Separation, Identification, and Antidiabetic Activity of Catechin Isolated from Arbutus unedo L. Plant Roots
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
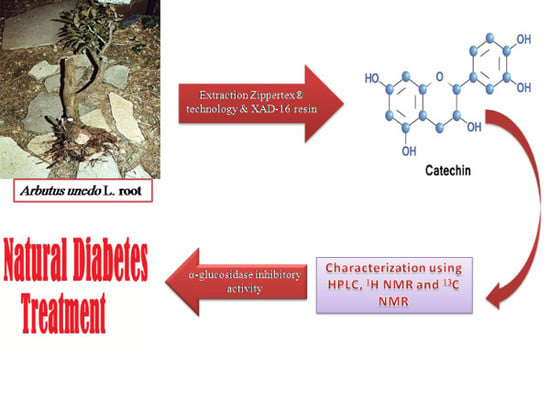
4.1. Plant Material
4.2. Extraction and Isolation Procedures
4.3. Analytical Methods
4.3.1. Analytical HPLC
4.3.2. HPLC Semi-Preparative
4.3.3. NMR and HRMS Analysis
4.4. Enzyme Inhibition Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin. Diabetes 2017, 35, 5–26. [Google Scholar]
- Crawford, K. Review of 2017 diabetes standards of care. Nurs. Clin. N. Am. 2017, 52, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, A.; Caletti, M.T.; Marchignoli, F.; Forlani, G.; Marchesini, G. Which treatment for type 2 diabetes associated with non-alcoholic fatty liver disease? Dig. Liver Dis. 2017, 49, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Ouahidi, M.; Farid, O.; Moufid, A.; Khalidi, A.; Lemhadri, A. L’utilisation des plantes médicinales dans le traitement du diabète au maroc. Phytothérapie 2007, 5, 194–203. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Nothias, L.-F.L.; Boutet-Mercey, S.P.; Cachet, X.; De La Torre, E.; Laboureur, L.; Gallard, J.-F.O.; Retailleau, P.; Brunelle, A.; Dorrestein, P.C.; Costa, J. Environmentally friendly procedure based on supercritical fluid chromatography and tandem mass spectrometry molecular networking for the discovery of potent antiviral compounds from Euphorbia semiperfoliata. J. Nat. Prod. 2017, 80, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. In Oxidative Stress and Inflammation in Non-Communicable Diseases-Molecular Mechanisms and Perspectives in Therapeutics; Springer: New York, NY, USA, 2014; pp. 141–159. [Google Scholar]
- Mrabti, H.N.; Sayah, K.; Jaradat, N.; Kichou, F.; Ed-Dra, A.; Belarj, B.; Cherrah, Y.; Faouzi, M.E.A. Antidiabetic and protective effects of the aqueous extract of Arbutus unedo L. in streptozotocin-nicotinamide-induced diabetic mice. J. Complement. Integr. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Marmouzi, I.; Sayah, K.; Chemlal, L.; El Ouadi, Y.; Elmsellem, H.; Cherrah, Y.; Faouzi, M.A. Arbutus unedo L. aqueous extract is associated with in vitro and in vivo antioxidant activity. J. Mater. Environ. Sci. 2017, 8, 217–224. [Google Scholar]
- Bento, I.; Pereira, J.A. Arbutus unedo L. and its benefits on human health. J. Food Nutr. Res. 2011, 50, 73–85. [Google Scholar]
- Bnouham, M.; Merhfour, F.Z.; Ziyyat, A.; Aziz, M.; Legssyer, A.; Mekhfi, H. Antidiabetic effect of some medicinal plants of oriental morocco in neonatal non-insulin-dependent diabetes mellitus rats. Hum. Exp. Toxicol. 2010, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Rivas-Gonzalo, J.; Del Castillo, M.; Cano, M.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.-M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Mariotto, S.; Esposito, E.; Di Paola, R.; Ciampa, A.; Mazzon, E.; de Prati, A.C.; Darra, E.; Vincenzi, S.; Cucinotta, G.; Caminiti, R. Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice. Pharmacol. Res. 2008, 57, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Afkir, S.; Nguelefack, T.B.; Aziz, M. Arbutus unedo prevents cardiovascular and morphological alterations in l-name-induced hypertensive rats: Part i: Cardiovascular and renal hemodynamic effects of Arbutus unedo in L-name-induced hypertensive rats. J. Ethnopharmacol. 2008, 116, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Novais, M.