The Proteome of the Murine Presynaptic Active Zone
Abstract
:1. Introduction
1.1. Subcellular Fractionation of the Presynaptic Active Zone
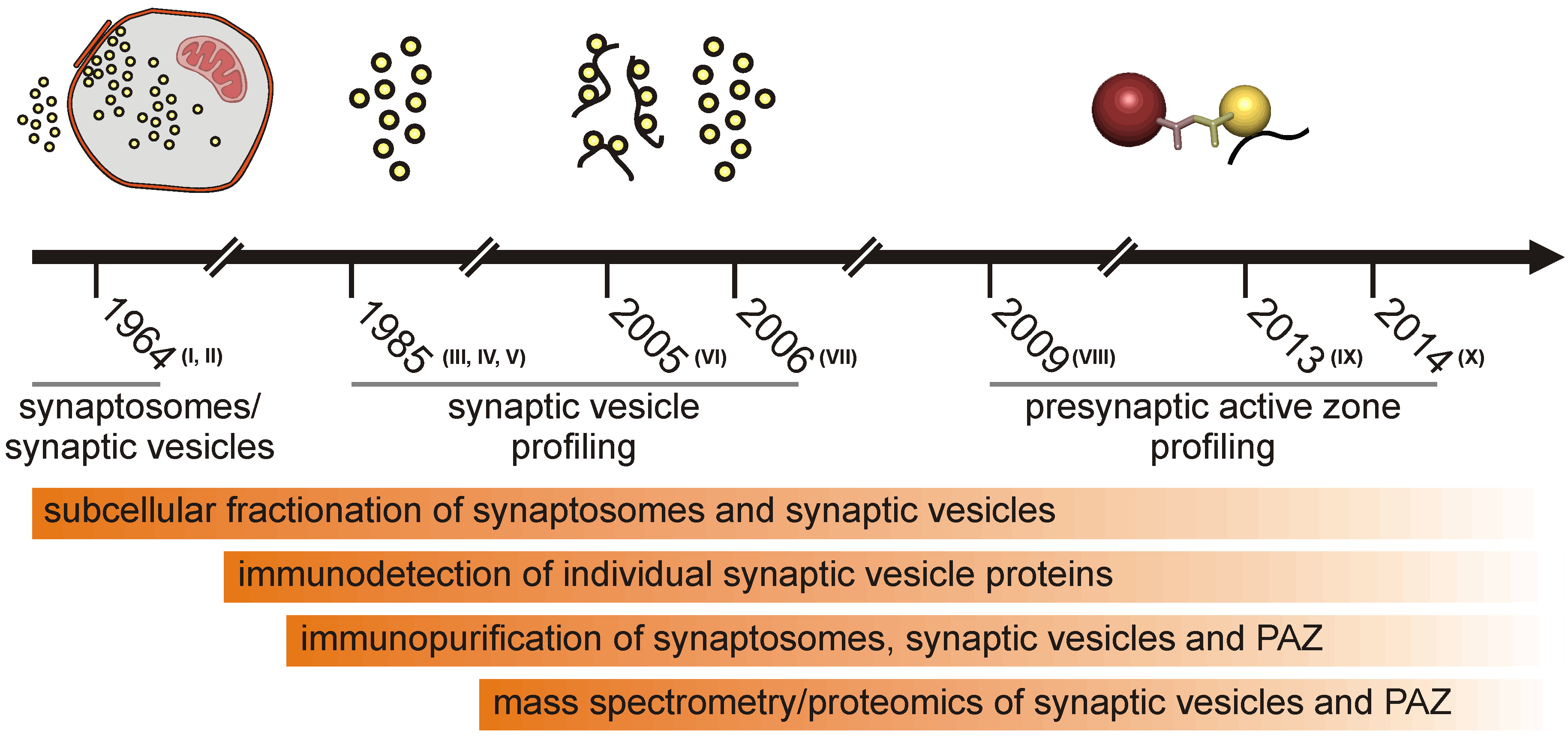
1.2. Proteomic Approaches
1.3. The Active Zone Is a Dynamic Focal Hot Spot
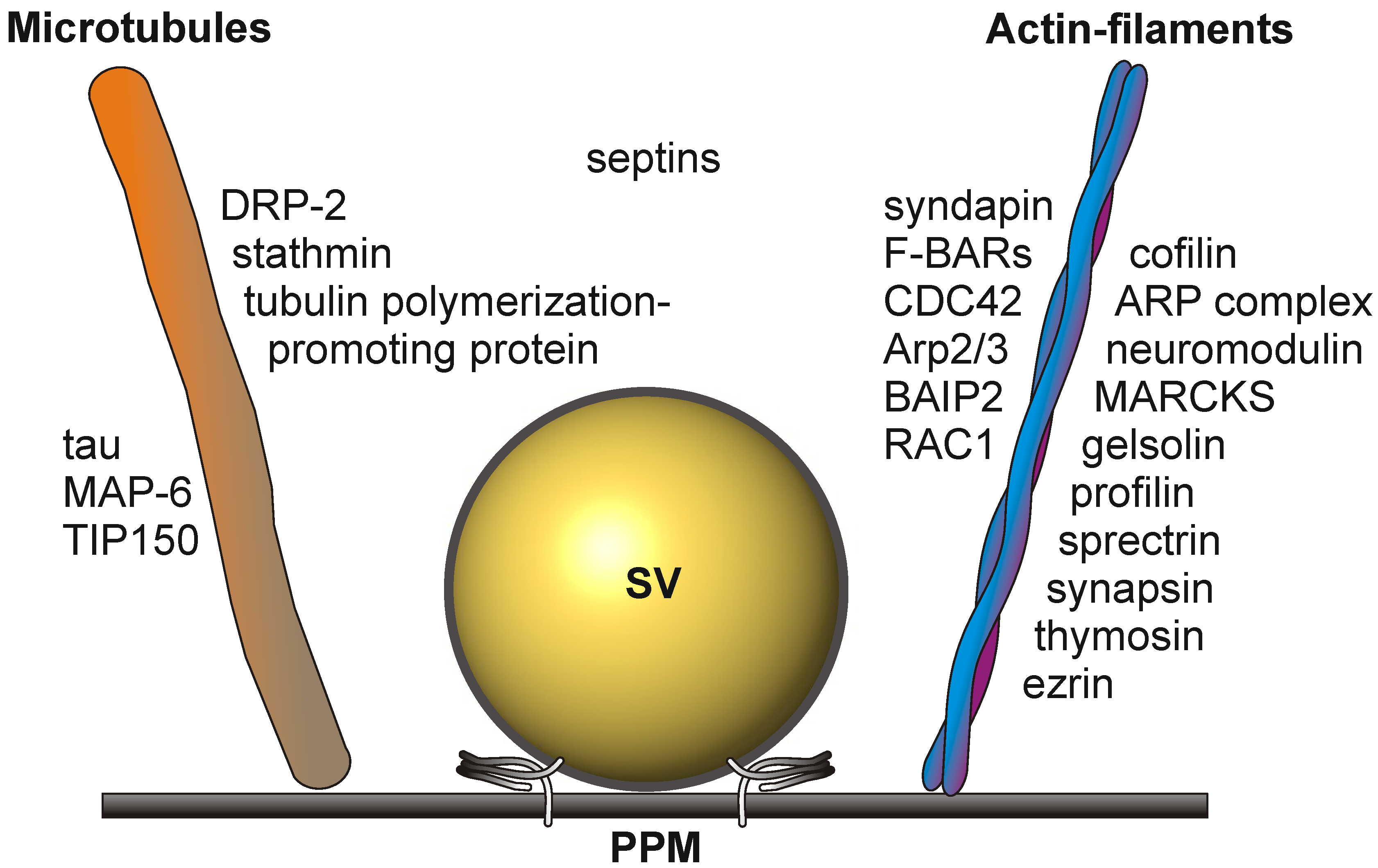
1.4. Signaling Events at the Presynaptic Active Zone
1.5. The Role of Calcium at the Presynaptic Active Zone

2. Additional Protein Constituents of PAZ
3. Conclusions
Abbreviations
| PAZ | presynaptic active zone |
| PPM | presynaptic plasma membrane |
| SV | synaptic vesicle |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morciano, M.; Burre, J.; Corvey, C.; Karas, M.; Zimmermann, H.; Volknandt, W. Immunoisolation of two synaptic vesicle pools from synaptosomes: A proteomics analysis. J. Neurochem. 2005, 95, 1732–1745. [Google Scholar] [CrossRef]
- Morciano, M.; Beckhaus, T.; Karas, M.; Zimmermann, H.; Volknandt, W. The proteome of the presynaptic active zone: From docked synaptic vesicles to adhesion molecules and maxi-channels. J. Neurochem. 2009, 108, 662–675. [Google Scholar] [CrossRef]
- Boyken, J.; Gronborg, M.; Riedel, D.; Urlaub, H.; Jahn, R.; Chua, J.J. Molecular profiling of synaptic vesicle docking sites reveals novel proteins but few differences between glutamatergic and gabaergic synapses. Neuron 2013, 78, 285–297. [Google Scholar] [CrossRef]
- Weingarten, J.; Lassek, M.; Mueller, B.F.; Rohmer, M.; Lunger, I.; Baeumlisberger, D.; Dudek, S.; Gogesch, P.; Karas, M.; Volknandt, W. The proteome of the presynaptic active zone from mouse brain. Mol. Cell. Neurosci. 2014, 59C, 106–118. [Google Scholar]
- Whittaker, V.P.; Michaelson, I.A.; Kirkland, R.J. The separation of synaptic vesicles from nerve-ending particles (synaptosomes). Biochem. J. 1964, 90, 293–303. [Google Scholar]
- De Robertis, E.; Rodriguez de Lores Arnaiz, G.; Pellegrino de Iraldi, A. Isolation of synaptic vesicles from nerve endings of the rat brain. Nature 1962, 194, 794–795. [Google Scholar] [CrossRef]
- Gray, E.G.; Whittaker, V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962, 96, 79–88. [Google Scholar]
- Clarke, G.L.; Chen, J.; Nishimune, H. Presynaptic active zone density during development and synaptic plasticity. Front. Mol. Neurosci. 2012, 5, e12. [Google Scholar]
- Maas, C.; Torres, V.I.; Altrock, W.D.; Leal-Ortiz, S.; Wagh, D.; Terry-Lorenzo, R.T.; Fejtova, A.; Gundelfinger, E.D.; Ziv, N.E.; Garner, C.C. Formation of golgi-derived active zone precursor vesicles. J. Neurosci. 2012, 32, 11095–11108. [Google Scholar] [CrossRef]
