Mulberry, an ecologically and economically important plant, has been cultivated by farmers as early as 5000 years ago [
18]. This plant is still being cultivated around the world due to its industrial value, especially in sericulture and other purposes. Hence, improved mulberry varieties are necessary for mulberry crop improvement. The mulberry, belonging to the genus
Morus, is a complicated species, containing many different and similar traits that vary depending on the cultivated region, environmental conditions, and developmental stages [
19]. The conventional breeding process has been undertaken to breed mulberry cultivar during the past few decades in order to develop the mulberry varieties with high yield crops [
5]. Thus, the evaluation of the genetic relationship of
Morus genotypes is important for such tasks as measuring the genetic divergence among different mulberry cultivars, predicting progeny performance, and selecting parents for hybridization.
Classifications and evolutionary relationships among
Morus species have been determined by the phenotypic features of the plants, such as height form, leaf structure, bark structure, syncarp, fruit properties, flour morphology, and other various characters. The morphological features of the vegetative and reproductive structures vary from cultivar to cultivar [
20], which is also evident from our study (
Table 1). Compared to the seven other cultivars, Gae-ryang (SM4) and Soosang (SM8) have a small leaf surface. In contrast, the Sangchon Jo Sang Saeng (SM5) cultivar has large leaves. The Hong-ol (SM3) and Soosang (SM8) cultivars have lobed leaves, while the Gwasang II (SM7), Soo hyang (SM6), and Dae-Saug (SM1) cultivars have unlobed leaves. However, the disadvantages of phenotype-based classification are overcome by advanced molecular techniques, such as the use of microsatellite markers, which are highly neutral and insensitive to environment influence [
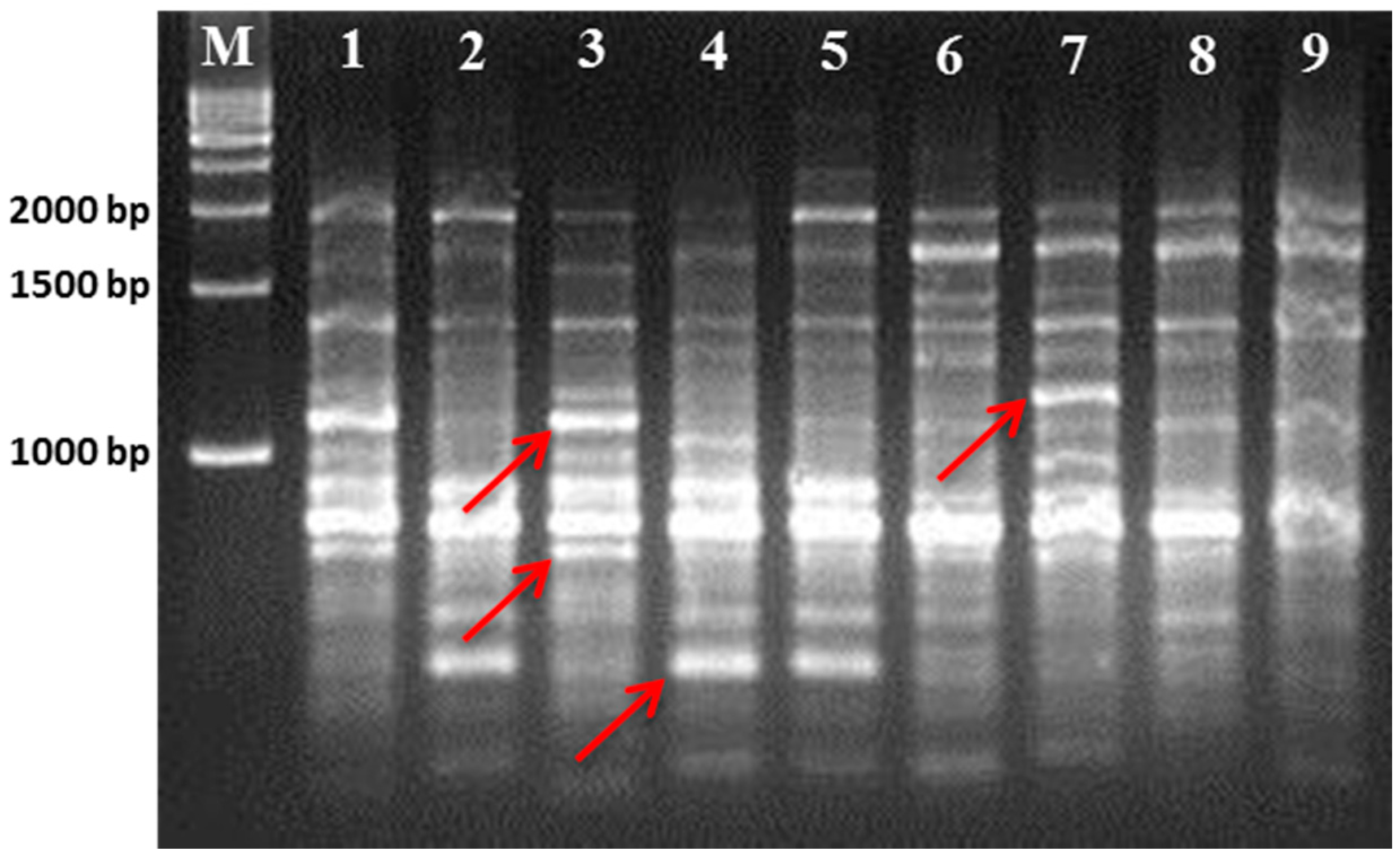
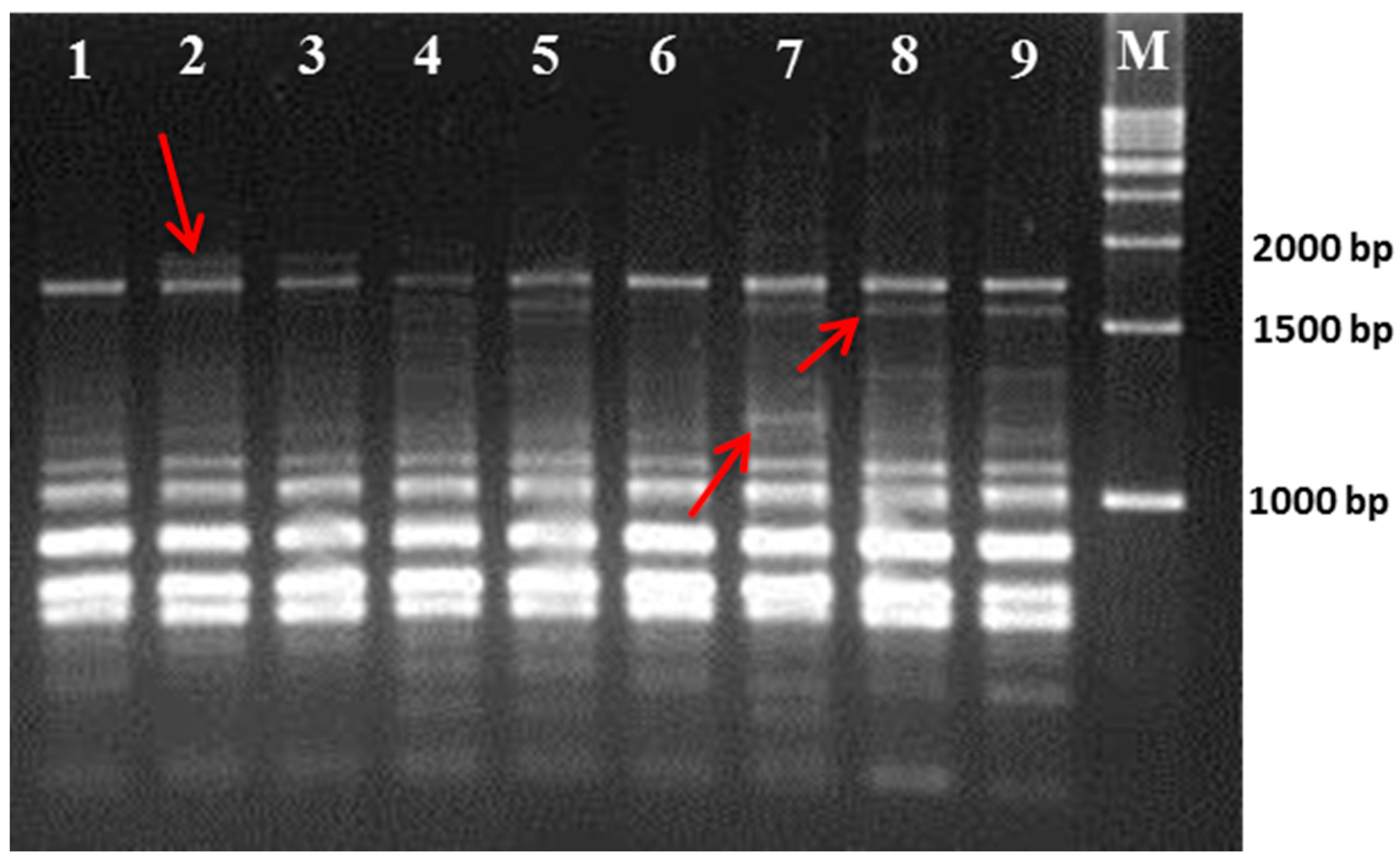
21]. Hence, we used RAPD- and ISSR-based molecular markers to determine the genetic relationships among those cultivars.
