Prevention of Diabetes after Gestational Diabetes: Better Translation of Nutrition and Lifestyle Messages Needed
Abstract
:1. Introduction
2. Best Practice Guidelines for Diabetes Prevention in Women Who Have Had GDM
| Guideline Topic | RACGP [27] | TG Ltd. [26] | ADIPS [25] | NICE [28] | ADA [29] |
|---|---|---|---|---|---|
| Postnatal screening | 6–12 weeks after delivery; OGTT | 6–12 weeks after delivery; 75 g OGTT | 6–12 weeks after delivery; OGTT | 6 weeks after delivery; FPG | 6–12 weeks after delivery; 75 g OGTT |
| Repeat screening | 3 Yearly | Yearly (Alternatively 75 g OGTT every 2 years or if contemplating further pregnancy) | Yearly; OGTT if contemplating further pregnancy | Yearly | 1–3 Yearly; yearly if IFG or IGT, otherwise 3 yearly |
| Repeat screening test | FPG | FBG or RBG | 75 g OGTT or FPG | FPG | 75 g OGTT |
| Lifestyle recommendations | General healthy eating Increase physical activity (30 min brisk walking 5 days a week) and/or weight loss. Encourage breastfeeding | Healthy diet and exercise | Weight control Healthy diet and exercise | Weight control Healthy diet and exercise Encourage breastfeeding | Weight loss 7% body weight Low fat Increase fibre at 14 g/1000 kcal and whole grains Increased PA to 150 min/week moderate activity |
Postpartum Screening for Type 2 Diabetes
3. Type 2 Diabetes Prevention
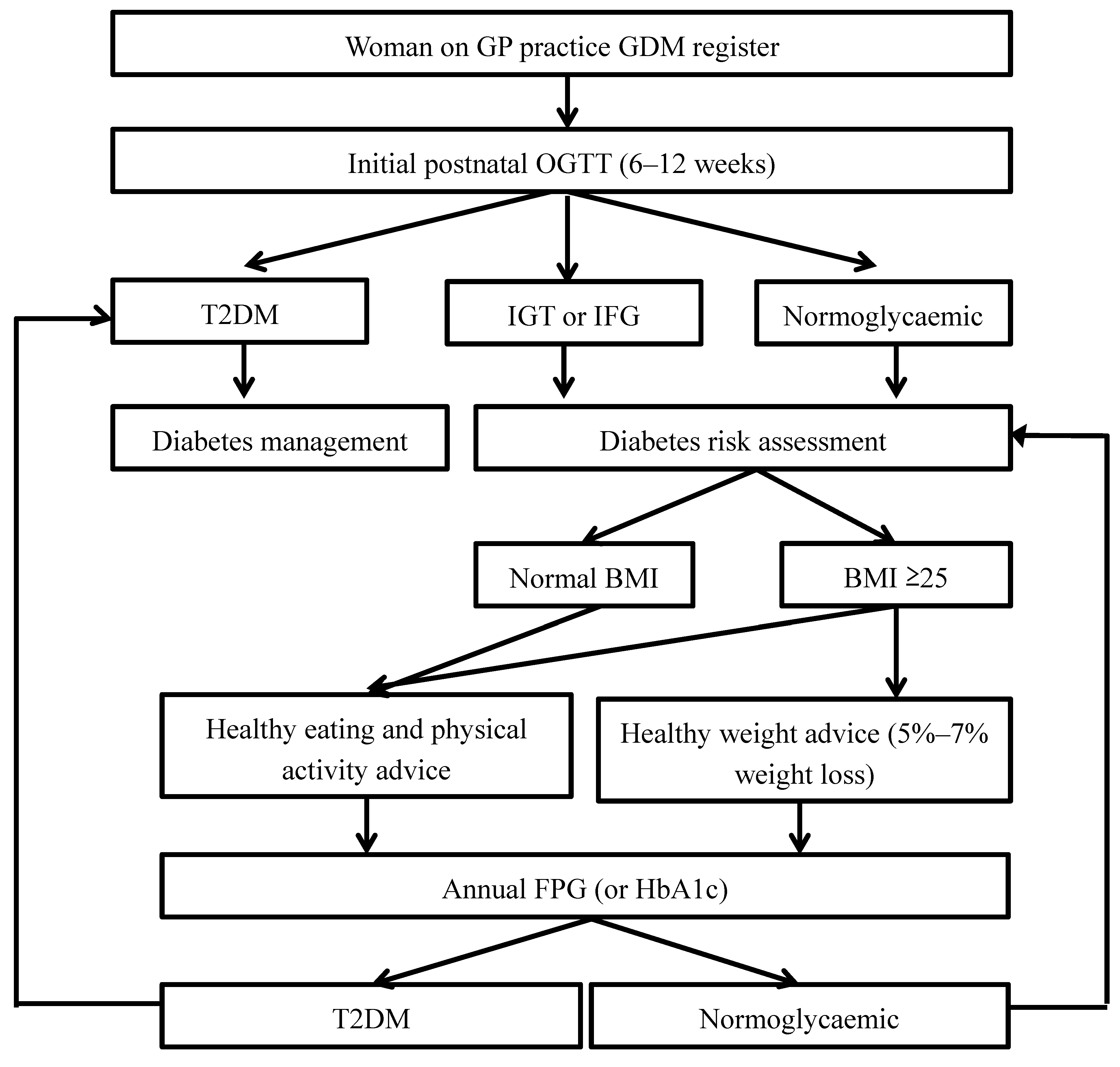
3.1. Risk Awareness
3.2. Lifestyle Modification
3.2.1. Weight Loss
3.2.2. Macronutrients
3.2.3. Physical Activity
3.3. Breastfeeding
4. Intervention Programs
| Study Title | Recruitment Target | Intervention | Follow Up Duration | Primary Outcome Measures | Estimated Completion |
|---|---|---|---|---|---|
| Mothers After Gestational Diabetes in Australia study (MAGDA) [111,112] | 574 Australian women (Victoria and South Australia) | 1 individual session and 5 group sessions (initial 3 months) and 2 follow-up telephone sessions. 5 goals: 5% weight reduction, <30% energy from fat, <10% energy from saturated fat, 15 g fibre/ 1000 kcal, >30 min daily moderate or vigorous exercise. | 1 year | Diabetes risk (FPG, weight or waist circumference) | 2015 |
| Tianjin Gestational Diabetes Prevention Program (TGDPP) [114] | 1180 Chinese women (Tianjin province) | 5 individual consults with dietitian (year 1) and 2 individual consults with dietitian (year 2). 6 goals: 5%–10% weight reduction in overweight women through > 10% reduction in total energy, <30% energy from fat, <10% energy from saturated fat, 55%–65% energy from carbohydrate, 20–30 g per day fibre, >30 min daily moderate or vigorous exercise. | 2 years | Incident T2DM | 2013–2014 |
| The DIAMIND study [110] | 276 Australian women (South Australia) | SMS text OGTT reminder at 6 weeks postpartum, with further reminders at 3 and 6 months if not tested. Control group receive single SMS text reminder at 6 months postpartum. | 6 months | OGTT attendance by 6 months post-partum | 2014 |