- Pitsch, J.; Opitz, T.; Borm, V.; Woitecki, A.; Staniek, M.; Beck, H.; Becker, A.J.; Schoch, S. The presynaptic active zone protein rim1alpha controls epileptogenesis following status epilepticus. J. Neurosci. 2012, 32, 12384–12395. [Google Scholar] [CrossRef]
- Sudhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef]
- Volknandt, W.; Karas, M. Proteomic analysis of the presynaptic active zone. Exp. Brain Res. 2012, 217, 449–461. [Google Scholar] [CrossRef]
- Ohtsuka, T. Cast: Functional scaffold for the integrity of the presynaptic active zone. Neurosci. Res. 2013, 76, 10–15. [Google Scholar] [CrossRef]
- Bai, F.; Witzmann, F.A. Synaptosome proteomics. Sub-Cell. Biochem. 2007, 43, 77–98. [Google Scholar] [CrossRef]
- Schrimpf, S.P.; Meskenaite, V.; Brunner, E.; Rutishauser, D.; Walther, P.; Eng, J.; Aebersold, R.; Sonderegger, P. Proteomic analysis of synaptosomes using isotope-coded affinity tags and mass spectrometry. Proteomics 2005, 5, 2531–2541. [Google Scholar] [CrossRef]
- Filiou, M.D.; Bisle, B.; Reckow, S.; Teplytska, L.; Maccarrone, G.; Turck, C.W. Profiling of mouse synaptosome proteome and phosphoproteome by IEF. Electrophoresis 2010, 31, 1294–1301. [Google Scholar] [CrossRef]
- Huttner, W.B.; Schiebler, W.; Greengard, P.; de Camilli, P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 1983, 96, 1374–1388. [Google Scholar] [CrossRef]
- Burre, J.; Volknandt, W. The synaptic vesicle proteome. J. Neurochem. 2007, 101, 1448–1462. [Google Scholar] [CrossRef]
- Sudhof, T.C.; Rizo, J. Synaptic vesicle exocytosis. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–14. [Google Scholar]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A rapid percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef]
- Booth, R.F.; Clark, J.B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem. J. 1978, 176, 365–370. [Google Scholar]
- Bajjalieh, S.M.; Frantz, G.D.; Weimann, J.M.; McConnell, S.K.; Scheller, R.H. Differential expression of synaptic vesicle protein 2 (sv2) isoforms. J. Neurosci. 1994, 14, 5223–5235. [Google Scholar]
- Burre, J.; Beckhaus, T.; Schagger, H.; Corvey, C.; Hofmann, S.; Karas, M.; Zimmermann, H.; Volknandt, W. Analysis of the synaptic vesicle proteome using three gel-based protein separation techniques. Proteomics 2006, 6, 6250–6262. [Google Scholar] [CrossRef]
- Yao, J.; Nowack, A.; Kensel-Hammes, P.; Gardner, R.G.; Bajjalieh, S.M. Cotrafficking of sv2 and synaptotagmin at the synapse. J. Neurosci. 2010, 30, 5569–5578. [Google Scholar]