The results of the present study again suggest that RAPD and ISSR markers are useful in determining the intra-genetic relationship within mulberry genotypes. Although the ISSR markers in the present study exhibited a higher polymorphism in comparison to the RAPD markers, both markers produced almost satisfactory polymorphisms among the closely related genotypes. This finding resembles that of a previously published study on
Morus species by Srivastava et al. (2004) [
17], who showed that ISSR primers were more efficient in revealing DNA polymorphisms. Similarly, Vijayan and Chatterjee (2003) also found high genetic deviation among 11 closely related local cultivars of mulberry after ISSR markers were used [
22]. PIC values have been estimated for both RAPD and ISSR markers, and the values have projected an estimate of the discriminating power of these markers [
23]. The average PIC value using ISSR primers was found to be higher than those using RAPD primers. This outcome was in agreement with findings from Medhi et al. (2014), who found high PIC values via ISSR during an analysis of the genetic diversity of
Zanthoxylum species [
24]. Taken together with other reports [
4], our findings further suggest that ISSR primers can be used as potential markers for discriminating inter-species of
Morus cultivars.
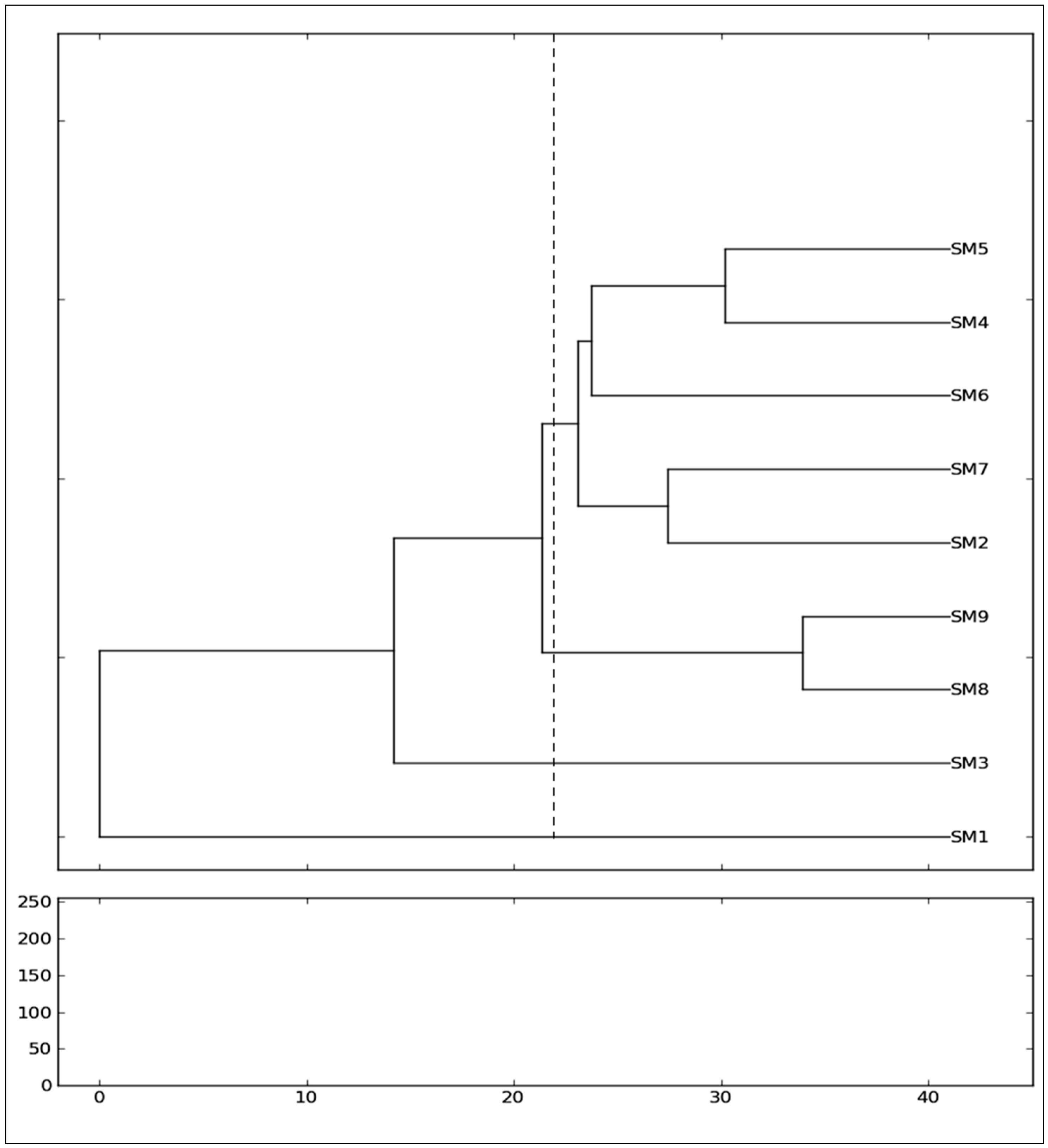
Regarding the genetic relationship of the mulberry genotypes, the assessment of genetic similarity coefficients and the consequent clustering of the genotypes exposed a high genetic variability among the genotypes of nine
Morus species, although they are collected from closely related local cultivars of the same origin. The correlation coefficient among matrices, based on Mantel’s test, was found to be high between RAPD and ISSR (
r = 0.574,
p = 0.000), RAPD and pooled combined matrices (
r = 0.784,
p = 0.001), and ISSR and pooled combined matrices (
r = 0.74,
p = 0.000). The dendrogram drawn using the UPGMA cluster method based on the combined pooled data of RAPD and ISSR markers divided the nine mulberry genotypes into two divergent major groups and the two individual independent accessions. The cophenetic correlation coefficient (
r = 0.785) between the dendrogram and its original matrix, as verified by Mantel’s Z-statistics, was highly significant (
p = 0.000). The grouping of five genotypes of
