Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update
Abstract
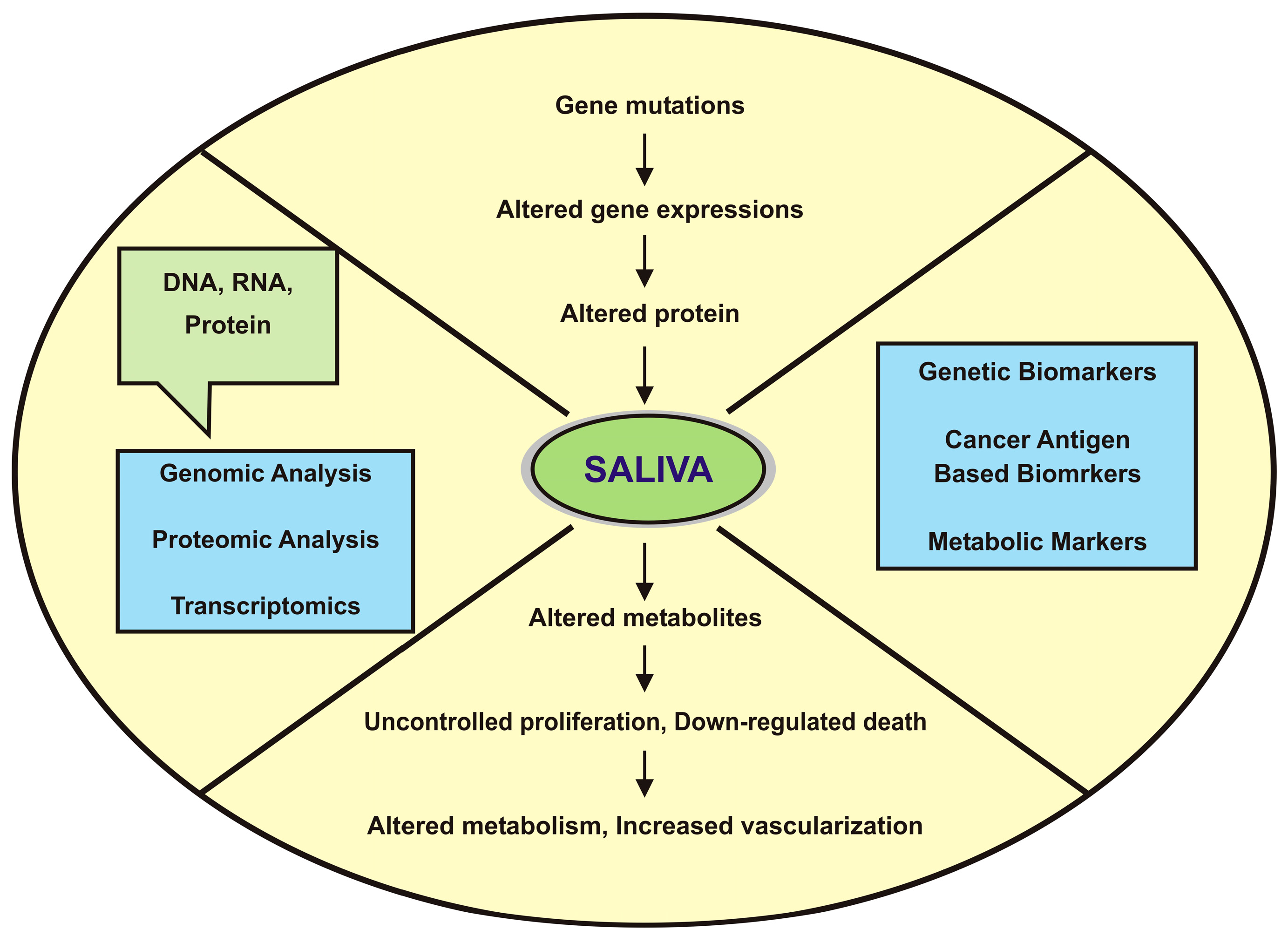
:1. Introduction
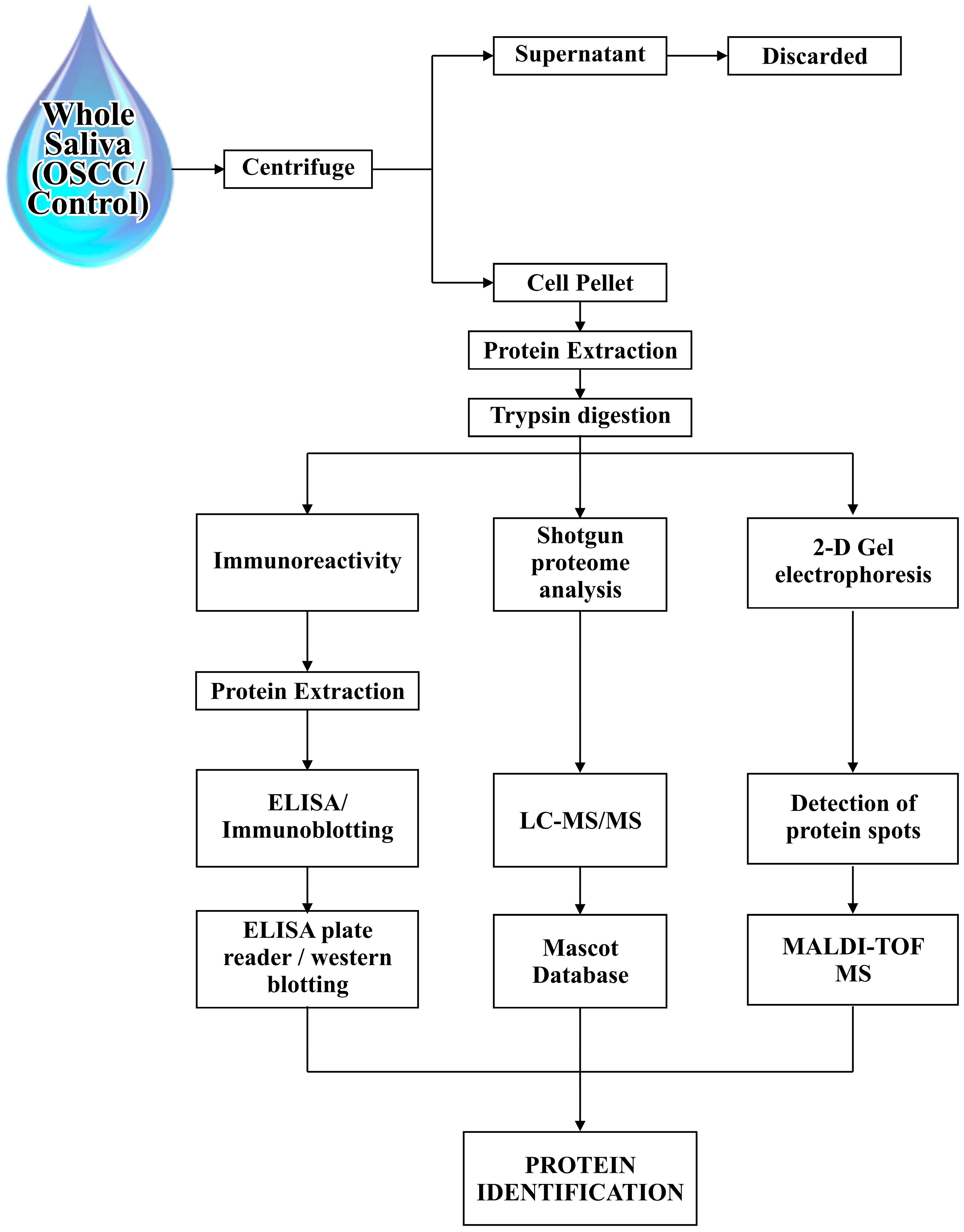
2. Proteomic Tools and Saliva Sampling
3. Potential Salivary Biomarkers for Oral Cancer Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feller, L.; Lemmer, J. Oral squamous cell carcinoma: Epidemiology, clinical presentation and treatment. J. Cancer Ther. 2012, 3, 263–268. [Google Scholar] [CrossRef]
- Granato, D.C.; Zanetti, M.R.; Kawahara, R.; Yokoo, S.; Domingues, R.R.; Aragão, A.Z.; Agostini, M.; Carazzolle, M.F.; Vidal, R.O.; Flores, I.L.; et al. Integrated proteomics identified up-regulated focal adhesion-mediated proteins in human squamous cell carcinoma in an orthotopic murine model. PLoS ONE 2014, 9, e98208. [Google Scholar] [CrossRef] [PubMed]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Yi, C.; Chung, H.-R.; Wang, D.-J.; Chang, W.-C.; Lee, S.-Y.; Lin, C.-T.; Yang, Y.-C.; Yang, W.-C.V. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. 2010, 46, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Langerman, A.; Zhang, Y.; Khalid, O.; Hu, S.; Cao, C.-X.; Lingen, M.W.; Wong, D.T.W. Quantitative proteomic analysis of microdissected oral epithelium for cancer biomarker discovery. Oral Oncol. 2015, 51, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Lihong, H.; Linlin, G.; Yiping, G.; Yang, S.; Xiaoyu, Q.; Zhuzhu, G.; Xiaohan, Y.; Xin, Z.; Liyan, X.; Shujuan, S. Proteomics approaches for identification of tumor relevant protein targets in pulmonary squamous cell carcinoma by 2D-DIGE-MS. PLoS ONE 2014, 9, e95121. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Hua, C.H.; Lin, C.-D.; Lai, C.H.; Huang, S.H.; Tsai, M.H.; Kao, J.Y.; Lin, C.W. S100A8 as potential salivary biomarker of oral squamous cell carcinoma using nanoLC-MS/MS. Clin. Chim. Acta 2014, 436, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.; Rehman, R.; Rehman, I. Advances of Proteomic Sciences in Dentistry. Int. J. Mol. Sci. 2016, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Chiappin, S.; Antonelli, G.; Gatti, R.; de Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.-D.H.-D.; Cvikl, B.B.; Lussi, A.A.; Gruber, R.R. Salivary pellets induce a pro-inflammatory response involving the TLR4–NF-kB pathway in gingival fibroblasts. BMC Oral Health 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Onsongo, G.; Popko, J.; de Jong, E.P.; Cao, J.; Carlis, J.V.; Griffin, R.J.; Rhodus, N.L.; Griffin, T.J. Proteomics analysis of cells in whole saliva from oral cancer patients via value-added three-dimensional peptide fractionation and tandem mass spectrometry. Mol. Cell. Proteom. 2007, 7, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, K.; Rosenthal, D.I.; Koomen, J.M.; Streckfus, C.F.; Chambers, M.; Kobayashi, R.; El-Naggar, A.K. Pre-analytic saliva processing affect proteomic results and biomarker screening of head and neck squamous carcinomta. Int. J. Oncol. 2007, 30, 743–749. [Google Scholar] [PubMed]
