A Statistical Analysis of the Relationship between Brown Haze and Surface Air Pollution Levels on Respiratory Hospital Admissions in Auckland, New Zealand
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Brown Haze Data
2.3. Air Pollution and Climate Data
2.4. Hospital Admissions
2.5. Statistical Methods
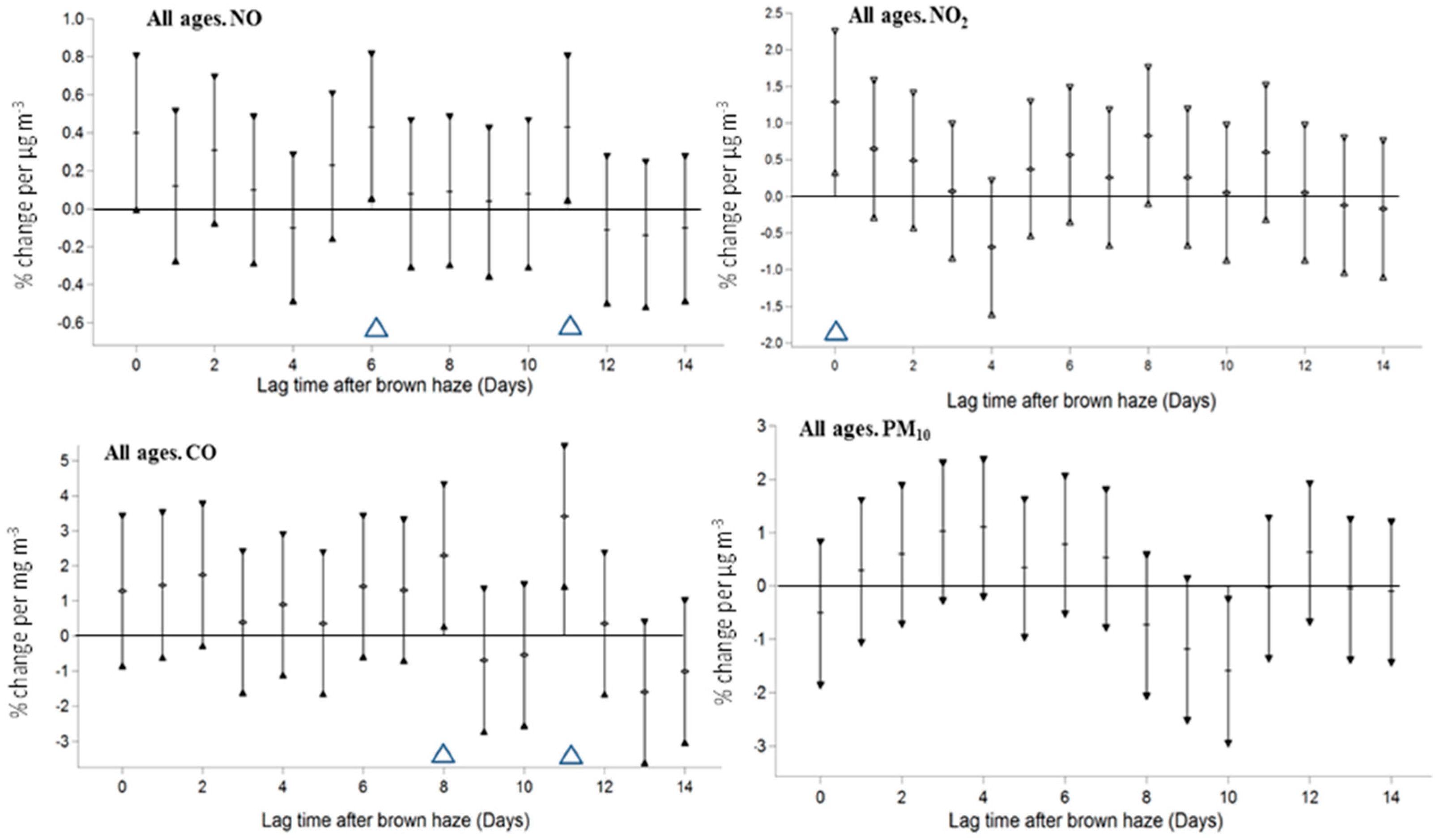
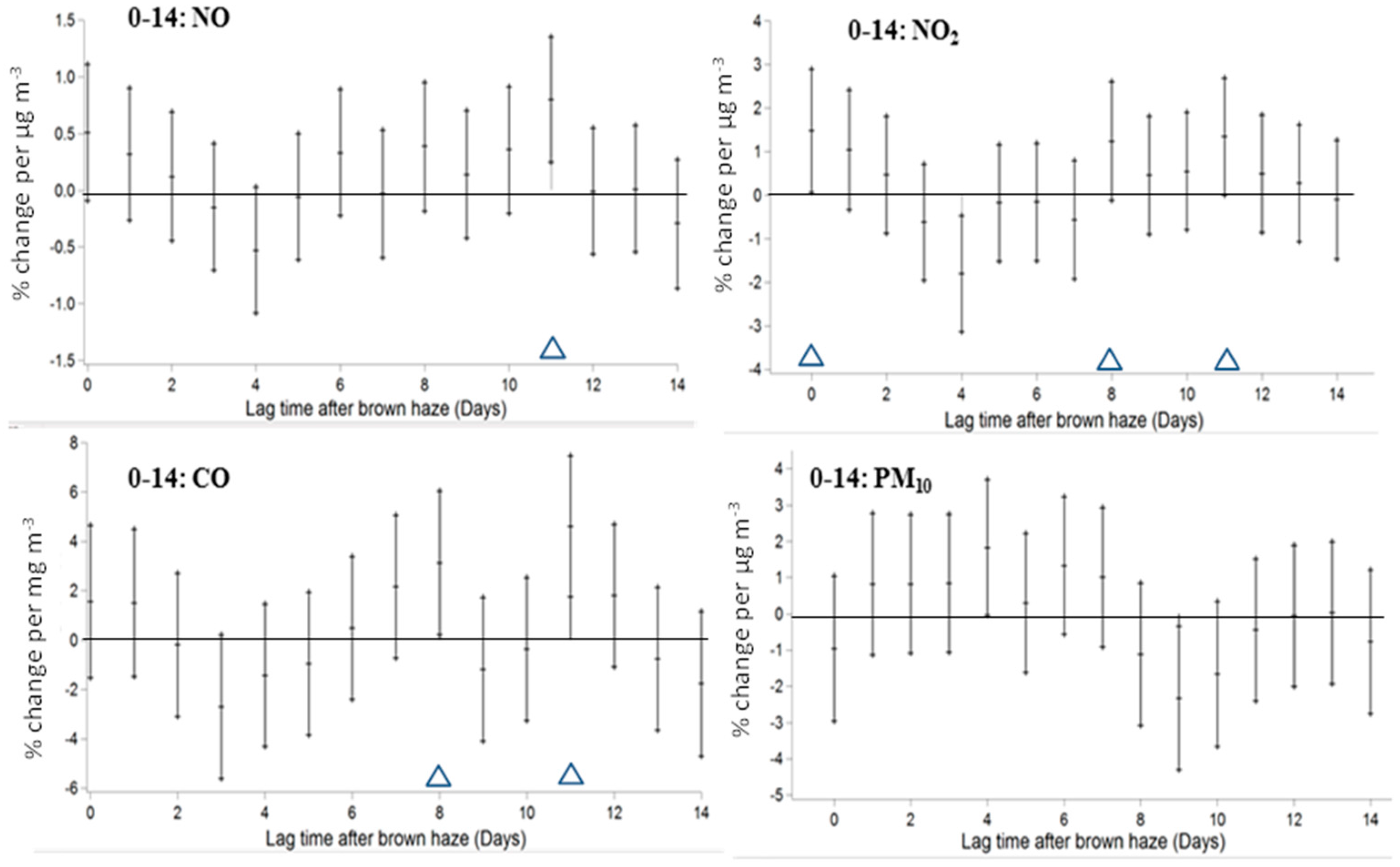
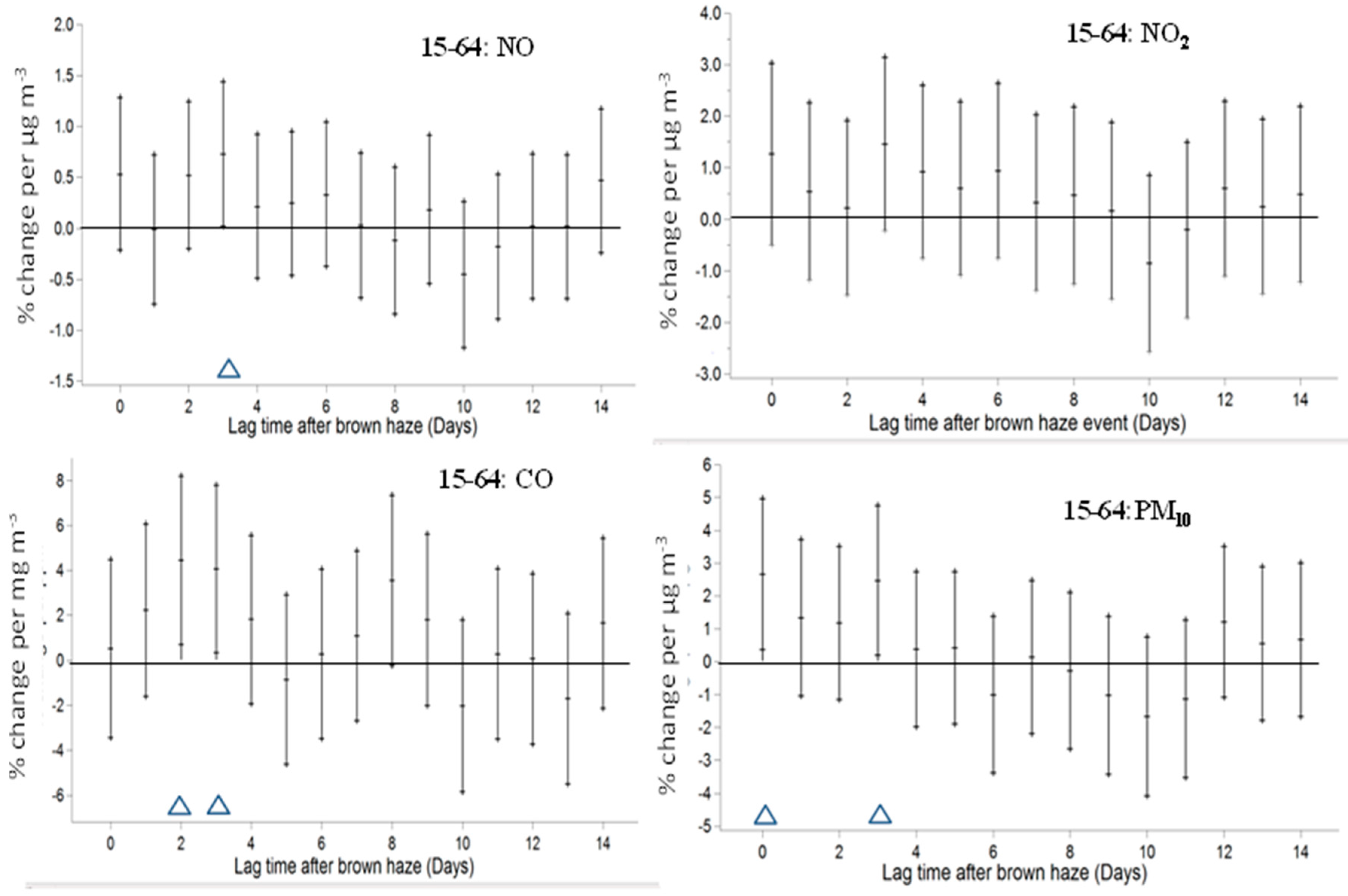
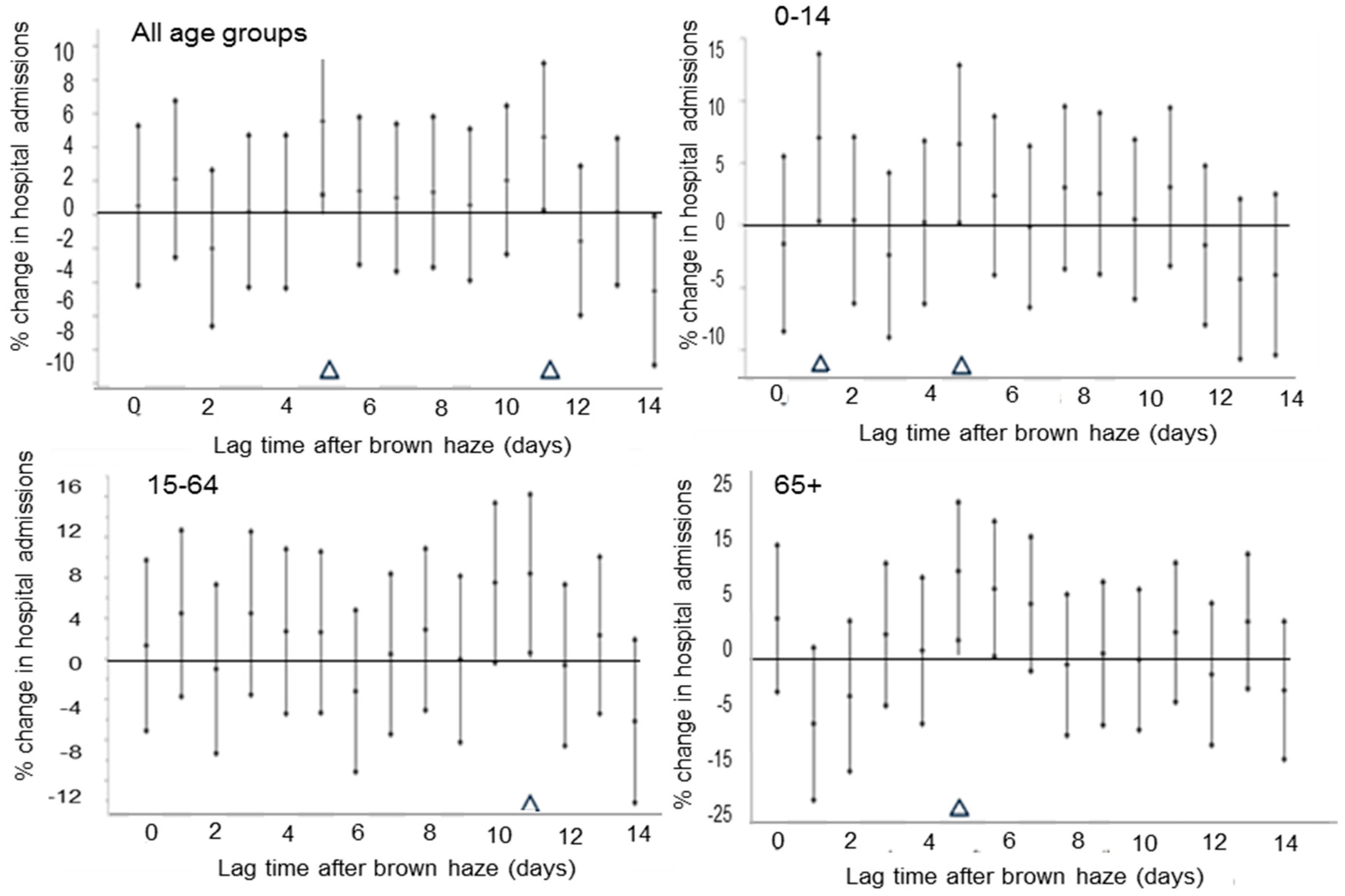
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution. Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities Study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Turner, M.C.; Burnett, R.T.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; Krewski, D.; Brook, R.D. Relationships between fine particulate air pollution, cardiometabolic disorders and cardio-vascular mortality. Circ. Res. 2014, 116, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Xun, W.W.; Katsouyanni, K.; Dimakopoulou, K.; Brunekreef, B.; Weinmayr, G.; Hoffmann, B.; et al. Long-term exposure to air pollution and cardiovascular mortality: An analysis of 22 European cohorts. Epidemiology 2014, 25, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Hoek, G.; Van Vliet, P.; Meliefste, K.; Fischer, P.H.; Wijga, A. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am. J. Respir. Crit. Care Med. 2002, 166, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Anderson, H.R.; Sunyer, J.; Qyres, J.; Baccini, M. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Am. J. Respir. Crit. Care Med. 2001, 164, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Nafstad, P.; Håheim, L.L.; Oftedal, B.; Gram, F.; Holme, I.; Hjermann, I. Lung cancer and air pollution: A 27 year follow up of 16,209 Norwegian men. Thorax 2003, 58, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Krewski, D.; Pope, C.A.; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Ezzati, M.; Dockery, D.W.; Pope, C.A., III; Ezzati, M. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, M.; Nicolich, M.J. Cumulative effects and threshold levels in air pollution mortality: Data analysis of nine large US cities using the NMMAPS dataset. Environ. Pollut. 2009, 157, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Gouveia, N.; Cifuentes, L.A.; de Leon, A.P.; Junger, W.; Vera, J.; Strappa, V.; Hurtado-Díaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res. Rep. Health Eff. Inst. 2012, 171, 5–86. [Google Scholar]
- Künzli, N.; Kaier, R.; Medina, S.; Studnicka, M.; Chanel, O.; Fillinger, P.; Herry, M.; Horak, F., Jr.; Puybonnieux-Texier, V.; Quénel, P.; et al. Public health impact of outdoor and traffic related air pollution: A European assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Wong, C.M.; Vichit-Vadakan, N.; Kan, H.; Qian, Z. Public