| Lifestyle Intervention Program for Women With Gestational Diabetes or Gestational Impaired Glucose Tolerance (APPLES) [124] | 350 American women (California) | 1 individual session and 3 telephone counseling sessions (Phase 1, during pregnancy). After 6 weeks post-partum, 3 individual sessions and 13 telephone counseling sessions (phase 2, 6 months). DPP goals (weight reduction and increased physical activity). | 2 years | Achievement of pre-pregnancy weight if normal BMI pre-pregnancy or 5% weight reduction on pre-pregnancy weight if overweight | 2016 |
| Croí MyAction program [125] | 54 Irish women (Galway) | 1 individual risk assessment and 2.5 h session weekly for 12 weeks (1 h group exercise, 1 h group education and 30min individual session). Support person participates in 12-week program. Goals: BMI >30 kg/m2, >30 minutes moderate intensity physical activity >5 days per week, Mediterranean dietary pattern. | 1 year | Mean FPG reduction on OGTT | 2014 |
| Optimizing Outcomes in Women with Gestational Diabetes Mellitus and their Infants study [116] | 100 American women (North Carolina) | Phase 1 (4 months): 1 group session (during pregnancy) and 13 group sessions (post-partum, includes 1 h exercise class). Phase 2: 3 group sessions at monthly intervals. Weekly SMS text from enrollment to study completion. | 10 months | FBG and weight (BMI) | 2014 |
| Dulce Mothers [113] | 84 Latina American women (California) | 8 weekly 2 h group sessions including 15–20 minutes exercise. DPP goals (weight reduction and increased physical activity). | 6 months | HbA1c, blood lipids and weight (BMI) | 2014 (completed) |
| Estudio Parto [117] | 300 Hispanic American women (Massachusetts) | Phase 1: 1 individual session (during pregnancy), 1 individual session (6 weeks post-partum), weekly/fortnightly/monthly print and telephone contact (6 months). Phase 2: monthly/bimonthly print and telephone contact (6 months) | 1 year | Weight (BMI), insulin resistance markers and cardiovascular disease risk markers | 2016 |
| Gestational Diabetes’ Effects on Moms (GEM) study [115] | 2320 American women (California) | Phase 1: Individual weight goal letter (during pregnancy). Phase 2: 13 telephone sessions and support materials (6 months). Phase 3: 3 newsletters and support materials (6 months) | 1 year | Achieving post-partum weight goal and total weight change | 2014 |
| Prevention of gestational diabetes through lifestyle modification (RADIEL) study [118] | 728 Finnish women (South Eastern region) | Individual counseling 3 monthly pre and during pregnancy and at 6 weeks, 6 months and 12 months. Goals: 5%–10% weight loss pre-pregnancy if BMI ≥25 kg/m2 or no weight gain during first 2 trimesters for pre-pregnancy BMI ≥30 kg/m2, >30 min daily moderate or vigorous exercise, 1600–1800 kcal/day, 40%–50% energy from carbohydrates, 30%–40% from fats and 20%–25% from protein. | 1 year | OGTT | 2014 |
