1. Background
Cancer is a disease of unregulated cell growth. Unlike normal adult cells that divide only to replace dead or dying cells, cancer cells continue to grow, forming more abnormal cells. Typically cancerous cells arise when proto-oncogenes undergo a genetic change and become oncogenes, or tumorcoding genes. Cancer cells are often found to contain broken or morphed chromosomes that can cause irregular gene expression. Cancer invades healthy cells to obtain nutrients by parasitising its host organism. According to the National Institute of Health’s National Cancer Institute, 1.6 million new cases of cancer are predicted to be diagnosed in 2017 and over 600,000 people would die from the disease [
1,
2].
Chemotherapy is a common treatment for cancer control. The chemotherapy process adds drugs into a person’s body to kill cancerous cells, prevent the cells from dividing, and prevent the spread of cancer into other parts of the body. The majority of chemotherapy agents do not target cancer cells selectively so they also harm healthy cells. Part of this is due to the drugs dispersal throughout the body and not centralized in the spot of the cancerous cells. The goal of chemotherapy is to kill the cancerous cells with minimal damage to healthy tissue. Chemotherapy agents’ lack of selectivity means that time is a factor when administering therapy. Time will help determine how much damage is done to healthy tissues. While there are many treatments out there for fighting cancer, there is no real cure for this disease. Although chemotherapy is an effective tool in the fight against cancer, the side effects of the compounds are a limiting factor for how much dose can be applied. The rapid cellular division of cancer also means that it can easily become resistant to the chemotherapeutic agents used to fight this disease. This has been argued to be the leading cause of cancer mortality [
3,
4]. If treatment resistance can be overcome, then cancer mortality would decrease. This implication has provided the impetus for further study into either reducing chemotherapy resistance or finding alternative therapies that would be immune to resistance [
5].
Chemotherapy is often coupled with radiation therapy and surgery to improve survival of patients with solid tumors. Fine et al. [
6] showed that coupling chemotherapy with post-operative radiation therapy has advantageous effects on patients with malignant gliomas. Li et al. [
7] verified Fine’s results with a three cohort study that showed coupling radiation and chemotherapy greatly reduces the risk of metastases. Fine concluded that hypoxic tumors are generally resistant to radiation therapy. The goal of radiation therapy is to create free radical oxygen agents that will enter the cell nucleus and destroy DNA molecules, effectively killing the tumor cells. Therfore hypoxic tumors are often termed
radioresistant since free radicals cannot be created. These conclusions have been studied extensively and now models are coming out to assess the extent of radioresistance a tumor has based on its level of hypoxia [
8]. Despite being an effective treatment to gain local control of solid tumors, radiation therapy often has side effects, including radiation burns, and short and long term damage to normal tissues and organs [
9].
One alternative to both chemotherapy and radiation therapy is termed
anti-angiogenesis therapy. This therapy can be used as a preoperative therapy or in combination with radiation and chemotherapy. There is currently debate whether or not anti-angiogenesis therapy alone can cure a patient of their tumor or if a combination of therapies is required to achieve this cure [
10].
Angiogenesis is the proliferation of endothelial cells from healthy human tissue to an organ. Endothelial cells carry blood, nutrients, and oxygen to vital organs and carry de-oxygenated blood away from these organs. While this process is vital in the embryonic stage of development for human life, it is also a means by which tumors survive, grow, and spread. Vascular endothelial growth factor (VEGF) plays the primary role in the development and migration of these tissues [
11]. Solid tumors use this phenomenon to attract blood vessels from normal tissues in order to acquire a supply of necessary nutrients and oxygen for growth. Solid tumors must attract blood vessels from their host as they cannot proliferate beyond 1–2 mm
without this supply of oxygen and nutrients [
12]. The four stages of angiogenesis are initiation, promotion, progression, and metastasis that are described below.
A tumor will send out chemicals during the initiation stage, such as VEGF, to promote endothelial cells to begin proliferation. The nearby endothelial cells are catalyzed by the VEGF molecules and begin to promote outwards towards the tumor. The endothelial cells reach the tumor in the progression stage and are able to supply it with necessary nutrients, such as glucose and oxygen. Metastasis can then occur when cells from the initial lesion break off and travel through the newly proliferated endothelial cells to anywhere in the body. Certain cancers tend to metastasize to different locations in the body, but in general a patient with metastatic cancer has a higher mortality rate than one with a non-metastatic tumor for a variety of reasons. It is believed that a metastatic tumor can grow a new tumor from just one cell that attaches itself to the host [
13]. Multiple tumors accelerate the rate of death of the patient through increased parasitism of nutrients. They also become increasingly harder to treat without greatly increasing the side effects of treatment.