- Liu, J.; Duan, Y. Saliva: A potential media for disease diagnostics and monitoring. Oral Oncol. 2012, 48, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Bhate, A.V.; Gangadaran, P.; Sawant, S.S.; Salot, S.; Sehgal, L.; Dange, P.P.; Chaukar, D.A.; D’cruz, A.K.; Kannanl, S.; et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Orrù, S.; Pagnozzi, D.; Pucci, P. Interaction proteomics. Biosci. Rep. 2005, 25, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Apweiler, R.; Balgley, B.M.; Boontheung, P.; Bundy, J.L.; Cargile, B.J.; Cole, S.; Fang, X.; Gonzalez-Begne, M.; Griffin, T.J.; et al. Systematic comparison of the human saliva and plasma proteomes. Proteom. Clin. Appl. 2009, 3, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.J.; Reategui, E.P.; Pedroso, F.; Pernas, F.G.; Karakullukcu, B.M.; Carraway, K.L.; Hamilton, K.; Singal, R.; Goodwin, W.J. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.; Bahar, G.; Shpitzer, T.; Feinmesser, R. Concomitant analysis of salivary tumor markers—A new diagnostic tool for oral cancer. Clin. Cancer Res. 2006, 12, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; Wormald, R.; Meleady, P.; Henry, M.; Curran, A.; Clynes, M. Analysis of the saliva proteome from patients with head and neck squamous cell carcinoma reveals differences in abundance levels of proteins associated with tumour progression and metastasis. J. Proteom. 2008, 71, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Pickering, V.; Jordan, R.C.K.; Schmidt, B.L. Elevated salivary endothelin levels in oral cancer patients—A pilot study. Oral Oncol. 2007, 43, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Principe, S.; Hui, A.B.-Y.; Bruce, J.; Sinha, A.; Liu, F.-F.; Kislinger, T. Tumor-derived exosomes and microvesicles in head and neck cancer: implications for tumor biology and biomarker discovery. Proteomics 2013, 13, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Ciavarella, D.; Santarelli, A.; Ranieri, E.; Colella, G.; Muzio, L.L.; Russo, L.L. Potential salivary proteomic markers of oral squamous cell carcinoma. Cancer Genom. Proteom. 2016, 13, 55–62. [Google Scholar]
- Almadori, G.; Bussu, F.; Galli, J.; Limongelli, A.; Persichilli, S.; Zappacosta, B.; Minucci, A.; Paludetti, G.; Giardina, B. Salivary glutathione and uric acid levels in patients with head and neck squamous cell carcinoma. Head Neck 2007, 29, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Katakura, A.; Kamiyama, I.; Takano, N.; Shibahara, T.; Muramatsu, T.; Ishihara, K.; Takagi, R.; Shouno, T. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull. Tokyo Dent. Coll. 2007, 48, 199–203. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.A.; Taylor, J.M.G.