- Gronborg, M.; Pavlos, N.J.; Brunk, I.; Chua, J.J.; Munster-Wandowski, A.; Riedel, D.; Ahnert-Hilger, G.; Urlaub, H.; Jahn, R. Quantitative comparison of glutamatergic and gabaergic synaptic vesicles unveils selectivity for few proteins including mal2, a novel synaptic vesicle protein. J. Neurosci. 2010, 30, 2–12. [Google Scholar] [CrossRef]
- Lassek, M.; Weingarten, J.; Einsfelder, U.; Brendel, P.; Muller, U.; Volknandt, W. Amyloid precursor proteins are constituents of the presynaptic active zone. J. Neurochem. 2013, 127, 48–56. [Google Scholar]
- De Robertis, E.; Rodriguez de Lores Arnaiz, G.; Salganicoff, L.; Pellegrino de Iraldi, A.; Zieher, L.M. Isolation of synaptic vesicles and structural organization of the acetycholine system within brain nerve endings. J. Neurochem. 1963, 10, 225–235. [Google Scholar] [CrossRef]
- Ueda, T.; Greengard, P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J. Biol. Chem. 1977, 252, 5155–5163. [Google Scholar]
- Buckley, K.; Kelly, R.B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 1985, 100, 1284–1294. [Google Scholar] [CrossRef]
- Jahn, R.; Schiebler, W.; Ouimet, C.; Greengard, P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc. Natl. Acad. Sci. USA 1985, 82, 4137–4141. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Gronborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brugger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- Burre, J.; Beckhaus, T.; Corvey, C.; Karas, M.; Zimmermann, H.; Volknandt, W. Synaptic vesicle proteins under conditions of rest and activation: Analysis by 2-D difference gel electrophoresis. Electrophoresis 2006, 27, 3488–3496. [Google Scholar] [CrossRef]
- Barth, J.; Volknandt, W. Proteomic investigations of the synaptic vesicle interactome. Expert Rev. Proteomics 2011, 8, 211–220. [Google Scholar] [CrossRef]
- Ramos-Ortolaza, D.L.; Bushlin, I.; Abul-Husn, N.; Annangudi, S.P.; Sweedler, J.; Devi, L.A. Quantitative neuroproteomics of the synapse. Methods Mol. Biol. 2010, 615, 227–246. [Google Scholar]
- Tannu, N.S.; Hemby, S.E. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat. Protoc. 2006, 1, 1732–1742. [Google Scholar] [CrossRef]
- Van Montfort, B.A.; Canas, B.; Duurkens, R.; Godovac-Zimmermann, J.; Robillard, G.T. Improved in-gel approaches to generate peptide maps of integral membrane proteins with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Mass Spectrom. 2002, 37, 322–330. [Google Scholar] [CrossRef]
- Rietschel, B.; Arrey, T.N.; Meyer, B.; Bornemann, S.; Schuerken, M.; Karas, M.; Poetsch, A. Elastase digests: New ammunition for shotgun membrane proteomics. Mol. Cell. Proteomics 2009, 8, 1029–1043. [Google Scholar] [CrossRef]