Anti-angiogenesis therapy is a means of preventing the proliferation of endothelial cells so that tumors will not metastasize. So far there are a variety of drugs that have been approved by the FDA which work to prevent angiogenesis, such as Bevacizumab for glioblastoma and Sorafenib for kidney cancer [
14]. Unlike other methods of cancer treatment, such as radiation and chemotherapy that target the cancer cells directly, anti-angiogenesis therapy targets normal endothelial cells that migrate to the tumor. Because normal cells do not divide rapidly mutations that could cause resistance are far less common. This treatment has therefore been described as being resistant to resistance. Since anti-angiogenesis therapy targets developing endothelial cells, it is an exceptionally good therapy at treating widely metastatic tumors that have many metastases throughout the body without additional toxicity to the patient [
15]. Zetter [
15] also concluded that anti-angiogenesis therapy may be most effective when delivered at low doses for long periods of time to prevent dormant micro-metastases from ever becoming active.
Anti-angiogenesis therapy does carry lower risk to the patient compared to chemotherapy and radiation therapy, but there are potential side effects [
16]. Some notable consquences include the drug interfering with surgical wounds healing, CNS syndromes, female reproductive tract damage, and damage to other organs that have a high turnover rate of endothelial cells. However, it is hard to predict which patients will exhibit side effects and most can be reversed by stopping treatment [
17]. Cook and Figg [
18] also discussed the potential side effects of anti-angiogenesis therapy and claimed that while long term therapy seems promising, there is little clinical data to show what the risk factors are for patients. Jain et al. [
19] claims that most side effects experienced by patients are due to anti-VEGF drugs and the most common side effect is hypertension. Elice and Rodeghiero claimed that the most severe side effect was hemorrhaging and showed that in patients with squamous cell carcinoma around nine percent of experienced life threatening pulmonary hemorrhage [
16]. However they also discussed that there has been no clear mechanism identified by which an anti-VEGF drug could cause a hemorrhage. A phase two trial of Bevacizumab, an anti-VEGF anti-angiogenesis drug, showed that 14 percent of participants experienced side effects at doses of 10 mg/kg in single doses administered intravenously [
20].
A variety of mathematical models have been developed over the last few decades to describe the evolution of tumor growth and endothelial cell migration under various cancer therapies [
21]. Although these models can focus on different aspects of the evolution of cancer, the modelling process leads to three distinct approaches: continuum models [
22]—typically formulated as either ordinary or partial differential equations, discrete models [
23], or hybrid models, a combination of the previous two [
24,
25].
The mathematical models that are most relevant to this study include the work by Hahnfeldt et al. [
26], who developed a simple mathematical model based on the notion that tumour growth is a process that ultimately relies on the development of the vasculature. This model assumed that tumour cells produce two distinct factors, one that exerts a stimulatory effect, and another that exerts an inhibitory effect on the vascular network. Sachs et al. [
27] proposed a logistic growth model using the clinical data that tumors decelerate in their growth rate as they get larger. They were also the first to develop a model to illustrate the effects of anti-angiogenesis therapy on the growth of angiogenic endothelial cells. To account for tumor vasculature, they include a variable carrying capacity in the overall dynamics that is a balance between stimulation and inhibition of tumor growth factors.
To determine an optimal protocol for anti-angiogenesis therapy, Ergun et al. [
28] applied optimal control theory to a slightly modified version of the mathematical model given in [
26]. In particular they implemented a bang-bang control in which a fixed amount of angiogenic inhibitors would be used for the duration of the treatment and assumed that maximizing the anti-angiogenic agent dose was equivalent to minimizing the tumor volume. By using this method, Ergun et al. concluded that, as opposed to traditional theory, the largest dose of angiogenesis inhibitors should be given during the second phase of treatment. This work validated the theory of dose escalation proposed by Norton and Simon [
29,
30]. D’Onofrio and Gandolfi [
31] also considered a modified version of the mathematical model given in [
26] in which they determined conditions that guarantee the eradication of the tumor under a protocol of periodic anti-angiogenic therapy.
Ledzewicz and Schättler [
32] considered the same mathematical model given in [
28] but attempted to characterize all parts of the angiogenesis treatment, not just the first two phases. In this work, the authors concluded that the optimal regimen for delivering treatment followed a “0asa0” pattern, where
a and
0 denote trajectories with full and no anti-angiogenic therapy, respectively, and
s denotes a segment along an optimal singular control. In other words, the solution to their problem is an optimal singular arc that is a concatenation of bang-bang controls—constant controls that give either a full or no dose of inhibitors—and the optimal singular control which administers the inhibitors using a time-dependent schedule at less than a maximum rate. In similar work Ledzewicz and Schättler [
33] highlighted that depending on the initial conditions not all of the pieces of the “0asa0” solution proposed in their earlier work are present. In fact, the authors point out that the most typical and medically most relevant scenarios are optimal protocols that take the simple form “a0” in which case all inhibitors are administered at full dose from the beginning or “as0” in which case the dosage is adjusted in a continuous manner until all inhibitors have been exhausted.