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008, 113, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Chiba, I.; Ishikawa, M.; Satoh, C.; Notani, K.I.; Ohiro, Y.; Totsuka, Y.; Mizuno, S.; Kitagawa, Y. Serum p53 antibodies as a prognostic indicator in oral squamous cell carcinoma. Odontology 2008, 96, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nemes, J.A.; Deli, L.; Nemes, Z.; Márton, I.J. Expression of p16INK4A, p53, and Rb proteins are independent from the presence of human papillomavirus genes in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 344–352. [Google Scholar]
- Kumar, M.; Srivastava, G.; Kaur, J.; Assi, J.; Alyass, A.; Leong, I.; MacMillan, C.; Witterick, I.; Shukla, N.K.; Thakar, A.; et al. Prognostic significance of cytoplasmic S100A2 overexpression in oral cancer patients. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.L.; Rees, T.; Jordan, L.; Oxford, L.; O’Brien, J.; Chen, H.S.; Wong, D. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 2011, 47, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, Y.S.; Lin, C.C.; Yang, Y.H.; Ho, Y.P.; Tsai, C.C. Elevated serum levels of a c-erbB-2 oncogene product in oral squamous cell carcinoma patients. J. Oral Pathol. Med. 2004, 33, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Metgud, R.; Patel, S. Serum and salivary levels of albumin as diagnostic tools for oral pre-malignancy and oral malignancy. Biotech. Histochem. 2013, 89, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-P.; Chen, G.-F.; Xu, Z.-F.; Zhang, X.; Ping, F.-Y.; Zhao, S.-F. Detection of telomerase activity in saliva from oral squamous cell carcinoma patients. Int. J. Oral Maxillofac. Surg. 2005, 34, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-P.; Zhou, X.-J.; Wei, K.-J.; Yang, X.; Ma, C.-Y.; Zhang, C.-P.; Zhang, Z.-Y. Application of serum tumor markers and support vector machine in the diagnosis of oral squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 2008, 17, 457–460. [Google Scholar] [PubMed]
- Sawant, S.S.; Zingde, S.M.; Vaidya, M.M. Cytokeratin fragments in the serum: Their utility for the management of oral cancer. Oral Oncol. 2008, 44, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Chen, C.H.; Kao, J.Y.; Chen, S.Y.; Tsai, M.H.; Huang, S.H.; Lin, C.W. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal. Chim. Acta 2010, 681, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.M.; Goodyear, P. Prognostic and predictive protein biomarkers in laryngeal squamous cell carcinoma—A systematic review. Int. J. Otolaryngol. Head Neck Surg. 2015, 4, 180–189. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Jesil, A.M.; Sabu, M.C.; Kuttan, R. A preliminary assessment of the acute and subchronic toxicity profile of phase2: An alpha-amylase inhibitor. Int. J. Toxicol. 2005, 24, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Tang, C.H.; Huang, S.H.; Tsai, M.H.; Chen, S.Y.; Kao, J.Y.; Lin, C.W. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin. Chim. Acta 2011, 412, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Drucker, E.; Thurnher, D. Identification of tumour-related proteins as potential screening markers by proteome analysis—Protein profiles of human saliva as a predictive and prognostic tool. EPMA J. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Hamakawa, H.; Ueyama, Y.; Hatori, M.; Toyoshima, T. Identification of a truncated cystatin SA-I as a saliva biomarker for oral squamous cell carcinoma using the SELDI ProteinChip platform. Int. J. Oral Maxillofac. Surg. 2010, 39, 68–74. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.P.; Xie, H.; Onsongo, G.; Stone, M.D.; Chen, X.-B.; Kooren, J.A.; Refsland, E.W.; Griffin, R.J.; Ondrey, F.G.; Wu, B.; et al. Quantitative proteomics reveals myosin and actin as promising saliva biomarkers for distinguishing pre-malignant and malignant oral lesions. PLoS ONE 2010, 5, e11148. [Google Scholar] [CrossRef] [PubMed]
- Shpitzer, T.; Hamzany, Y.; Bahar, G.; Feinmesser, R.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R.M. Salivary analysis of oral cancer biomarkers. Br. J. Cancer 2009, 101, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Jessie, K.; Jayapalan, J.J.; Ong, K.-C.; Abdul Rahim, Z.H.; Zain, R.M.; Wong, K.-T.; Hashim, O.H. Aberrant proteins in the saliva of patients with oral squamous cell carcinoma. Electrophoresis 2013, 34, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yu, T.; Xie, Y.; Yang, Y.; Li, Y.; Zhou, X.; Tsung, S.; Loo, R.R.; Loo, J.R.; Wong, D.T. Discovery of oral fluid biomarkers for human oral cancer by mass spectrometry. Cancer Genom. Proteom. 2007, 4, 55–64. [Google Scholar]
- Yu, J.-S.; Chen, Y.-T.; Chiang, W.-F.; Hsiao, Y.-C.; Chu, L.J.; See, L.-C.; Wu, C.-S.; Tu, H.-T.; Chen, H.-W.; Chen, C.-C.; et al. Saliva protein biomarkers to detect oral squamous cell carcinoma in a high-risk population in Taiwan. Proc. Natl. Acad. Sci. USA 2016, 113, 11549–11554. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortuño, R.; Martínez-Sánchez, J.M.; Fu, M.; Ballbè, M.; Quirós, N.; Fernández, E.; Pascual, J.A. Assessment of tobacco specific nitrosamines (TSNAs) in oral fluid as biomarkers of cancer risk: A population-based study. Environ. Res. 2016, 151, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Righini, C.A.; de Fraipont, F.; Timsit, J.F.; Faure, C.; Brambilla, E.; Reyt, E.; Favrot, M.C. Tumor-specific methylation in saliva: A promising biomarker for early detection of head and neck cancer recurrence. Clin. Cancer Res. 2007, 13, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rhodus, N.L.; Ondrey, F.G.; Wuertz, B.R.K.; Chen, X.; Zhu, Y.; Griffin, T.J. Quantitative proteomic analysis of oral brush biopsies identifies secretory leukocyte protease inhibitor as a promising, mechanism-based oral cancer biomarker. PLoS ONE 2014, 9, e95389. [Google Scholar] [CrossRef] [PubMed]
