Conditioning Machine Learning Models to Adjust Lowbush Blueberry Crop Management to the Local Agroecosystem
Abstract
:1. Introduction
- nutrient variables are intrinsically multivariate—compositions should be interpreted as a whole, not as a collection of parts [30],
- descriptive statistical tests compare nutrient status of high and low yielders based on arbitrary yield threshold—they are designed to test differences, not to predict optimal compositions.
2. Materials and Methods
2.1. Experimental Setup
2.2. Soil and Tissue Analyses
2.3. Meteorological Indices
2.4. Investigative Models
2.5. Statistical Analysis
2.5.1. Isometric Log-Ratio
2.5.2. Analysis and Modelling
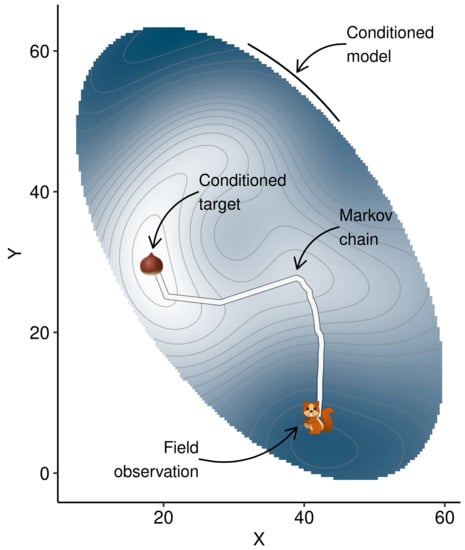
- use the model to predict yield from initial conditions,
- generate n random samples within a fixed radius around the point,
- to avoid extrapolation, compute the Mahalanobis distance between each random sample and the center and covariance of the training data set, then filter out random samples where the Mahalanobis distance is higher than a critical distance,
- use the model to predict yields from the remaining samples,
- extract the sample returning the highest yield,
- if yield is increased compared to the previous value, retain the current vector for the next round and shorten the radius by a factor—else, keep the previous vector for the next round, then increase the radius by a factor.
3. Results
3.1. Variability of Tissue and Yield Data at Regional State
3.2. Investigative Models at Regional Scale
3.2.1. Effects over 2-Years Cropping Cycles
3.2.2. Effects during the Fruit-Bearing Year
3.3. Predictive Model at Local Scale
3.4. Portrait of Optimal Leaf Nutrients at Regional Scale
4. Discussion
4.1. Model Features
4.2. Weather Indices
4.3. Fertilization
4.4. Agronomic Features Optimisation
- Regional guidelines deny the importance of local conditions on plant epigenetics.
- A collection of reference ranges relies on the assumption that the space of successful nutrient dosage and leaf and soil compositions have the shape of hypercube. As illustrated by Parent [46], the shape of such space is irregular and blob- or cloud-like.
- According to Parent [46], interpreting a perturbation between a nutritionally imbalanced specimen and its optimum or successful target “should be done with a multivariate and compositional data perspective in mind. This implies that (1) a univariate or an incomplete multivariate perspective (e.g., focusing on extreme excesses and deficiencies) could miss a high yield region (a parachutist adjusting her fall following only one axis will likely miss the enchanting island and fall into the sea) and (2) changes of concentrations in a closed system are relative, i.e., increasing the concentration of a component will inevitably decrease the concentration of at least another one”.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Brazelton, C. World Blueberry Acreage & Production; U.S. Highbush Blueberry Council: Folsom, CA, USA, 2011; p. 51. [Google Scholar]
- Gagnon, B.; Simard, R.R.; Lalande, R.; Lafond, J. Improvement of soil properties and fruit yield of native lowbush blueberry by papermill sludge addition. Can. J. Soil Sci. 2003, 83, 1–9. [Google Scholar] [CrossRef]
- Lafond, J.; Ziadi, N. Fertilisation azotée et phosphatée dans la production du bleuet nain sauvage au Québec. Can. J. Plant Sci. 2011, 91, 535–544. [Google Scholar] [CrossRef]
- White, S.N.; Boyd, N.S.; Acker, R.C.V. Growing Degree-day Models for Predicting Lowbush Blueberry (Vaccinium angustifolium Ait.) Ramet Emergence, Tip Dieback, and Flowering in Nova Scotia, Canada. HortScience 2012, 47, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Drummond, F. Reproductive Biology of Wild Blueberry (Vaccinium angustifolium Aiton). Agriculture 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, I.M.; Thomsen, C.J.M.; Sargent, R.D. Forested field edges support a greater diversity of wild pollinators in lowbush blueberry (Vaccinium angustifolium). Agric. Ecosyst. Environ. 2017, 237, 154–161. [Google Scholar] [CrossRef]
- MAPAQ. Monographie de l’Industrie du Bleuet Auvage au Québec; Gouvernement du Québec: Québec, QC, Canada, 2016; ISBN 978-2-550-75899-0.