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013. [Google Scholar]
- Hunt, K.J.; Schuller, K.L. The increasing prevalence of diabetes in pregnancy. Obstet. Gynecol. Clin. North. Am. 2007, 34, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). Diabetes Prevalence in Australia: Detailed Estimates for 2007–2008; AIHW: Canberra, Austrilia, 2011. [Google Scholar]
- Goss, J. Projection of Australian Health Care Expenditure by Disease: 2003 to 2033. Cat. no. HWE 43; AIHW: Canberra, Austrilia, 2008. [Google Scholar]
- Australian Institute of Health and Welfare (AIHW). Diabetes in Pregnancy: Its Impact on Australian Women and Their Babies; AIHW: Canberra, Austrilia, 2010. [Google Scholar]
- Bullard, K.M.; Saydah, S.H.; Imperatore, G.; Cowie, C.C.; Gregg, E.W.; Geiss, L.S.; Cheng, Y.J.; Rolka, D.B.; Williams, D.E.; Caspersen, C.J. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care 2013, 36, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.A.; Hadden, D.R.; Maresh, M.; Deerochanawong, C.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Coustan, D.R.; Hod, M.; Oats, J.J.N.; et al. For the HAPO Study Cooperative Research Group: Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel–Recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Lin, L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J. Perinatol. 2014, 34, 100–104. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Almario, C.V.; Ecker, T.; Moroz, L.A.; Bucovetsky, L.; Berghella, V.; Baxter, J.K. Obstetricians seldom provide postpartum diabetes screening for women with gestational diabetes. Am. J. Obstet. Gynecol. 2008, 198, e521–e528. [Google Scholar] [CrossRef]
- Shah, B.R.; Lipscombe, L.L.; Feig, D.S.; Lowe, J.M. Missed opportunities for type 2 diabetes testing following gestational diabetes: A population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1484–1490. [Google Scholar] [CrossRef]
- Shah, B.R.; Retnakaran, R.; Booth, G.L. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008, 31, 1668–1669. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Tong, J.; Wallace, T.M.; Kodama, K.; Shofer, J.B.; Heckbert, S.R.; Boyko, E.J.; Fujimoto, W.Y.; et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006, 29, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S251–S260. [Google Scholar] [CrossRef] [PubMed]
- Damm, P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int. J. Gynecol. Obstet. 2009, 104, S25–S26. [Google Scholar] [CrossRef]
- Chamberlain, C.; McLean, A.; Oats, J.; Oldenburg, B.; Eades, S.; Sinha, A.; Wolfe, R. Low rates of postpartum glucose screening among indigenous and non-indigenous women in Australia with gestational diabetes. Matern. Child Health J. 2014. [Google Scholar] [CrossRef]