| Identified Salivary Biomarkers | Proteomic Tools | Inference | References |
|---|---|---|---|
| Glutathione | HPLC | Epidemiological marker to identify subjects with an increased risk of developing oral squamous cell carcinoma (OSCC) to submit strict follow up and chemoprevention. | [25] |
| Interleukin 1a (IL-1a), Interleukin 1b (IL-1b), Interleukin-6(IL-6), Interleukin-8(IL-8), TNF-a | ELISA | These pro-angiogenic, pro-inflammatory cytokines were found to be elevated in the whole saliva of oral cancer patients and oral pre-cancers as compared to controls, which suggested its utility as surrogate indicators of carcinogenic transformation from oral pre-cancer to oral cancer. | [26,27,28] |
| CD44 | Immunoblot | CD44 is elevated in the majority of head and neck squamous cell carcinoma (HNSCC) and distinguishes cancer from benign diseases with high specificity; these markers will detect HNSCC with very high sensitivity and specificity. | [19] |
| CD59 | Immunoblot | Non-invasive method for the diagnosis of oral cancer. | [15] |
| Immunoglobulin heavy chain constant region gamma (IgG) | LC/MS | Significantly altered in OSCC patients as compared with healthy controls; they are inhibitors of apoptosis. | [21] |
| Mac-2 binding protein (M2BP) | ELISA | Provide a sensitivity of 90% and a specificity of 83% for OSCC detection. | [15] |
| MRP14 | Immunoblotting | MRP14 is a calcium-binding protein that has been implicated in different types of human cancers. Provides a sensitivity of 90% and a specificity of 83% for OSCC detection. | [15] |
| p53 antibodies | ELISA | Presence of p53 autoantibodies in saliva, as well as serum of oral cancer patients demonstrated that its detection in saliva can offer a non-invasive method for the detection of a subset of tumors with p53 aberrations. | [29,30] |
| Profilin | Immunoblot | The data proved that these new targets may lead to a simple clinical tool for the non-invasive diagnosis of oral cancer and suggested that patient-based salivary proteomics is a promising approach to the discovery of biomarkers for oral cancer detection. | [15] |
| S100 calcium binding protein | LC/MS | S100A2, an 11.4 kDa protein, is a member of the S100 family of calcium-binding proteins that have diverse functions, regulating a variety of cellular processes such as differentiation, regeneration, cell growth, and signal transduction in neoplastic cells and is a prognostic marker for oral cancer patients. | [21,31] |
| Endothelin-1 | Quantitative real time RT-PCR | Salivary ET-1is a good biomarker for OSCC development in oral lichen planus (OLP) patients regardless of the degree of OLP disease activity. However, it appeared not to be a good biomarker for detecting recurrence of OSCC in patients in remission. | [22,32] |
| Cofilin-1 | LC/MS | These proteins are involved in tumour progression, metastasis and angiogenesis. | [21] |