- Plouffe, D.; Bourgeois, G.; Beaudry, N.; Chouinard, G.; Choquette, D. CIPRA—Centre Informatique de Prévision des Ravageurs en Agriculture: Guide des Cultures, 2018; Agriculture et Agroalimentaire Canada: Ottawa, ON, Canada, 2018; ISBN 978-0-660-28720-1.
- Coulibali, Z.; Cambouris, A.N.; Parent, S.-É. Site-specific machine learning predictive fertilization models for potato crops in Eastern Canada. PLoS ONE 2020, 15, e0230888. [Google Scholar] [CrossRef]
- Coulibali, Z.; Cambouris, A.N.; Parent, S.-É. Cultivar-specific nutritional status of potato (Solanum tuberosum L.) crops. PLoS ONE 2020, 15, e0230458. [Google Scholar] [CrossRef] [Green Version]
- Samborska, I.A.; Alexandrov, V.; Sieczko, L.; Kornatowska, B.; Cetner, M.D.; Kalaji, H.M. Artificial neural networks and their application in biological and agricultural research. Signpost Open Access J. NanoPhotoBioSciences 2014, 2, 010409. [Google Scholar]
- Keppel, G.; Kreft, H. Integration and synthesis of quantitative data: Alexander von Humboldt’s renewed relevance in modern biogeography and ecology. Front. Biogeogr. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Rayment, A.F. The response of native stands of lowbush blueberry in newfoundland to nitrogen, phosphorus, and potassium fertilizers. Can. J. Plant Sci. 1965, 45, 145–152. [Google Scholar] [CrossRef]
- Smagula, J.M.; Ismail, A.A. Effects of fertilizer application, preceded by terbacil, on growth, leaf nutrient concentration, and yield of the lowbush blueberry, Vaccinium angustifolium Ait. Can. J. Plant Sci. 1981, 61, 961–964. [Google Scholar] [CrossRef]
- Penney, B.G.; Mcrae, K.B. Herbicidal weed control and crop-year NPK fertilization improves lowbush blueberry (Vaccinium angustifolium Ait.) production. Can. J. Plant Sci. 2000, 80, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, K.R.; Eaton, L.J. Wild blueberry response to phosphorus applied to Prince Edward Island soils. Can. J. Plant Sci. 2008, 88, 363–366. [Google Scholar] [CrossRef]
- Lafond, J. Fractionnement de la fertilisation azotée dans la production du bleuet nain sauvage et suivi de l’azote du sol. Can. J. Soil Sci. 2010, 90, 189–199. [Google Scholar] [CrossRef]
- Smagula, J.M.; Dunham, S. Diammonium Phosphate Corrects Phosphorus Deficiency in Lowbush Blueberry. J. Small Fruit Vitic. 1996, 3, 183–191. [Google Scholar] [CrossRef]
- Warman, P.R. The effects of pruning, fertilizers, and organic amendments on lowbush blueberry production. Plant Soil 1987, 101, 67–72. [Google Scholar] [CrossRef]
- Starast, M.; Karp, K.; Vool, E.; Paal, T.; Tairi, A. Effect of NPK fertilization and elemental sulphur on growth and yield of lowbush blueberry. Agric. Food Sci. 2007, 16, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Eaton, L.J.; Sanderson, K.R.; Fillmore, S.A.E. Comparison of consecutive and alternate fertilizer applications in wild blueberry production. Can. J. Plant Sci. 2009, 89, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Yarborough, D.E.; Smagula, J.M. Fertilizing with Nitrogen and Phosphorus; University of Maine: Orono, ME, USA, 2013. [Google Scholar]
