | 0.82 | ||
| 1st LRD (#/mm) | =0.016 − 0.74 (IAA) | 0.024 | 0.28 | ||
| Solidago canadensis | NO3− | Root Mass (g) | =−0.10 + 0.82 (CK(ZR+R)) | 0.018 | 0.47 |
| 1st ORL (mm) | |||||
| 1st LRD (#/mm) | =−0.14 + 0.90 (CK(ZR+R)) | 0.002 | 0.71 | ||
| NH4+ | Root Mass (g) | =0.22 + 0.71 (IAA) | 0.012 | 0.51 | |
| 1st ORL (mm) | =0.23 + 0.71 (IAA) − 0.45 (CK(ZR+R)) | 0.004 | 0.60 | ||
| 1st LRD (#/mm) | |||||
| Ailanthus altissima | NO3− | Root Mass (g) | =0.28 + 0.68 (IAA) + 0.42 (ABA) | 0.01 | 0.44 |
| 1st ORL (mm) | =0.031 + 0.44 (IAA) − 0.40 (CK(ZR+R)) | 0.009 | 0.54 | ||
| 1st LRD (#/mm) | |||||
| NH4+ | Root Mass (g) | =−0.23 + 0.56 (IAA) | 0.050 | 0.40 | |
| 1st ORL (mm) | =−0.39 − 0.94 (ABA) | 0.015 | 0.60 | ||
| 1st LRD (#/mm) | |||||
| Oryza sativa | NO3− | Root Mass (g) | =−0.097 + 0.97 (IAA) | 0.049 | 0.39 |
| #Seminal Roots (SR) | =−0.14 + 0.50 (IAA) + 0.26 (ABA) − 0.42 (CK(ZR+R)) | 0.00 | 0.92 | ||
| Length of SR (mm) | =−0.19 + 0.88 (IAA) + 0.28 (ABA) | 0.00 | 0.91 | ||
| IBLLR | |||||
| NH4+ | Root Mass (g) | =−0.082 + 0.37 (IAA) − 0.36 (ABA) | 0.005 | 0.48 | |
| #Seminal Roots (SR) | |||||
| Length of SR (mm) | |||||
| IBLLR |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Jones, R.H.; Mou, P. Relationships between Nutrient Heterogeneity, Root Growth, and Hormones: Evidence for Interspecific Variation. Plants 2018, 7, 15. https://doi.org/10.3390/plants7010015
Dong J, Jones RH, Mou P. Relationships between Nutrient Heterogeneity, Root Growth, and Hormones: Evidence for Interspecific Variation. Plants. 2018; 7(1):15. https://doi.org/10.3390/plants7010015
Chicago/Turabian StyleDong, Jia, Robert H. Jones, and Pu Mou. 2018. "Relationships between Nutrient Heterogeneity, Root Growth, and Hormones: Evidence for Interspecific Variation" Plants 7, no. 1: 15. https://doi.org/10.3390/plants7010015