- Rietschel, B.; Bornemann, S.; Arrey, T.N.; Baeumlisberger, D.; Karas, M.; Meyer, B. Membrane protein analysis using an improved peptic in-solution digestion protocol. Proteomics 2009, 9, 5553–5557. [Google Scholar] [CrossRef]
- Schoch, S.; Gundelfinger, E.D. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006, 326, 379–391. [Google Scholar] [CrossRef]
- Cingolani, L.A.; Goda, Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 2008, 9, 344–356. [Google Scholar] [CrossRef]
- Dent, E.W.; Kalil, K. Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 2001, 21, 9757–9769. [Google Scholar]
- Nelson, J.C.; Stavoe, A.K.; Colon-Ramos, D.A. The actin cytoskeleton in presynaptic assembly. Cell Adh. Migr. 2013, 7, 379–387. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, J.; Liu, J.; Ward, T.; Wordeman, L.; Davidson, A.; Wang, F.; Yao, X. Tip150 interacts with and targets mcak at the microtubule plus ends. EMBO Rep. 2009, 10, 857–865. [Google Scholar] [CrossRef]
- Marsick, B.M.; San Miguel-Ruiz, J.E.; Letourneau, P.C. Activation of ezrin/radixin/moesin mediates attractive growth cone guidance through regulation of growth cone actin and adhesion receptors. J. Neurosci. 2012, 32, 282–296. [Google Scholar] [CrossRef]
- Goode, B.L.; Drubin, D.G.; Barnes, G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 2000, 12, 63–71. [Google Scholar] [CrossRef]
- Oda, K.; Shiratsuchi, T.; Nishimori, H.; Inazawa, J.; Yoshikawa, H.; Taketani, Y.; Nakamura, Y.; Tokino, T. Identification of baiap2 (bai-associated protein 2), a novel human homologue of hamster irsp53, whose sh3 domain interacts with the cytoplasmic domain of bai1. Cytogenetics Cell Genet. 1999, 84, 75–82. [Google Scholar] [CrossRef]
- Tsang, C.W.; Estey, M.P.; DiCiccio, J.E.; Xie, H.; Patterson, D.; Trimble, W.S. Characterization of presynaptic septin complexes in mammalian hippocampal neurons. Biol. Chem. 2011, 392, 739–749. [Google Scholar]
- Franzen, B.; Yang, Y.; Sunnemark, D.; Wickman, M.; Ottervald, J.; Oppermann, M.; Sandberg, K. Dihydropyrimidinase related protein-2 as a biomarker for temperature and time dependent post mortem changes in the mouse brain proteome. Proteomics 2003, 3, 1920–1929. [Google Scholar] [CrossRef]
- Castegna, A.; Aksenov, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of oxidatively modified proteins in alzheimer's disease brain. Part II: Dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J. Neurochem. 2002, 82, 1524–1532. [Google Scholar] [CrossRef]