Health and Air Pollution in Asia (PAPA): A multicity study of short-term effects of air pollution on mortality. Environ. Health Perspect. 2008, 116, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, S.A.; Nongkynrih, B.; Gupta, S.K. Air pollution in Delhi: Its Magnitude and Effects on Health. Indian J. Community Med. 2013, 38, 4–8. [Google Scholar] [PubMed]
- Chow, J.C.; Watson, J.G.; Mauderly, J.L.; Costa, D.L.; Wyzya, R.E.; Vedal, S.; Hidy, G.M.; Altshuler, S.L.; Marrack, D.; Heuss, J.M.; et al. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Samet, J.M.; Anderson, H.R.; Atkinson, R.; Lertre, A.; Medina, S.; Samoli, E.; Touloumi, G.; Burnett, R.T.; Krewski, D.; et al. Air pollution and health: A European and North American approach (APHENA). Res. Rep. Health Eff. Inst. 2009, 142, 5–90. [Google Scholar]
- Nidhi, J.G. Air quality and respiratory health in Delhi. Environ. Monit. Assess. 2007, 135, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Siscovick, D.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The Effect of Air Pollution on Lung Development from 10 to 18 Years of Age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-W.; Vivian, E.; Mohammed, K.A.; Jakhar, S.; Vaughn, M.; Huang, J.; Zelicoff, A.; Xaverius, P.; Bai, Z.; Lin, S.; et al. Long-term ambient air pollution and lung function impairment in Chinese children from a high air pollution range area: The Seven Northeastern Cities (SNEC) study. Atmos. Environ. 2016, 138, 144–151. [Google Scholar] [CrossRef]
- Kuschel, G.; Metcalfe, J.; Wilton, E.; Guria, J.; Hales, S.; Rolfe, K.; Woodward, A. Updated Health and Air Pollution in New Zealand Study—Volume 1: Summary Report. 2012, p. 89. Available online: http://www.hapinz.org.nz/HAPINZ%20Update_Vol%201%20Summary%20Report.pdf (accessed on 24 May 2017).