| Albumin | MALDI-MS | Serum albumin levels decreased in oral pre-malignancy and oral malignancy cases compared to healthy individuals. Salivary albumin levels increased in oral pre-malignancy and oral malignancy cases compared to healthy individuals, suggesting that albumin may play a role in the early diagnosis and prognosis of oral pre-malignant and oral malignant tissues. | [33,34] |
| Telomerase | PCR and ELISA | Telomerase is required for the maintenance of telomere length during chromosome replication; telomerase activity has been detected in tumor cells. | [35] |
| Tissue polypeptide antigen (TPA), Cyfra 21-1, Cancer antigen 125 (CA-125) | ELISA, TRFIA, Immuno-radiometric assay | Significant increases in salivary concentrations of Cyfra 21-1, CA-125 and tissue polypeptide antigen markers revealed sensitivity, specificity, and it is used as a diagnostic tool, especially when a concurrent analysis for significantly increased markers is done. | [20,36,37] |
| Transferrin | LC/MS | Salivary transferrin levels in patients are strongly correlated with the size and stage of the tumor. | [21,38] |
| Fibrin | LC/MS | Similarly, the use of the fibrin SCC biomarker is limited by its non-specificity, even though it is involved with various carcinogenic processes. | [21,39] |
| α-Amylase | MALDI-MS | α-amylase (57 kDa) dominated the high mass range in the MALDI mass spectra of the saliva from healthy subjects, but the peak was suppressed for patients with oral cancer. SDS-PAGE results show that concentrations of alpha-amylase in patients' saliva were significantly higher than those in healthy subjects. MALDI-MS thus has potential as a possible rapid diagnostic screening tool for oral cancer. | [40] |
| Salivary zinc finger, Protein 510 peptide | MALDI-TOF MS Technology | ZNF510 peptides, as OSCC-related salivary biomarkers via the proteomic approach, proved useful in adjunct diagnosis for early detection rather than as a specific diagnosis marker for progression of OSCC patients. | [41] |
| Keratin 36, cystatin A. | MS-based proteomics | Keratin overexpression in OSCC cells may have important molecular functions as structural constituents of the cytoskeleton as well as implications on cell shape and cell size. A 14 kDa protein detected in pre-treatment saliva from the OSCC patients was identified as a truncated cystatin SA-I, with deletion of three amino acids from the N-terminus, proposing that Protein-Chip analysis may provide a reliable screening test and cystatin SA-I might be a useful tumor biomarker for OSCC. | [42,43] |
| Truncated cystatin SA-I | Anion exchange (Q10), cation-exchange (CM10), reversed phase (H50), and immobilized affinity capture (IMAC3) Protein-Chip array | A 14 kDa protein detected in pre-treatment saliva from the OSCC patients was identified as a truncated cystatin SA-I, with deletion of three amino acids from the N-terminus. Truncated cystatin SA-I is a useful tumor biomarker for OSCC. | [43] |