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kivçak, B.; Mert, T.; Denizci, A. Antimicrobial activity of Arbutus unedo L. J. Pharm. Sci. 2001, 26, 125–128. [Google Scholar]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauza, R.; Zougagh, M.; Ríos, Á. Fast supercritical fluid extraction of low-and high-density polyethylene additives: Comparison with conventional reflux and automatic soxhlet extraction. J. Supercrit. Fluids 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C. Catechin-based extract optimization obtained from Arbutus unedo L. Fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crop. Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef]
- Junior, O.V.; Dantas, J.H.; Barão, C.E.; Zanoelo, E.F.; Cardozo-Filho, L.; de Moraes, F.F. Formation of inclusion compounds of (+) catechin with β-cyclodextrin in different complexation media: Spectral, thermal and antioxidant properties. J. Supercrit. Fluids 2017, 121, 10–18. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. α-glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-glucosidase and α-amylase inhibitory activities of nepalese medicinal herb pakhanbhed (Bergenia ciliata, haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, A.; Frosali, S.; Andreassi, M.; Di Stefano, A. Lycopene phytocomplex, but not pure lycopene, is able to trigger apoptosis and improve the efficacy of photodynamic therapy in HL60 human leukemia cells. Exp. Biol. Med. 2010, 235, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2010, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Selga, A.; Torres, J.L. Efficient preparation of catechin thio conjugates by one step extraction/depolymerization of pine (Pinus pinaster) bark procyanidins. J. Agric. Food Chem. 2005, 53, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, C.; Arcile, G.; Cariel, L.; Lenoir, C.; Bignon, J.; Wdzieczak-Bakala, J.; Ouazzani, J. Antiangiogenic-like properties of fermented extracts of ayurvedic medicinal plants. J. Med. Food 2015, 18, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Fichtali, I.; Laaboudi, W.; Hadrami, E.E.; Aroussi, F.E.; Ben-Tama, A.; Benlemlih, M.; Stiriba, S. Synthesis, characterization and antimicrobial activity of novel benzophenone derived 1, 2, 3-triazoles. J. Mater. Environ. Sci. 2016, 7, 1633–1641. [Google Scholar]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]


| Compounds | α-Glucosidase Inhibitory Activity IC50 (μg/mL), ±SD |
|---|---|
| Catechin | 87.55 ± 2.23 |
| Acarbose | 199.53 ± 1.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrabti, H.N.; Jaradat, N.; Fichtali, I.; Ouedrhiri, W.; Jodeh, S.; Ayesh, S.; Cherrah, Y.; Faouzi, M.E.A. Separation, Identification, and Antidiabetic Activity of Catechin Isolated from Arbutus unedo L. Plant Roots. Plants 2018, 7, 31. https://doi.org/10.3390/plants7020031
Mrabti HN, Jaradat N, Fichtali I, Ouedrhiri W, Jodeh S, Ayesh S, Cherrah Y, Faouzi MEA. Separation, Identification, and Antidiabetic Activity of Catechin Isolated from Arbutus unedo L. Plant Roots. Plants. 2018; 7(2):31. https://doi.org/10.3390/plants7020031
Chicago/Turabian StyleMrabti, Hanae Naceiri, Nidal Jaradat, Ismail Fichtali, Wessal Ouedrhiri, Shehdeh Jodeh, Samar Ayesh, Yahia Cherrah, and My El Abbes Faouzi. 2018. "Separation, Identification, and Antidiabetic Activity of Catechin Isolated from Arbutus unedo L. Plant Roots" Plants 7, no. 2: 31. https://doi.org/10.3390/plants7020031