- Fukata, Y.; Itoh, T.J.; Kimura, T.; Menager, C.; Nishimura, T.; Shiromizu, T.; Watanabe, H.; Inagaki, N.; Iwamatsu, A.; Hotani, H.; et al. Crmp-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 2002, 4, 583–591. [Google Scholar]
- Dharmalingam, E.; Haeckel, A.; Pinyol, R.; Schwintzer, L.; Koch, D.; Kessels, M.M.; Qualmann, B. F-bar proteins of the syndapin family shape the plasma membrane and are crucial for neuromorphogenesis. J. Neurosci. 2009, 29, 13315–13327. [Google Scholar] [CrossRef]
- Koch, D.; Spiwoks-Becker, I.; Sabanov, V.; Sinning, A.; Dugladze, T.; Stellmacher, A.; Ahuja, R.; Grimm, J.; Schuler, S.; Muller, A.; et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. 2011, 30, 4955–4969. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Free, R.B.; Cabrera, D.M.; Skinbjerg, M.; Sibley, D.R. Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase. J. Biol. Chem. 2008, 283, 36441–36453. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Bolshakov, A.E.; Abushik, P.A.; Krivoi, II; Antonov, S.M. Na+,K+-ATPase functionally interacts with the plasma membrane Na+,Ca2+ exchanger to prevent Ca2+ overload and neuronal apoptosis in excitotoxic stress. J. Pharmacol. Exp. Ther 2012, 343, 596–607. [Google Scholar] [CrossRef]
- Tidow, H.; Aperia, A.; Nissen, P. How are ion pumps and agrin signaling integrated? Trends Biochem. Sci. 2010, 35, 653–659. [Google Scholar] [CrossRef]
- Xie, Z.; Askari, A. Na+/K+-ATPase as a signal transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [Google Scholar] [CrossRef]
- Mohammadi, K.; Kometiani, P.; Xie, Z.; Askari, A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J. Biol. Chem. 2001, 276, 42050–42056. [Google Scholar]
- Desfrere, L.; Karlsson, M.; Hiyoshi, H.; Malmersjo, S.; Nanou, E.; Estrada, M.; Miyakawa, A.; Lagercrantz, H.; El Manira, A.; Lal, M.; et al. Na,K-ATPase signal transduction triggers creb activation and dendritic growth. Proc. Natl. Acad. Sci. USA 2009, 106, 2212–2217. [Google Scholar] [CrossRef]
- Kizhatil, K.; Sandhu, N.K.; Peachey, N.S.; Bennett, V. Ankyrin-B is required for coordinated expression of beta-2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Exp. Eye Res. 2009, 88, 57–64. [Google Scholar] [CrossRef]
- Koticha, D.; Babiarz, J.; Kane-Goldsmith, N.; Jacob, J.; Raju, K.; Grumet, M. Cell adhesion and neurite outgrowth are promoted by neurofascin NF155 and inhibited by NF186. Mol. Cell. Neurosci. 2005, 30, 137–148. [Google Scholar] [CrossRef]
- Seki, M.; Nawa, H.; Morioka, T.; Fukuchi, T.; Oite, T.; Abe, H.; Takei, N. Establishment of a novel enzyme-linked immunosorbent assay for thy-1; quantitative assessment of neuronal degeneration. Neurosci. Lett. 2002, 329, 185–188. [Google Scholar] [CrossRef]