- Keely, E. An opportunity not to be missed—How do we improve postpartum screening rates for women with gestational diabetes? Diabetes Metab. Res. Rev. 2012, 28, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Keely, E.; Clark, H.; Karovitch, A.; Graham, I. Screening for type 2 diabetes following gestational diabetes: Family physician and patient perspectives. Can. Fam. Physician 2010, 56, 558–563. [Google Scholar] [PubMed]
- Nielsen, K.K.; Kapur, A.; Damm, P.; de Courten, M.; Bygbjerg, I.C. From screening to postpartum follow-up—The determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy Childbirth 2014, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Gabbe, S.G.; Landon, M.B.; Warren-Boulton, E.; Fradkin, J. Promoting health after gestational diabetes: A National Diabetes Education Program call to action. Obstet. gynecol. 2012, 119, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.A.; Lim, S.S.; Upham, S.; Pennington, A.; O’Reilly, S.L.; Asproloupos, D.; McIntyre, D.H.; Dunbar, J.A. Who’s responsible for the care of women during and after a pregnancy affected by gestational diabetes? Med. J. Aust. 2014, 201, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Grol, R. Beliefs and evidence in changing clinical practice. Br. Med. J. 1997, 315, 418–421. [Google Scholar] [CrossRef]
- Grol, R.; Grimshaw, J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet 2003, 362, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Nankervis, A.; McIntyre, H.D.; Moses, R.; Ross, G.P.; Callaway, L.; Porter, C.; Jeffries, W.; Boorman, C.; de Vries, B.; McElduff, A. Australian Diabetes in Pregnancy Society, Consensus guidelines for the testing and diagnosis of Gestational Diabetes Mellitus in Australia. Avaliable online: http://adips.org/downloads/2014ADIPSGDMGuidelinesVJune2014FINALforWEB.pdf (accessed on 10 October 2014).
- Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, 5th ed.; Therapeutic Guidelines Limited: Melbourne, Austrilia, 2014. [Google Scholar]
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice (the Red Book), 8th ed.; Royal Australian College of General Practitioners House: Melbourne, Austrilia, 2012. [Google Scholar]
- National Institute for Health and Care Excellence. CG63 Diabetes in Pregnancy: Management of Diabetes and Its Complication from Pre-Conception to Postnatal Period; NICE: London, UK, 2008. [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar]
- Kachalia, A.; Mello, M.M. Breast cancer screening: Conflicting guidelines and medicolegal risk. JAMA 2013, 309, 2555–2556. [Google Scholar] [CrossRef]
- O’Reilly, S.L.; Janus, E.; Dunbar, J. Diabetes prevention in high-risk women: Many guidelines do not make light work. In Proccedings of the 11th Guidelines International Network Conference, Melbourne, Australia, 20–24 August 2014; p. 72.