- Saleem, S.R.; Zaman, Q.U.; Schumann, A.W.; Madani, A.; Percival, D.C.; Farooque, A.A. Impact of Variable Rate Fertilization on Wild Blueberry Plant Growth and Fruit Yield. Appl. Eng. Agric. 2013, 2929, 683–690. [Google Scholar]
- Sanderson, K.R.; Carter, M.R.; Ivany, J.A. Effects of gypsum on yield and nutrient status of native lowbush blueberry. Can. J. Plant Sci. 1996, 76, 361–366. [Google Scholar] [CrossRef]
- Munson, R.D.; Nelson, W.L. Principles and practices in plant analysis. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 359–387. [Google Scholar]
- Sauz, M.; Heras, L.; Montañés, L. Relationships between yield and leaf nutrient contents in peach trees: Early nutritional status diagnosis. J. Plant Nutr. 1992, 15, 1457–1466. [Google Scholar] [CrossRef]
- Beaufils, E.R. Diagnosis and recommendation integrated system (DRIS). Soil Sci. Bull. 1973, 1, 1–132. [Google Scholar]
- Parent, L.E.; Dafir, M. A Theoretical Concept of Compositional Nutrient Diagnosis. J. Amer. Soc. Hort. Sci. 1992, 117, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Montañés, L.; Heras, L.; Abadía, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J. Plant Nutr. 1993, 16, 1289–1308. [Google Scholar] [CrossRef] [Green Version]
- Parent, S.-É.; Parent, L.E.; Egozcue, J.J.; Rozane, D.-E.; Hernandes, A.; Lapointe, L.; Hébert-Gentile, V.; Naess, K.; Marchand, S.; Lafond, J.; et al. The plant ionome revisited by the nutrient balance concept. Front. Plant Sci. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, C.L.; Langille, W.M. The mineral content of lowbush blueberry. Can. Plant Dis. Surv. 1962, 42, 124–128. [Google Scholar]
- Trevett, M.F. A second approximation of leaf analysis standards for lowbush blueberries. Maine Agric. Exp. Stn. Res. Life Sci. 1972, 19, 15–16. [Google Scholar]
- Bouchard, A.R.; Gagnon, M.J. Nutrient status of the lowbush blueberry, Lac-Saint-Jean area, Québec, Canada. Commun. Soil Sci. Plant Anal. 1987, 18, 675–686. [Google Scholar] [CrossRef]
- Lafond, J. Optimum leaf nutrient concentrations of wild lowbush blueberry in Quebec. Can. J. Plant Sci. 2009, 89, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Raymond, R.; Mailloux, A.; Dubé, A. Pédologie de la Région du Lac-Saint-Jean; Ministère de l’Agriculture et de la Colonisation du Québec, Division des sols: Québec, QC, Canada, 1965; p. 159.
- Burgher-Maclellan, K.; Mackenzie, K. An Overview of RAPD Analysis to Estimate Genetic Relationships in Lowbush Blueberry. Small Fruits Rev. 2004, 3, 295–305. [Google Scholar] [CrossRef]
- Marty, C.; Lévesque, J.-A.; Bradley, R.L.; Lafond, J.; Paré, M.C. Lowbush blueberry fruit yield and growth response to inorganic and organic N-fertilization when competing with two common weed species. PLoS ONE 2019, 14, e0226619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, R.; Tremblay, L.; Savard, J.; Savard, G.; Grenon, G.; Trépanier, R. Trousse d’Information et de Démarrage en Production Du bleuet Nain Semi-Cultivé; Ministère de l’Agriculture, des Pêcheries et de l’Alimentation: Quebec, QC, Canada, 2000.