- Jeng, C.J.; McCarroll, S.A.; Martin, T.F.; Floor, E.; Adams, J.; Krantz, D.; Butz, S.; Edwards, R.; Schweitzer, E.S. Thy-1 is a component common to multiple populations of synaptic vesicles. J. Cell Biol. 1998, 140, 685–698. [Google Scholar] [CrossRef]
- Almqvist, P.; Carlsson, S.R.; Hardy, J.A.; Winblad, B. Regional and subcellular distribution of thy-1 in human brain assayed by a solid-phase radioimmunoassay. J. Neurochem. 1986, 46, 681–685. [Google Scholar] [CrossRef]
- Paoloni-Giacobino, A.; Chen, H.; Antonarakis, S.E. Cloning of a novel human neural cell adhesion molecule gene (ncam2) that maps to chromosome region 21q21 and is potentially involved in down syndrome. Genomics 1997, 43, 43–51. [Google Scholar] [CrossRef]
- Korshunova, I.; Caroni, P.; Kolkova, K.; Berezin, V.; Bock, E.; Walmod, P.S. Characterization of basp1-mediated neurite outgrowth. J. Neurosci. Res. 2008, 86, 2201–2213. [Google Scholar] [CrossRef]
- Frey, D.; Laux, T.; Xu, L.; Schneider, C.; Caroni, P. Shared and unique roles of cap23 and gap43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 2000, 149, 1443–1454. [Google Scholar] [CrossRef]
- Falk, J.; Bonnon, C.; Girault, J.A.; Faivre-Sarrailh, C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol. Cell 2002, 94, 327–334. [Google Scholar] [CrossRef]
- Smalla, K.H.; Matthies, H.; Langnase, K.; Shabir, S.; Bockers, T.M.; Wyneken, U.; Staak, S.; Krug, M.; Beesley, P.W.; Gundelfinger, E.D. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal ca1 synapses. Proc. Natl. Acad. Sci. USA 2000, 97, 4327–4332. [Google Scholar] [CrossRef]
- Ray, A.; Treloar, H.B. Igsf8: A developmentally and functionally regulated cell adhesion molecule in olfactory sensory neuron axons and synapses. Mol. Cell. Neurosci. 2012, 50, 238–249. [Google Scholar] [CrossRef]
- Takeuchi, T.; Misaki, A.; Liang, S.B.; Tachibana, A.; Hayashi, N.; Sonobe, H.; Ohtsuki, Y. Expression of T-cadherin (CDH13, h-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J. Neurochem. 2000, 74, 1489–1497. [Google Scholar]
- Miyashita, M.; Ohnishi, H.; Okazawa, H.; Tomonaga, H.; Hayashi, A.; Fujimoto, T.T.; Furuya, N.; Matozaki, T. Promotion of neurite and filopodium formation by CD47: Roles of integrins, Rac, and Cdc42. Mol. Biol. Cell 2004, 15, 3950–3963. [Google Scholar] [CrossRef]
- Gil, O.D.; Zanazzi, G.; Struyk, A.F.; Salzer, J.L. Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J. Neurosci. 1998, 18, 9312–9325. [Google Scholar]