As mentioned previously, there are limitations to how large a tumor can grow. Depending on the site of the lesion, a tumor will only grow to a threshold volume before it metastasizes throughout the patient’s body or the patient dies. Current clinical treatments using angiogenesis inhibitors to prevent either of these from happening favor the results from the bang-bang control where a patient receives an injection of a drug over discrete time periods, generally 1–2 injections per week for several weeks, but our research shows that great tumor control can be achieved using the continuous administration of a drug, or daily injections over the course of treatment. Nilsson [
34] used single IV injections of scFv(L19)-tTF, an experimental anti-angiogenesis drug for small tumors or three IV injections for large solid tumors, but in both cases, while the tumor growth was inhibited, the tumor did not disappear completely. On the other hand, d’Onofrio et al. [
35] did a thorough analysis of the different ways that anti-angiogenesis treatment can be administered. This work concluded that continuous, low doses of the therapeutic drug would produce a better outcome in the eradication of the solid tumor, verifying the conclusions made in [
15]. Because of the unfavorable results with bang-bang control and singular arcs for tumors depending on initial conditions, we investigate the two compartment model investigated in [
32] but subject it to a continuous optimal control protocol. In [
33] there were problems with a linear control where the model deviates from a singular arc which means that the bang-bang control fails to be optimal. We predict that using a quadratic control and following the conclusion in [
35] we will resolve these issues.
3. Results and Discussion
Three different initial conditions are chosen from [
26] to define the initial tumor volume ranging between 100 mm
and 300 mm
. The initial amount of endothelial cells were taken from [
32] and is equal to 8000 mm
, yielding
mm. These numbers are representative of a typical scenario in which a patient begins anti-angiogenesis therapy. The time frame for this study was taken from Goldman et al. [
38], Klement et al. [
39], and Holash et al. [
11] who all performed analyses of anti-angiogenesis drugs on mice over the course of two to three weeks. Shih [
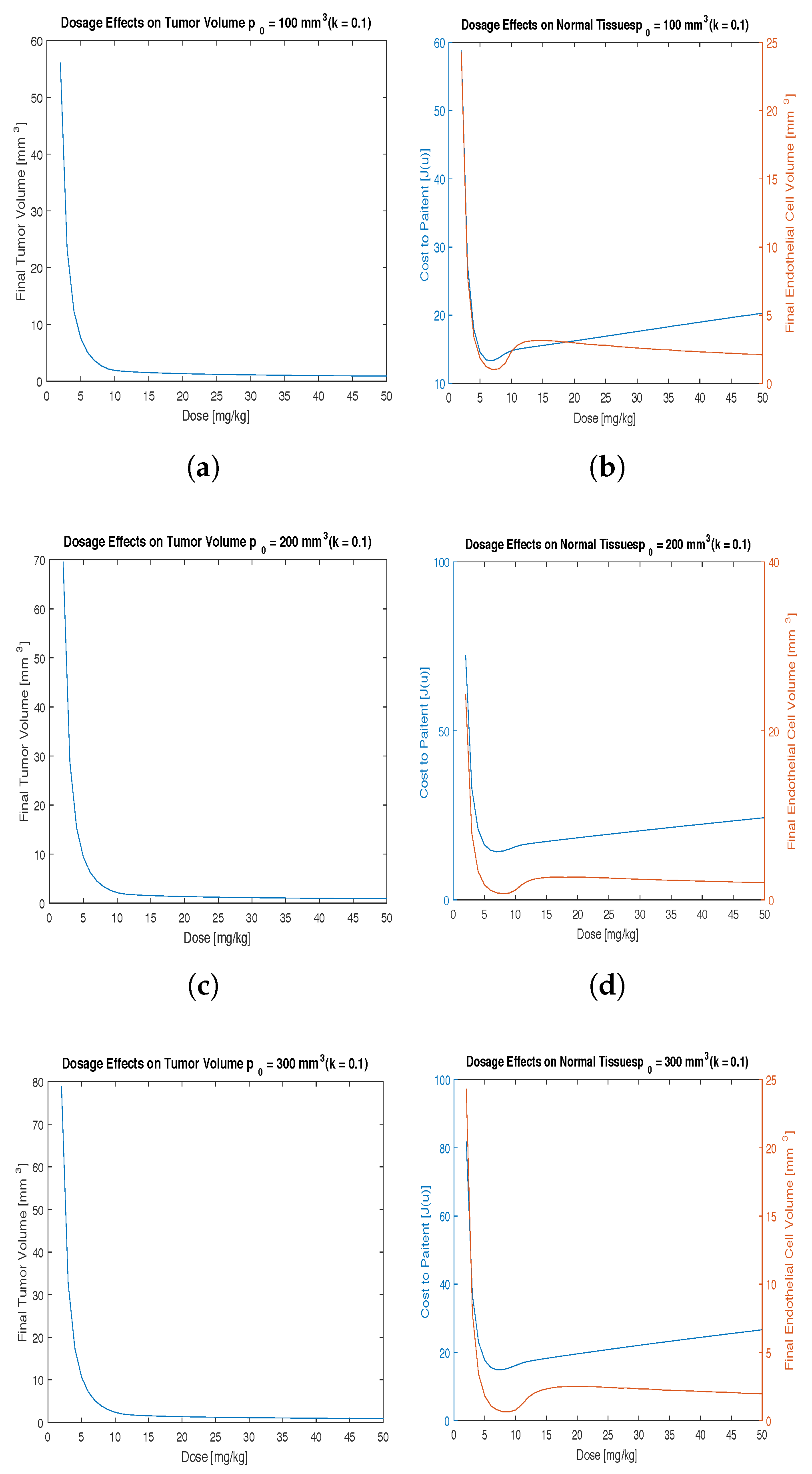
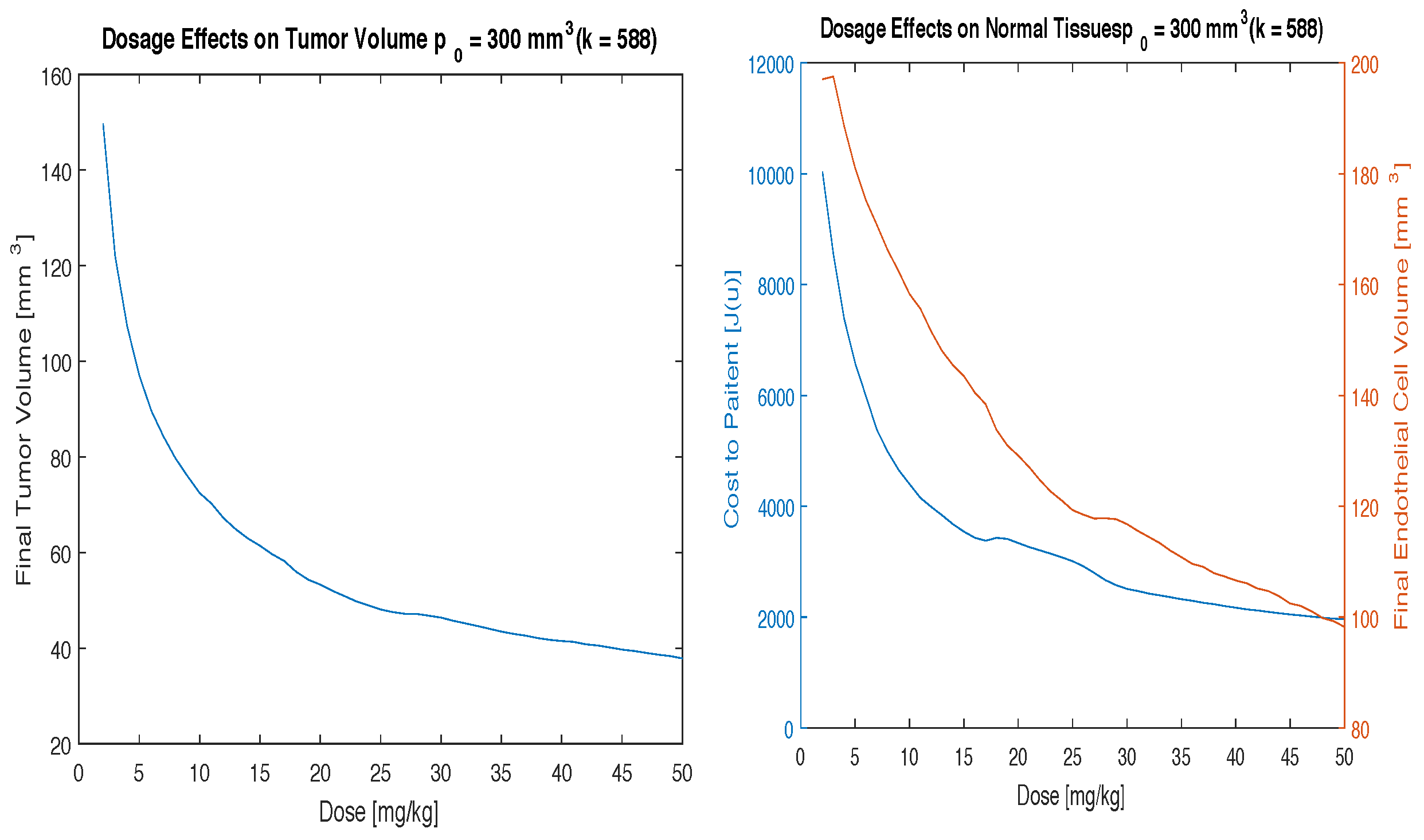
40] investigated the effects of anti-angiogensis therapy on patients with a variety of doses ranging from 0.3 to 10 mg/kg also over a two to three week period. This study was performed using a two-week time period assuming that the minimal treatment time would help minimize toxicity to the patient. The doses to study were determined by calculating the cost function over a range of therapeutic doses (
Figure 1). Ideally a dose that minimizes cost to the patient and maximizes endotheliel cells at the end of the 2 weeks treatment period will be chosen. It is worth noting that a low cost function represents both a low tumor volume and a low total treatment dose. This investigation resulted in the doses of 10, 15 and 20 mg/kg having low cost and high endothelial volume.