- Wilkinson, S.A.; Brodribb, W.E.; Upham, S.; Janamian, T.; Nicholson, C.; Jackson, C.L. Primary care of women after gestational diabetes mellitus: Mapping evidence-practice gap. Med. J. Aust. 2014, 201, S74–S77. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.; Modder, J.; Mortagy, I.; Springett, A.; Hughes, H.; Baldeweg, S. Missed opportunities for diabetes prevention: Post-pregnancy follow-up of women with gestational diabetes mellitus in England. Br. J. Gen. Pract. 2011, 61, e611–e619. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Collins, C.; Lowe, J. Postnatal testing for diabetes in Australian women following gestational diabetes mellitus. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Devsam, B.U.; Bogossian, F.E.; Peacock, A.S. An interpretive review of women’s experiences of gestational diabetes mellitus: Proposing a framework to enhance midwifery assessment. Women Birth 2013, 26, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.K.; Lowe, J.M.; Collins, C.E. Australian women’s experiences of living with gestational diabetes. Women Birth 2014, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Australia National Gestational Diabetes Register. Available online: http://www.diabetesaustralia.com.au/GD/Home/Headline-Containers-for-L2/National-Gestational-Diabetes-Register/ (accessed on 10 October 2014).
- Sterne, V.L.; Logan, T.; Palmer, M.A. Factors affecting attendance at postpartum diabetes screening in women with gestational diabetes mellitus. Pract. Diabetes Int. 2011, 28, 64a–68a. [Google Scholar]
- Swan, W.; Kilmartin, G.; Liaw, S.T. Assessment of readiness to prevent type 2 diabetes in a population of rural women with a history of gestational diabetes. Rural Remote Health 2007, 7, 802. [Google Scholar] [PubMed]
- Stuebe, A.; Ecker, J.; Bates, D.W.; Zera, C.; Bentley-Lewis, R.; Seely, E. Barriers to follow-up for women with a history of gestational diabetes. Am. J. Perinatol. 2010, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Public Health Guidance 49 Behaviour Change: Individual Approaches; NICE: London, UK, 2014. [Google Scholar]
- D’emden, M. Glycated haemoglobin for the diagnosis of diabetes. Aust. Prescriber 2014, 37, 98–100. [Google Scholar]
- McGrath, N.M.; Coats, A.; Barach, O. Improved post-partum follow-up of patients with gestational diabetes mellitus using HbA1c. Diabet. Med. 2013, 30, 1264–1265. [Google Scholar] [CrossRef] [PubMed]
- Chittleborough, C.R.; Baldock, K.L.; Taylor, A.W.; Hague, W.M.; Willson, T.; Martin, W.; Wood, J.; Phillips, P.J. Long-term follow-up of women with gestational diabetes mellitus: The South Australian Gestational Diabetes Mellitus Recall Register. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 127–131. [Google Scholar] [PubMed]
- Swan, W.E.; Liaw, S.T.; Dunning, T.; Pallant, J.F.; Kilmartin, G. Diabetes risk reduction behaviours of rural postpartum women with a recent history of gestational diabetes. Rural Remote Health 2010, 10, 1461. [Google Scholar] [PubMed]
- Clark, H.D.; Graham, I.D.; Karovitch, A.; Keely, E.J. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. Am. J. Obstet. Gynecol. 2009, 200, e631–e634, e637. [Google Scholar]
- Clark, H.D.; Walraven, C.V.; Code, C.; Karovitch, A.; Keely, E. Did Publication of a clinical practice guideline recommendation to screen for Type 2 diabetes in women with gestational diabetes change practice? Diabetes Care 2003, 26, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Vesco, K.K.; Dietz, P.M.; Bulkley, J.; Bruce, F.C.; Callaghan, W.M.; England, L.; Kimes, T.; Bachman, D.J.; Hartinger, K.J.; Hornbrook, M.C. A system-based intervention to improve postpartum diabetes screening among women with gestational diabetes. Am. J. Obstet. Gynecol. 2012, 207, e281–e283, e286. [Google Scholar]
- Reilly, S.L.; Threlfall, H.; Ford, D.; Dunbar, J. Adapting primary care collaboratives for diabetes prevention in high risk women. In Proceedinds of the 2nd Biennial Australian Implementation Conference, Sydney, Australia, 17–18 September 2014; p. 108.