- Fogel, A.I.; Akins, M.R.; Krupp, A.J.; Stagi, M.; Stein, V.; Biederer, T. Syncams organize synapses through heterophilic adhesion. J. Neurosci. 2007, 27, 12516–12530. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Juhaszova, M.; Golovina, V.A.; Church, P.J.; Stanley, E.F. Na/Ca exchanger and PMCA localization in neurons and astrocytes: Functional implications. Ann. N.Y. Acad. Sci. 2002, 976, 356–366. [Google Scholar]
- Houben, M.P.; Lankhorst, A.J.; van Dalen, J.J.; Veldman, H.; Joosten, E.A.; Hamers, F.P.; Gispen, W.H.; Schrama, L.H. Pre- and postsynaptic localization of rc3/neurogranin in the adult rat spinal cord: An immunohistochemical study. J. Neurosci. Res. 2000, 59, 750–759. [Google Scholar] [CrossRef]
- Clifton, J.G.; Brown, M.K.; Huang, F.; Li, X.; Reutter, W.; Hofmann, W.; Hixson, D.C.; Josic, D. Identification of members of the annexin family in the detergent-insoluble fraction of rat morris hepatoma plasma membranes. J. Chromatogr. A 2006, 1123, 205–211. [Google Scholar] [CrossRef]
- Hosoya, H.; Kobayashi, R.; Tsukita, S.; Matsumura, F. Ca(2+)-regulated actin and phospholipid binding protein (68 kD-protein) from bovine liver: Identification as a homologue for annexin VI and intracellular localization. Cell Motil. Cytoskeleton 1992, 22, 200–210. [Google Scholar] [CrossRef]
- Tanaka, T.; Kadowaki, K.; Lazarides, E.; Sobue, K. Ca2+-dependent regulation of the spectrin/actin interaction by calmodulin and protein 4.1. J. Biol. Chem. 1991, 266, 1134–1140. [Google Scholar]
- Miller, K.E.; Sheetz, M.P. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004, 117, 2791–2804. [Google Scholar] [CrossRef]
- Ly, C.V.; Verstreken, P. Mitochondria at the synapse. Neuroscientist 2006, 12, 291–299. [Google Scholar] [CrossRef]
- Werth, J.L.; Thayer, S.A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J. Neurosci. 1994, 14, 348–356. [Google Scholar]
- Billups, B.; Forsythe, I.D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 2002, 22, 5840–5847. [Google Scholar]
- Levy, M.; Faas, G.C.; Saggau, P.; Craigen, W.J.; Sweatt, J.D. Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 2003, 278, 17727–17734. [Google Scholar]
- Tang, Y.; Zucker, R.S. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 1997, 18, 483–491. [Google Scholar] [CrossRef]
- Yang, F.; He, X.P.; Russell, J.; Lu, B. Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na+-Ca2+ exchanger and protein kinase C. J. Cell Biol. 2003, 163, 511–523. [Google Scholar] [CrossRef]
- Rowland, K.C.; Irby, N.K.; Spirou, G.A. Specialized synapse-associated structures within the calyx of held. J. Neurosci. 2000, 20, 9135–9144. [Google Scholar]