The optimality system given in
Section 2 is solved using parameter values defined in
Table 1 using a standard forward-backward sweep method as described in [
41]. Starting with an initial guess for the control and state variables, the state system is solved forward in time. Using the newly obtained values of the state variables, the adjoint system is solved backward in time. Both systems of ordinary differential equations (ODE) are solved with MATLAB ODE solver
ode45 (MathWorks, Natick, MA, USA). The control is updated using a convex combination of the old control values and the new control values from the characterization. The iterative method is repeated until convergence. It is noted that the uniqueness of the optimal control can be proven for a final time
T that is sufficiently small. Nonetheless, the numerical simulations performed did not provide any indication of a solution that is not unique.
The effect of the dosage of angiogenesis inhibitors was investigated first. Numerical simulations were performed by varying the dosage in 1 mg/kg increments to determine the optimal dosage that minimizes the final tumor volume with a relatively low cost function,
. These results are presented in
Figure 1.
As can be seen in
Figure 1, subjecting the model given by Equations (
3) and (4) to our optimal control problem, a continuous optimal control protocol can effectively reduce the volume of the tumor. Since the cost function represents the potential side effects of the drug, it is important to choose the best treatment regimen that minimizes both the tumor cells
and the toxicity
. Running several simulations we can find the optimal dosage for administering the angiogenesis inhibitors, validating both mathematical parameters proposed in past work and clinical observations reported in medical literature.
This investigation begins with a small value of
k (
k = 0.1) which signifies low side effects from the administration of the drug.
Figure 1 shows that with this continuous model the cost function and the tumor volume can be minimized for all initial tumor volumes. This figure also provides doses of interest for further investigation between 10 mg/kg and 20 mg/kg of drug for all initial conditions considered.
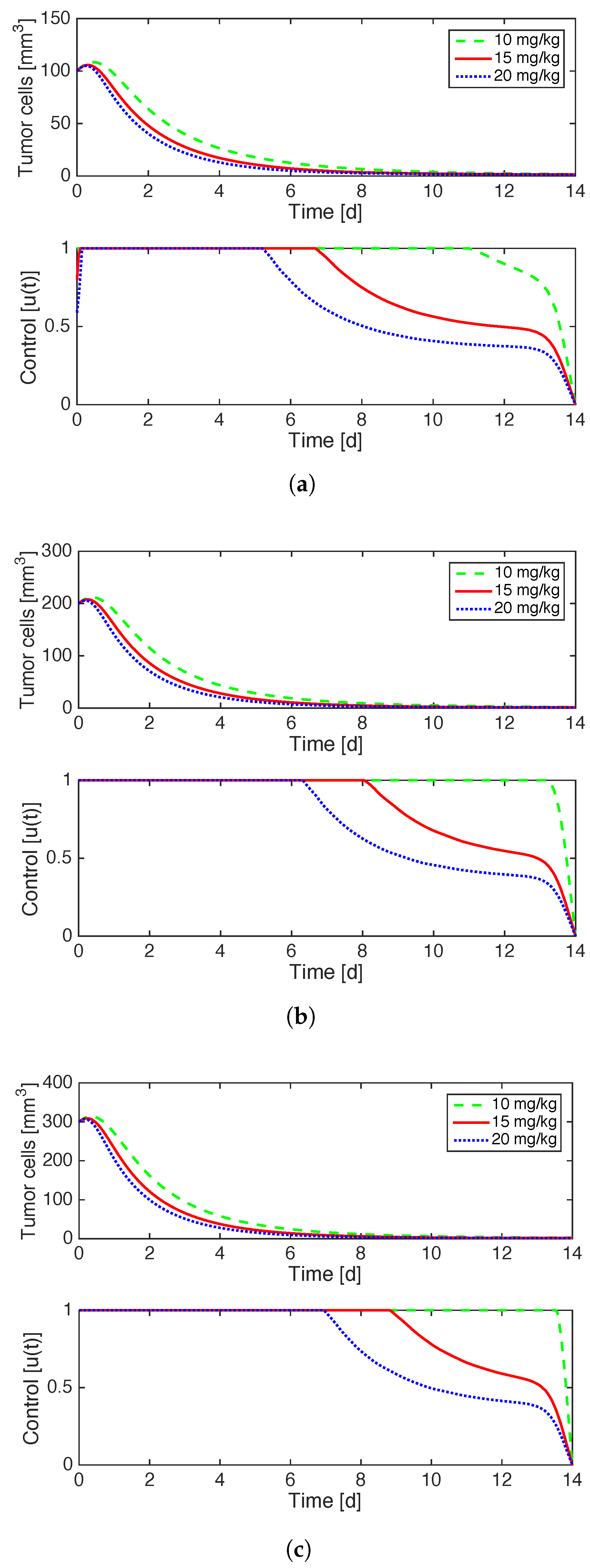
Figure 2 illustrates the effects of implementing optimal control on a case by case basis for the distinct initial values of tumor volume in consideration. The optimal administration of anti-angiogenesis drugs is at full strength for the beginning of treatment and then decays gradually until the end of treatment. The decay happens sooner for higher doses
G and lower values of
. The fact that the control is a continuous function is in contrast to the “0asa0” results in [
33] in which the bang-bang control produced a discontinuous control function.
For larger values of
k, the results become significantly more complex. Since