- Townsend, L.R.; Hall, L.V. Trends in nutrient levels of lowbush blueberry leaves during four consecutive years of sampling. Nat. Can. 1970, 97, 416–466. [Google Scholar]
- Isaac, R.A.; Johnson, W.C. Determination of Total Nitrogen in Plant Tissue, Using a Block Digestor. J. Assoc. Int. 1976, 59, 98–100. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- LaZerte, S.; Albers, S.; Brown, N. Weathercan: Download and format weather data from Environment and Climate Change Canada. J. Open Source Softw. 2018, 3, 571. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.L. Etude de la Résistance au Gel des Tiges et des Bourgeons de Bleuets Sauvages nains (Vaccinium Sp.). Master’s Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2019. [Google Scholar]
- Fournier, M.-P.; Paré, M.C.; Buttò, V.; Delagrange, S.; Lafond, J.; Deslauriers, A. How plant allometry influences bud phenology and fruit yield in two Vaccinium species. Ann. Bot. 2020, 126, 825–835. [Google Scholar] [CrossRef]
- Parent, S.-É. Why we should use balances and machine learning to diagnose ionomes. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Parent, L.E.; Parent, S.-É.; Hébert-Gentile, V.; Naess, K.; Lapointe, L. Mineral balance plasticity of cloudberry (Rubus chamaemorus) in Quebec-Labrador. Am. J. Plant Sci. 2013, 4, 1509–1520. [Google Scholar] [CrossRef]
- Parent, S.-É.; Parent, L.E.; Rozane, D.-E.; Hernandes, A.; Natale, W. Nutrient Balance as Paradigm of Soil and Plant Chemometrics. In Soil Fertility; Issaka, R.N., Ed.; Intech: New York, NY, USA, 2012; pp. 83–114. [Google Scholar]
- Egozcue, J.J.; Pawlowsky-Glahn, V.; Mateu-Figueras, G.; Barceló-Vidal, C. Isometric logratio transformations for compositional data analysis. Math. Geol. 2003, 35, 279–300. [Google Scholar] [CrossRef]
- Aitchison, J.; Greenacre, M. Biplots of compositional data. J. R. Stat. Soc. Ser. C Appl. Stat. 2002, 51, 375–392. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. 2020. Available online: https://cran.r-project.org/web/packages/compositions/compositions.pdf (accessed on 15 October 2020).
- Gabry, J.; Ali, I.; Brilleman, S.; Novik, J.B.; AstraZeneca; University, T. of C.; Wood, S.; Team, R.C.D.; Bates, D.; Maechler, M.; et al. rstanarm: Bayesian Applied Regression Modeling via Stan. 2020. Available online: http://mc-stan.org/ (accessed on 15 October 2020).
- Karatzoglou, A.; Smola, A.; Hornik, K. Kernlab: Kernel-Based Machine Learning Lab. Available online: https://cran.r-project.org/web/packages/kernlab/index.html (accessed on 15 October 2020).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Team; et al. Caret: Classification and Regression Training, R package version 6.0-47; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Codling, E.A.; Plank, M.J.; Benhamou, S. Random walk models in biology. J. R. Soc. Interface 2008, 5, 813–834. [Google Scholar] [CrossRef] [PubMed]
- R: Topographic Information on Auckland’s Maunga Whau Volcano. Available online: https://stat.ethz.ch/R-manual/R-devel/library/datasets/html/volcano.html (accessed on 2 April 2020).