- Fisher, G.; Rolfe, K.; Kjellstrom, T.; Woodward, A.; Hales, S.; Sturman, A.P.; Kingham, S.; Petersen, L.; Shrestha, R.; King, D. Health effects due to motor vehicle air pollution in New Zealand. Report for the Ministry for the Environment, New Zealand. 2002. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.600.8685&rep=rep1&type=pdf (accessed on 24 May 2017).
- McGowan, J.A.; Hider, P.N.; Chacko, E.; Town, G.I. Particulate air pollution and hospital admissions in Christchurch, New Zealand. Aust. N. Z. J. Public Health 2002, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hider, P.N.; Epton, M.J.; Jennings, L.C.; Kingham, S.P. Including viral infection data supports an association between particulate pollution and respiratory admissions. Aust. N. Z. J. Public Health 2011, 35, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Williams, G.M.; Schwartz, J.; Neller, A.H.; Best, T.L.; Petroeschevsky, A.L.; Simpson, R.W. Air pollution and child respiratory health: A case-crossover study in Australia and New Zealand. Am. J. Respir. Crit. Care Med. 2005, 171, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Scoggins, A.; Kjellstrom, T.; Fisher, G.; Connor, J.; Gimson, N. Spatial analysis of annual air pollution exposure and mortality. Sci. Total Environ. 2004, 321, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Coulson, G.; Olivares, G.; Talbot, N. Aerosol Size Distributions in Auckland. Air Qual. Clim. Chang. 2016, 50, 23–28. [Google Scholar]
- Talbot, N. An Investigation into the Occurrence of Brown Haze Events over Auckland. Master’s Thesis, University of Auckland, Auckland, New Zealand, 2011. [Google Scholar]
- Salmond, J.A.; Dirks, K.N.; Fiddes, S.; Pezza, A.; Talbot, N.; Scarfe, J.; Renwick, J.; Petersen, J. A climatological analysis of the incidence of brown haze in Auckland, New Zealand. Int. J. Climatol. 2015, 36, 2516–2526. [Google Scholar] [CrossRef]
- Jiang, N.; Hay, J.E.; Fisher, G.W. Synoptic weather types and morning rush hour nitrogen oxides concentrations during Auckland winters. Weather Clim. 2005, 25, 43–69. [Google Scholar]
- Scarfe, J. An Investigation into Surface Air Pollution, Brown Haze and Hospital Admissions in Auckland. Master’s Thesis, University of Auckland, Auckland, New Zealand, 2014. [Google Scholar]
- Senaratne, I.; Shooter, D. Elemental composition in source identification of brown haze in Auckland, New Zealand. Atmos. Environ. 2004, 38, 3049–3059. [Google Scholar] [CrossRef]
- Wang, K. The Changing Demography of Counties Manukau District Health Board; Heal: San Francisco, CA, USA, 2008. [Google Scholar]
- Symons, J.K.; Leksmono, N.S.; Hayes, E.T.; Chatterton, T.J.; Longhurst, J.W.S. Air quality management in Auckland, New Zealand. WIT Trans. Ecol. Environ. 2007, 101, 169–179. [Google Scholar]
- NIWA. The National Climate Database (CliFlo). Available online: http://cliflo.niwa.co.nz/ (accessed on 1 January 2012).