k is a weighting factor on how much the treatment will negatively effect the patient, it can be assumed that as
k increases, the optimal control
u will change the dosing scheme to compensate for the increasing negative effects of the drug. The effects of
k are investigated by choosing a broad range of values as presented in
Table 1. We include a case in which
k is a function of
, the initial tumor volume, and
T, the duration of the study, based on the results found by Garcia et al. [
20]. In particular, we use the term
as a means to compensate for the observation that fourteen percent of patients receiving angiogenesis inhibitors with their tumor volume were shown to have negative side effects at the end of treatment. The worst case scenario for
k (
,
T = 2 weeks) is shown to illustrate the importance of choosing a meaningful value of
k.
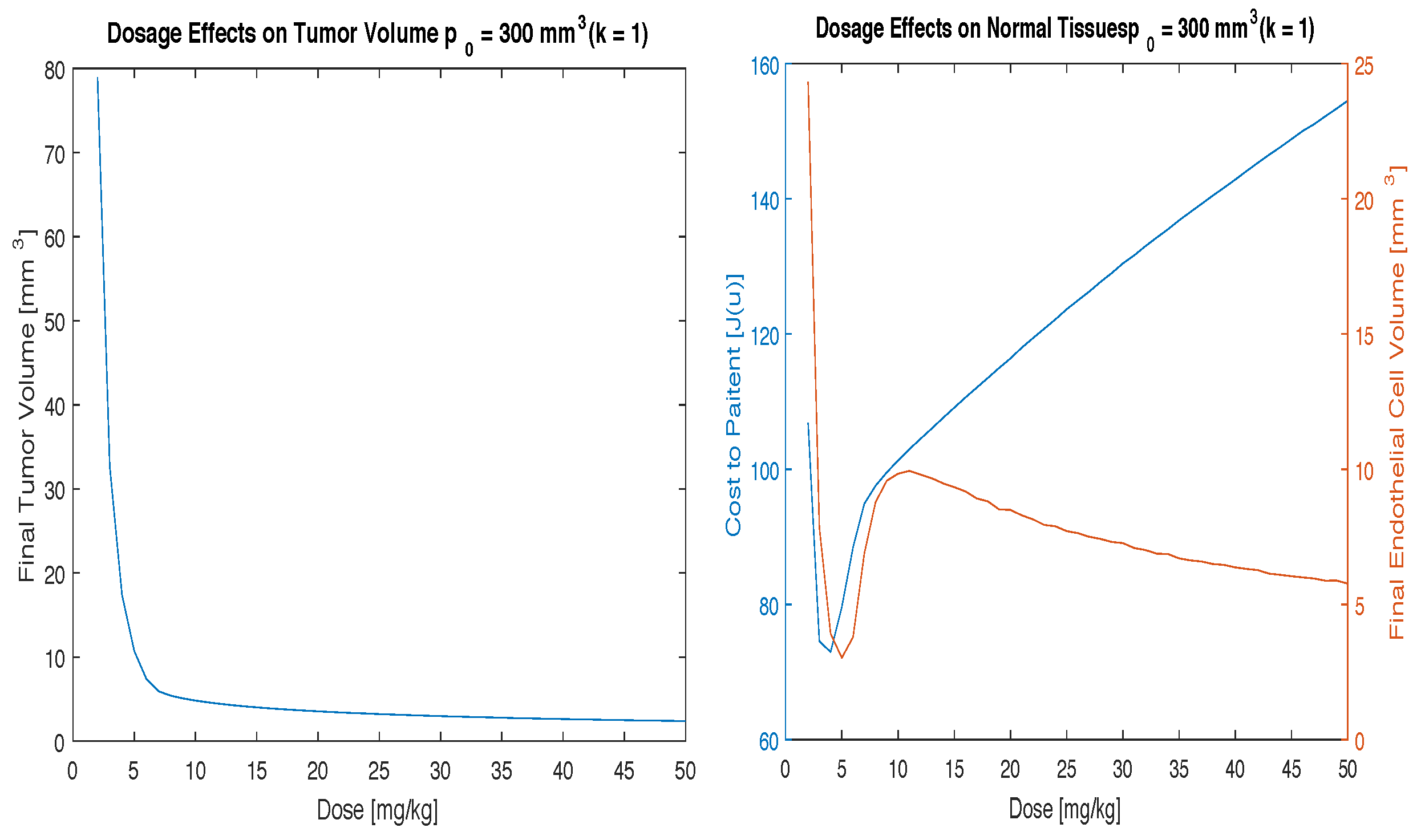
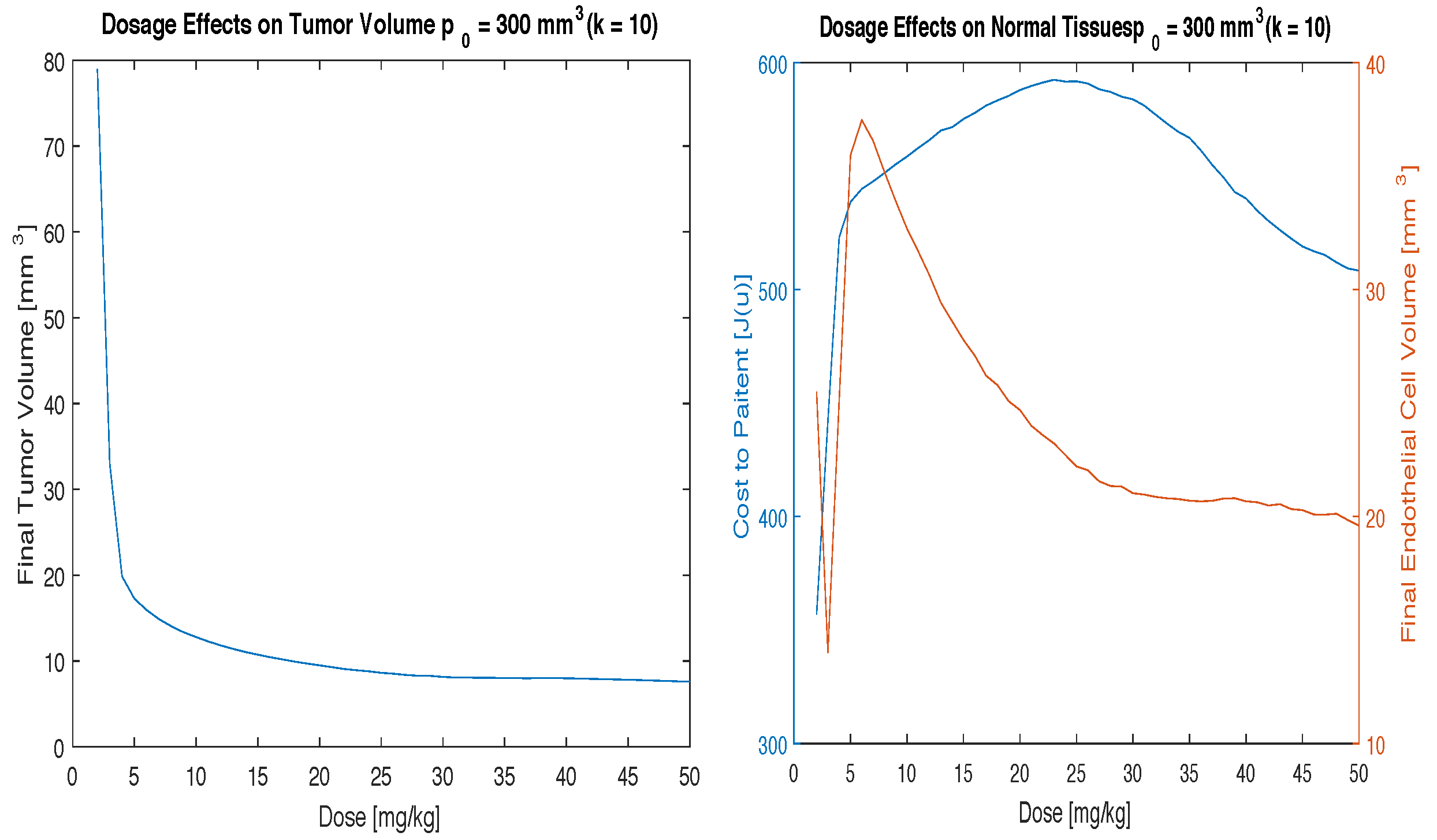
Figure 3,
Figure 4 and
Figure 5 illustrate the effects of varying the parameter
k. For a small increase in
k (
), the cost function and endothelial volume graphs in
Figure 3 look similar to that of the case where
with the same initial condition given in
Figure 1. However, for larger increases in
k the cost function and endothelial volume graphs change dramatically. For these cases it can no longer be concluded that the clinically useful range of doses (10–20 mg/kg) is the optimal dosing scheme.