| Myosin, actin, S100A7, keratin-19 and catalase | iTRAQ labeling and Mass spectrometric analysis, Immunoblot | Actin and myosin are promising salivary biomarkers for distinguishing premalignant and malignant oral lesions. It is highly beneficial and noninvasive, being an effective alternative to serum testing, and it provides the possibility of developing self-, home-testing kits for such markers, further facilitating it as a diagnostic aid. | [44,45] |
| Signal transducer and activator of transcription 3(STAT3), Serpin B3 (SCCA1) | (1) preparative IEF using free flow electrophoresis (FFE), (2) SCX chromatography, and (3) LC on line with ESI-MS/MS | Transcription factor that binds to the interleukin-6-responsive elements, may act as a protease inhibitor to modulate the host immune response against tumor cells. | [12] |
| α-1-antitrypsin (AAT), haptoglobin (HAP) | 2DE and MS | The patients' saliva α1-antitrypsin (AAT) and haptoglobin (HAP) β-chains were resolved into polypeptide spots with increased micro heterogeneity. A strong association of AAT and HAP with OSCC was further supported by immunohistochemical staining of cancer tissues. | [46] |
| Thioredoxin | MALDI–MS and LC-MS/MS | Saliva thioredoxin mRNA level was concordantly up-regulated in OSCC subjects. In addition, thioredoxin was found over-expressed in human cancers such as non-small cell lung, gastric, cervical and hepatocellular carcinomas. | [47] |
| KNG1, ANA2, and HSPA5 | Multiple reaction monitoring-MS | Four-protein panel offers a clinically effective tool for detecting OSCC and monitoring high-risk oral premalignant diseases (OPMDs). | [48] |
| Tobacco specific nitrosamines (TSNAs), N'-nitrosonornicotine (NNN) | A simple method with an alkaline single liquid–liquid extraction with dichloromethane/isopropanol was used for quantification. | Biomarker of cancer risk associated with exposure to tobacco smoke. | [49] |
| AAT and HAP | Two-Dimensional Electrophoresis, Mass spectrometry | Panel of proteins is useful for the prediction of aggressive phenotypes in OSCC; and the distinctive expression of proteins and tumor size parameter shows the aggression of cancer. | [50] |
| Secretory leukocyte peptidase inhibitor, keratin 36, cystatin A. | Mass spectrometry | Non-invasive biomarker of oral cancer progression with potential in preventive treatment. | [51] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update. Proteomes 2016, 4, 41. https://doi.org/10.3390/proteomes4040041
Sannam Khan R, Khurshid Z, Akhbar S, Faraz Moin S. Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update. Proteomes. 2016; 4(4):41. https://doi.org/10.3390/proteomes4040041
Chicago/Turabian StyleSannam Khan, Rabia, Zohaib Khurshid, Shazia Akhbar, and Syed Faraz Moin. 2016. "Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update" Proteomes 4, no. 4: 41. https://doi.org/10.3390/proteomes4040041