- Saaristo, T.; Moilanen, L.; Korpi-Hyovalti, E.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Jokelainen, J.; Peltonen, M.; Oksa, H.; Tuomilehto, J.; et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010, 33, 2146–2151. [Google Scholar] [CrossRef]
- Molitch, M.E.; Fujimoto, W.; Hamman, R.F.; Knowler, W.C. The diabetes prevention program and its global implications. J. Am. Soc. Nephrol. 2003, 14, S103–S107. [Google Scholar] [PubMed]
- Qiao, Q.; Pang, Z.; Gao, W.; Wang, S.; Dong, Y.; Zhang, L.; Nan, H.; Ren, J. A large-scale diabetes prevention program in real-life settings in Qingdao of China (2006–2012). Prim. Care Diabetes 2010, 4, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hernan, W.H.; Brandle, M.; Zhang, P.; Williamson, D.F.; Matulik, M.J.; Ratner, R.E.; Lachin, J.M.; Engelgau, M.M. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 2003, 26, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, P.; Barker, L.E.; Chowdhury, F.M.; Zhang, X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: A systematic review. Diabetes Care 2010, 33, 1872–1894. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Christophi, C.A.; Metzger, B.E.; Dabelea, D.; Bennett, P.H.; Pi-Sunyer, X.; Fowler, S.; Kahn, S.E. Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: Effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 2008, 93, 4774–4779. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [PubMed]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Infanti, J.; O’Dea, A.; Gibson, I.; McGuire, B.; Newell, J.; Glynn, L.; O’Neill, C.; Connolly, S.; Dunne, F. Reasons for participation and non-participation in a diabetes prevention trial among women with prior gestational diabetes mellitus (GDM). BMC Med. Res. Methodol. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, K.; da Costa, D.; Pillay, S.; de Civita, M.; Gougeon, R.; Leong, A.; Bacon, S.; Stotland, S.; Chetty, V.T.; Garfield, N.; Majdan, A.; Meltzer, S. Strategies to optimize participation in diabetes prevention programs following gestational diabetes: A Focus Group Study. PLoS One 2013, 8, e67878. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.; Ennen, C.; Carrese, J.; Hill-Briggs, F.; Levine, D.; Nicholson, W.; Clark, J. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: A qualitative study. J. Womens Health 2011, 20, 239–245. [Google Scholar] [CrossRef]
- Lie, M.L.S.; Hayes, L.; Lewis-Barned, N.J.; May, C.; White, M.; Bell, R. Preventing Type 2 diabetes after gestational diabetes: Women’s experiences and implications for diabetes prevention interventions. Diabetic Medicine 2013, 30, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Banerjee, A.T.; McTavish, S.; Mukerji, G.; Lowe, J.; Ray, J.; Evans, M.; Feig, D.S. Readiness for diabetes prevention and barriers to lifestyle change in women with a history of gestational diabetes mellitus: Rationale and study design. Diabetes Res. Clin. Pract. 2014, 106, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; McEwen, L.N.; Piette, J.D.; Goewey, J.; Ferrara, A.; Walker, E.A. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 2007, 30, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.; Lawson, M.L.; Gaboury, I.; Keely, E. Risk perception and unrecognized type 2 diabetes in women with previous gestational diabetes mellitus. Obstet. Med. 2009, 2, 107–110. [Google Scholar] [CrossRef]
- Gallivan, J.; Brown, C.; Greenberg, R.; Clark, C.M. Predictors of perceived risk of the development of diabetes. Diabetes Spectr. 2009, 22, 163–169. [Google Scholar] [CrossRef]
- Grimshaw, J.; Eccles, M.; Lavis, J.; Hill, S.; Squires, J. Knowledge translation of research findings. Implement. Sci. 2012, 7, 50. [Google Scholar] [CrossRef]