- Gosai, A.; Salinger, J.; Dirks, K. Climate and respiratory disease in Auckland, New Zealand. ANZJPH 2009, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.D.; Dominici, F. Statistical Methods for Environmental Epidemiology with R: A Case Study in Air Pollution and Health; Springer: New York, NY, USA; London, UK, 2008. [Google Scholar]
- Domicini, F.; McDermott, A.; Hastie, T.J. Improved semiparametric time series models of air pollution and mortality. J. Am. Stat. Assoc. 2004, 99, 938–948. [Google Scholar] [CrossRef]


| Variable | Statistic | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Min | Q1 | Med | Q3 | Max | |
| Annual respiratory hospital admissions count | ||||||||
| All age groups | 72,429 | 18.0 | 7.6 | 1 | 12 | 17 | 22 | 54 |
| 0–14 years | 33,490 | 8.3 | 4.8 | 0 | 5 | 7 | 11 | 28 |
| 15–64 years | 22,763 | 5.7 | 2.9 | 0 | 4 | 5 | 7 | 21 |
| 65+ years | 16,203 | 4.0 | 2.5 | 0 | 2 | 4 | 5 | 22 |
| Cool season respiratory hospital admission count | ||||||||
| All age groups | 31,245 | 23.1 | 7.7 | 6 | 18 | 22 | 28 | 54 |
| 0–14 years | 15,368 | 11.4 | 5.1 | 1 | 8 | 11 | 14 | 28 |
| 15–64 years | 9275 | 6.9 | 3.2 | 0 | 5 | 6 | 9 | 21 |
| 65+ years | 6602 | 4.9 | 3 | 0 | 3 | 5 | 6 | 15 |
| Annual atmospheric and pollution data | ||||||||
| NO (μg·m−3) | 3150 | 20.8 | 21.1 | 0.0 | 4.5 | 14.9 | 29.1 | 139.0 |
| NO2 (μg·m−3) | 3149 | 14.8 | 9.3 | 0.3 | 7.2 | 13.5 | 20.8 | 53.1 |
| CO (mg·m−3) | 3895 | 0.41 | 0.38 | 0.00 | 0.14 | 0.30 | 0.56 | 2.99 |
| PM10 (μg·m−3) | 3248 | 15.2 | 6.4 | 2.0 | 11.1 | 14.0 | 18.0 | 45.3 |
| Temperature (°C) | 4017 | 15.3 | 3.5 | 4.9 | 12.7 | 15.3 | 18.1 | 24.4 |
| Cool season atmospheric and pollution data | ||||||||
| NO (μg·m−3) | 1094 | 31.7 | 27.4 | 0.0 | 10.0 | 24.4 | 47.7 | 139.0 |
| NO2 (μg·m−3) | 1094 | 19.9 | 9.7 | 0.6 | 12.7 | 20.0 | 26.4 | 53.1 |
| CO (mg·m−3) | 1303 | 0.64 | 0.48 | 0.0 | 0.28 | 0.54 | 0.87 | 2.99 |
| PM10 (μg·m−3) | 1093 | 17.7 | 7.4 | 3.7 | 12.9 | 16.6 | 21.5 | 44.7 |
| Temperature (°C) | 1353 | 12.1 | 2.5 | 4.9 | 10.5 | 12.2 | 13.7 | NA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirks, K.N.; Scarfe, J.; Talbot, N.P.; Marshall, R.; Salmond, J.A. A Statistical Analysis of the Relationship between Brown Haze and Surface Air Pollution Levels on Respiratory Hospital Admissions in Auckland, New Zealand. Climate 2017, 5, 86. https://doi.org/10.3390/cli5040086
Dirks KN, Scarfe J, Talbot NP, Marshall R, Salmond JA. A Statistical Analysis of the Relationship between Brown Haze and Surface Air Pollution Levels on Respiratory Hospital Admissions in Auckland, New Zealand. Climate. 2017; 5(4):86. https://doi.org/10.3390/cli5040086
Chicago/Turabian StyleDirks, Kim Natasha, James Scarfe, Nicholas Philip Talbot, Roger Marshall, and Jennifer Anne Salmond. 2017. "A Statistical Analysis of the Relationship between Brown Haze and Surface Air Pollution Levels on Respiratory Hospital Admissions in Auckland, New Zealand" Climate 5, no. 4: 86. https://doi.org/10.3390/cli5040086