Morus alba from different cultivars (SangchonJo Sang Saeng (SM5), Gae-ryang (SM4), Soo hyang (SM6), Gwasang II (SM7), and Chung IL (SM2)) as a major cluster revealed close genetic similarities among them (
Figure 3), even though the genotypes have different morphological leaf characteristics (
Table 1). Zhao et al. (2007) also reported that the same clustered genotypes contain differences even though they originated from the same ecotype [
25]. It is a well-known fact that the sustainability of any plant population depends on their genetic diversity. Moreover, factors such as population isolation, genetic drift, mating system, gene flow, selection, and shifts in distribution are responsible for alterations in complex genetic constituting and further cause variations in hereditary multiplicity within the population [
26,
27]. The position of the genotype from Soo hyang (SM6) in relation to other genotypes merits special mention, as this genotype was found to have an intermediate genetic relationship between four other genotypes within the major cluster. The two genotypes of
Morus alba collected from Soosang (SM8) and Hong-Saug (SM9) cultivars formed a separate group, which indicated its genetic divergence from the above genotypes (
Figure 3). The separate independent position in the tree of
Morus alba from Hong-ol (SM3) showed its greater genetic divergence from other
Morus alba genotypes (
Figure 3). Being a different species, the
Morus lhou from Dae-Saug showed a separate identity which was observed in the phylogenetic tree. Thus, the present study showed the efficiency of unravelling the genetic correlation between closely related genotypes. The more closeness observed among
Morus alba in a group that crosses among these individuals may not produce a substantial amount of heterosis. Hence, the cross-breeding within the cultivars in a group may not generate greater variety with higher seed fertility, and it will not be beneficial in transgressive breeding [
17]. It is advisable that genetically diverse cultivars (e.g.,
Morus alba (SangchonJo Sang Saeng, (SM5)) and
Morus lhou (SM1) or the cultivars from different clusters) can be interspecifically hybridize for a greater improvement of the mulberry cultivars in South Korea. Our study with the two markers endorse this view to a certain extent; however, considering the small number of genotypes and markers used for this analysis, a study with different types of markers and a higher number of genotypes needs to be undertaken.