- Eaton, L.J.; Nams, V.O. Second cropping of wild blueberries—Effects of management practices. Can. J. Plant Sci. 2006, 86, 1189–1195. [Google Scholar] [CrossRef]
- Sanderson, K.; Jordan, C.; Fillmore, S. Leaf Nutrient Ranges for Wild Blueberries in Prince Edward Island. Int. J. Fruit Sci. 2008, 8, 63–68. [Google Scholar] [CrossRef]
- Hepler, P.R.; Yarborough, D.E. Natural Variability in Yield of Lowbush Blueberries. HortScience 1991, 26, 245–246. [Google Scholar] [CrossRef] [Green Version]
- De Wit, C.T. Resource use efficiency in agriculture. Agric. Syst. 1992, 40, 125–151. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Bell, D.J.; Drummond, F.A. Pollen-mediated gene flow in managed fields of lowbush blueberry. Can. J. Plant Sci. 2019, 100, 95–102. [Google Scholar] [CrossRef]
- Hall, I.V.; Aalders, L.E.; McRAE, K.B. Lowbush blueberry production in eastern Canada as related to certain weather data. Can. J. Plant Sci. 1982, 62, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Glass, V.M.; Percival, D.C.; Proctor, J.T.A. Tolerance of lowbush blueberries (Vaccinium angustifolium Ait.) to drought stress. I. Soil water and yield component analysis. Can. J. Plant Sci. 2005, 85, 911–917. [Google Scholar] [CrossRef]
- Parent, L.E. Diagnosis of the nutrient compositional space of fruit crops. Rev. Bras. De Frutic. 2011, 33, 321–334. [Google Scholar] [CrossRef]
- Penney, B.G.; McRae, K.B.; Bishop, G.A. Second-crop N fertilization improves lowbush blueberry (Vaccinium angustifolium Ait.) production. Can. J. Plant Sci. 2003, 83, 149–155. [Google Scholar] [CrossRef]
- Nestby, R.; Krogstad, T.; Joner, E.; Vohník, M. The effect of NP fertilization on European blueberry (Vaccinium myrtillus L.) development on cultivated land in mid-Norway. J. Berry Res. 2014, 4, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, R.; Percival, D.; Zaman, Q.; Astatkie, T.; Adl, S.; Buszard, D. Improved Growth and Harvestable Yield through Optimization of Fertilizer Rates of Soil-applied Nitrogen, Phosphorus, and Potassium in Wild Blueberry (Vaccinium angustifolium Ait.). HortScience 2016, 51, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 1995; Volume 11, ISBN 0-12-473542-8. [Google Scholar]
- Chapin III, F.S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Tagliavini, M.; Zavalloni, C.; Rombolà, A.D.; Quartieri, M.; Malaguti, D.; Mazzanti, F.; Millard, P.; Marangoni, B. Mineral nutrient partitioning to fruits of decidious trees. Acta Hortic. 2000, 131–140. [Google Scholar] [CrossRef]
- Sandler, H.A.; DeMoranville, C.J. Cranberry Production Guide—A Guide for Massachusetts. Available online: https://scholarworks.umass.edu/cgi/viewcontent.cgi?article=1000&context=cranberry_prod_guide (accessed on 15 October 2020).
- Lafond, J. Fertilization in Wild Blueberry Production. In Wild Blueberry Production Guide in a Context of Sustainable Development; CRAAQ: Québec, QC, Canada, 2000. [Google Scholar]
- Ochmian, I.; Oszmiański, J.; Jaśkiewicz, B.; Szczepanek, M. Soil and highbush blueberry responses to fertilization with urea phosphate. Folia Hortic. 2018, 30, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Lafond, J.; Ziadi, N. Biodisponibilité de l’azote et du phosphore dans les sols de bleuetières du Québec. Can. J. Soil Sci. 2013, 93, 33–44. [Google Scholar] [CrossRef]
- Lafond, J.; Ziadi, N. Phosphorus mobility in acidic wild blueberry soils in Québec, Canada. 2018. Available online: https://digitalcommons.library.umaine.edu/nabrew2018/proceedingpapers/proceedingpapers/17/ (accessed on 15 October 2020).