Figure 4 shows that good tumor control can still be obtained using the clinically useful range of 10–20 mg/kg but that the cost to the patient will increase significantly and the patient will experience more severe side effects. In the lower dose range, where the cost function is a minimal, tumor control can be achieved but the volume will not reduce as significantly.
Figure 5, the highest value of
k, shows that despite the cost being so high you can still achieve good tumor volume reduction, but you will need a higher dosing scheme to do so. In this case, it makes more sense to use a high dose of 50 mg/kg to eradicate as much of the tumor as possible before the negative side effects take place. In other words, since the cost to the patient is high no matter what the dosing scheme you want to use is going to be, it makes more sense to use a high dose of drug. This is akin to what is done in other cancer therapies, such as radiation, where high doses are given over a short amount of time for patients that are at high risk for negative side effects or at high risk for metastatic disease spreading.
It is important to find the correct value of k to achieve the correct results. k represents the biological side effects of the administered drugs. This is not a fixed value and will change based on the biology of the system, the drug being used, the time-frame of the treatment, and the initial conditions of the tumor volume. Further study into determining the amount of side effects in a specific patient with a specific drug must be done to create a system for evaluating the correct value of k. A database could be created to include the frequency and severity of side effects based on the initial and final conditions. Then correction factors can be determined based on biologically relevant statistics such as age, sex, and other predetermined factors. With this information, the model presented here can be used in a case-by-case basis to optimize the cancer treatment with angiogenesis inhibitors.
4. Conclusions
When discussing any form of treatment for cancer it is important to consider the negative side effects that treatment will have on the patient. The goal of any treatment is to destroy as many cancer cells as possible while limiting toxicity of the treatment to a reasonable minimum. Since developing vasculature is most important to fetus development [
42], there are limited side effects to angiogensis therapy for adults. However, there still are side effects as even adults require new vasculature to maintain blood flow to vital organs for oxygen and nutrient transfer.
These results show that in the clinically relevant dosage range of 10–20 mg/kg one can achieve good tumor response with continuous drug concentration while minimizing the toxicity which represents negative side effects of angiogenesis inhibitors by tapering off the concentration at the end of treatment. This is true for all initial tumor volumes and most values of the parameter k.
Figure 3,
Figure 4 and
Figure 5 show the importance of studying the side effects of anti-angiogenesis therapy from both a biological and mathematical perspective. Doing so will allow physicians to choose the precise value of
k that is representative of biological side effects. Further work must be done to determine a biologically relevant range of values of
k that take into consideration factors that lead to side effects in patients. Once a method for determining
k is created, this model can be used to optimize the treatment of solid tumors with angiogenesis inhibitors on a case-by-case basis.
The work presented in this paper shows that good tumor control can be achieved using a continuous dosing strategy for anti-angiogenesis therapy. The model presented has solved the singularity issue of the bang-bang control model presented in [
32] and shown that continuous amounts of anti-angiogenesis drugs in a system reduces tumor volume greatly over a two week time frame. Technology currently exists to administer drugs in such a way that the concentration in blood is at the desired level [
43]. These infusion pumps can be used to administer the dosing scheme developed in this paper.
Tumor angiogenesis is a convenient method for tumors to attract endothelial vessels to provide them with the necessary nutrients and oxygen to grow and metastasize. Since all drugs have side effects, it is important to find the optimal timing and dosage to give to a patient to reduce the angiogenesis process as a potential treatment for cancerous tumors. These findings show that given a continuous low dose regimen of angiogensis inhibitors a tumor volume present in a body can be greatly reduced by attacking the endothelial cells, preventing the tumor from receiving the necessary nutrients for survival and growth. While the current practice is to administer angiogensis inhibitors in 1–2 injections per week, given these promising results, perhaps one day the delivery prescription of these drugs will be changed to match what has been simulated, creating one more tool in the fight against cancer.