- Perkins, G.A.; Tjong, J.; Brown, J.M.; Poquiz, P.H.; Scott, R.T.; Kolson, D.R.; Ellisman, M.H.; Spirou, G.A. The micro-architecture of mitochondria at active zones: Electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J. Neurosci. 2010, 30, 1015–1026. [Google Scholar] [CrossRef]
- Nautiyal, M.; Sweatt, A.J.; MacKenzie, J.A.; Mark Payne, R.; Szucs, S.; Matalon, R.; Wallin, R.; Hutson, S.M. Neuronal localization of the mitochondrial protein nipsnap1 in rat nervous system. Eur. J. Neurosci. 2010, 32, 560–569. [Google Scholar] [CrossRef]
- Elinder, F.; Akanda, N.; Tofighi, R.; Shimizu, S.; Tsujimoto, Y.; Orrenius, S.; Ceccatelli, S. Opening of plasma membrane voltage-dependent anion channels (VDAC) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell Death Differ. 2005, 12, 1134–1140. [Google Scholar] [CrossRef]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef]
- Marin, R.; Ramirez, C.M.; Gonzalez, M.; Gonzalez-Munoz, E.; Zorzano, A.; Camps, M.; Alonso, R.; Diaz, M. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons. Mol. Membr. Biol. 2007, 24, 148–160. [Google Scholar] [CrossRef]
- Phillips, G.R.; Huang, J.K.; Wang, Y.; Tanaka, H.; Shapiro, L.; Zhang, W.; Shan, W.S.; Arndt, K.; Frank, M.; Gordon, R.E.; et al. The presynaptic particle web: Ultrastructure, composition, dissolution, and reconstitution. Neuron 2001, 32, 63–77. [Google Scholar] [CrossRef]
- Soltys, B.J.; Gupta, R.S. Mitochondrial-matrix proteins at unexpected locations: Are they exported? Trends Biochem. Sci. 1999, 24, 174–177. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Israelson, A.; Brdiczka, D.; Sheu, S.S. The voltage-dependent anion channel (VDAC): Function in intracellular signalling, cell life and cell death. Curr. Pharm. Des. 2006, 12, 2249–2270. [Google Scholar] [CrossRef]
- Gundelfinger, E.D.; Kessels, M.M.; Qualmann, B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 2003, 4, 127–139. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Laßek, M.; Weingarten, J.; Volknandt, W. The Proteome of the Murine Presynaptic Active Zone. Proteomes 2014, 2, 243-257. https://doi.org/10.3390/proteomes2020243
Laßek M, Weingarten J, Volknandt W. The Proteome of the Murine Presynaptic Active Zone. Proteomes. 2014; 2(2):243-257. https://doi.org/10.3390/proteomes2020243
Chicago/Turabian StyleLaßek, Melanie, Jens Weingarten, and Walter Volknandt. 2014. "The Proteome of the Murine Presynaptic Active Zone" Proteomes 2, no. 2: 243-257. https://doi.org/10.3390/proteomes2020243