- Nowaki, R.H.D.; Parent, S.-É.; Cecílio Filho, A.B.; Rozane, D.E.; Meneses, N.B.; dos Santos da Silva, J.A.; Natale, W.; Parent, L.E. Phosphorus Over-Fertilization and Nutrient Misbalance of Irrigated Tomato Crops in Brazil. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Eaton, L.J.; Ju, H.-Y.; Sanderson, K.R. Effects of summer and fall applications of foliar boron on fruit bud winter injury in wild blueberry (Vaccinium angustifolium Ait.). Can. J. Plant Sci. 2007, 87, 923–925. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Smagula, J.M. Evaluation of the leaf boron standard for Vaccinium angustifolium Ait. Acta Hortic. 2006, 365–370. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupré, R.L.C.; Khiari, L.; Gallichand, J.; Joseph, C.A. Multi-Factor Diagnostic and Recommendation System for Boron in Neutral and Acidic Soils. Agronomy 2019, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, A.; Parent, L.E.; Fortin, J.; Tremblay, C.; Khiari, L.; Giroux, M. Environmental Mehlich-III soil phosphorus saturation indices for Quebec acid to near neutral mineral soils varying in texture and genesis. Can. J. Soil Sci. 2006, 86, 711–723. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Van Lierop, W. Soil pH and lime requirement determination. In Soil Testing and Plant Analysis; America Book Series: Madison, WI, USA, 1990; Volume 3, pp. 73–126. [Google Scholar]
- Dong, D.; Xie, Z.; Du, Y.; Liu, C.; Wang, S. Influence of soil ph on aluminum availability in the soil and aluminum in tea leaves. Commun. Soil Sci. Plant Anal. 1999, 30, 873–883. [Google Scholar] [CrossRef]
- Townsend, L.R.; Hall, I.V. Chemical composition of lowbush blueberry cultivars. Proc. Am. Soc. Hort. Sci. 1968, 248–253. [Google Scholar]
- Parent, S.-É.; Parent, L.E.; Rozane, D.E.; Natale, W. Plant ionome diagnosis using sound balances: Case study with mango (Mangifera Indica). Front. Plant Sci. 2013, 4, 449. [Google Scholar] [CrossRef] [Green Version]
- Modesto, V.C.; Parent, S.-É.; Natale, W.; Parent, L.E. Foliar Nutrient Balance Standards for Maize (Zea mays L.) at High-Yield Level. Am. J. Plant Sci. 2014, 5, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Parent, S.-É.; Barlow, P.; Parent, L.E. Nutrient Balances of New Zealand Kiwifruit (Actinidia deliciosa cv. Hayward) at High Yield Level. Commun. Soil Sci. Plant Anal. 2015, 46, 256–271. [Google Scholar] [CrossRef]
- Vahl de Paula, B.; Squizani Arruda, W.; Etienne Parent, L.; Frank de Araujo, E.; Brunetto, G. Nutrient Diagnosis of Eucalyptus at the Factor-Specific Level Using Machine Learning and Compositional Methods. Plants 2020, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Leitzke Betemps, D.; Vahl de Paula, B.; Parent, S.-É.; Galarça, S.P.; Mayer, N.A.; Marodin, G.A.B.; Rozane, D.E.; Natale, W.; Melo, G.W.B.; Parent, L.E.; et al. Humboldtian Diagnosis of Peach Tree (Prunus persica) Nutrition Using Machine-Learning and Compositional Methods. Agronomy 2020, 10, 900. [Google Scholar] [CrossRef]













| Phenological Stage | Julian Day | Calendar Dates |
|---|---|---|
| Before flower bud opening | [92 to 125] | 1 April to 5 May |
| Flower bud opening | [126 to 163] | 5 May to 11 June |
| Flower open (Pollination period) | [164 to 180] | 12 June to 28 June |
| Fruit maturation | [181 to 220] | 29 June to 7 August |
| After fruit maturation (Harvest) | [221 to 244] | 7 August to 31 August |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parent, S.-É.; Lafond, J.; Paré, M.C.; Parent, L.E.; Ziadi, N. Conditioning Machine Learning Models to Adjust Lowbush Blueberry Crop Management to the Local Agroecosystem. Plants 2020, 9, 1401. https://doi.org/10.3390/plants9101401
Parent S-É, Lafond J, Paré MC, Parent LE, Ziadi N. Conditioning Machine Learning Models to Adjust Lowbush Blueberry Crop Management to the Local Agroecosystem. Plants. 2020; 9(10):1401. https://doi.org/10.3390/plants9101401
Chicago/Turabian StyleParent, Serge-Étienne, Jean Lafond, Maxime C. Paré, Léon Etienne Parent, and Noura Ziadi. 2020. "Conditioning Machine Learning Models to Adjust Lowbush Blueberry Crop Management to the Local Agroecosystem" Plants 9, no. 10: 1401. https://doi.org/10.3390/plants9101401