- Schwarz, P.E.; Li, J.; Lindstrom, J.; Tuomilehto, J. Tools for predicting the risk of type 2 diabetes in daily practice. Horm Metab. Res. 2009, 41, 86–97. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Balkau, B.; Colagiuri, S.; Zimmet, P.Z.; Tonkin, A.M.; Mitchell, P.; Phillips, P.J.; Shaw, J.E. AUSDRISK: An Australian type 2 diabetes risk assessment tool based on demographic, lifestyle and simple anthropometric measures. Med. J. Aust. 2010, 192, 197–202. [Google Scholar]
- Colagiuri, S.; Vita, P.; Cardona-Morrell, M.; Singh, M.F.; Farrell, L.; Milat, A.; Haas, M.; Bauman, A. The Sydney Diabetes Prevention Program: A community-based translational study. BMC Public Health 2010, 10, 328. [Google Scholar] [CrossRef]
- Wing, R.R.; Hamman, R.F.; Bray, G.A.; Delahanty, L.; Edelstein, S.L.; Hill, J.O.; Horton, E.S.; Hoskin, M.A.; Kriska, A.; Lachin, J.; et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes. Res. 2004, 12, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, T.; Dunbar, J.; Chapman, A.; Kilkkinen, A.; Vartiainen, E.; Heistaro, S.; Philpot, B.; Absetz, P.; Bunker, S.; O’Neil, A.; et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Temprosa, M.; Barrett-Connor, E.; Fowler, S.E.; Goldberg, R.B.; Mather, K.J.; Marcovina, S.M.; Montez, M.; Ratner, R.E.; Saudek, C.D.; et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: A report from the DPP Outcomes Study. Diabetic Med. 2013, 30, 46–55. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Patient experiences in Australia: Summary of findings, 2012–13; Australian Bureau of Statistics: Canberra, Australia, 2013.
- Britt, H.; Miller, G.; Charles, J.; Henderson, J.; Bayram, C.; Valenti, L.; Pan, Y.; Harrison, C.; O’Halloran, J.; Fahridin, S.; et al. General Practice Activity in Australia 2000–01 to 2009–10: 10 Year Data Tables; Australian Institute of Health and Welfare: Canberra, Austrilia, 2010. [Google Scholar]
- Nicholas, L.G.; Pond, C.D.; Roberts, D.C. Dietitian-general practitioner interface: a pilot study on what influences the provision of effective nutrition management. Am. J. Clin. Nutrit. 2003, 77, 1039S–1042S. [Google Scholar] [PubMed]
- Dunbar, J.A.; Jayawardena, A.; Johnson, G.; Roger, K.; Timoshanko, A.; Versace, V.L.; Shill, J.; Philpot, B.; Vartiainen, E.; Laatikainen, T.; et al. Scaling up diabetes prevention in victoria, australia: Policy development, implementation, and evaluation. Diabetes Care 2014, 37, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.; Ball, L.; Wall, C.; Leveritt, M. Nutrition beyond drugs and devices: A review of the approaches to enhance the capacity of nutrition care provision by general practitioners. Aust. J. Prim. Health 2012, 18, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Hughes, R.; Desbrow, B.; Leveritt, M. Patients’ perceptions of nutrition care provided by general practitioners: Focus on type 2 diabetes. Family Pract. 2012, 29, 719–725. [Google Scholar] [CrossRef]
- Walker, C.; Hernan, A.; Reddy, P.; Dunbar, J.A. Sustaining modified behaviours learnt in a diabetes prevention program in regional Australia: The role of social context. BMC Health Serv. Res. 2012, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.E.; Hughes, R.M.; Leveritt, M.D. Nutrition in general practice: Role and workforce preparation expectations of medical educators. Aust. J. Prim. Health 2010, 16, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.L.; Herring, S.J.; Sood, M.; Shah, N.R.; Kalet, A.L. What do resident physicians know about nutrition? An evaluation of attitudes, self-perceived proficiency and knowledge. J. Am. College Nutr. 2008, 27, 287–298. [Google Scholar] [CrossRef]
- Helman, A. Nutrition and general practice: An Australian perspective. Am. J. Clin. Nutr. 1997, 65, 1939S–1942S. [Google Scholar] [PubMed]
- Ball, L.; Johnson, C.; Desbrow, B.; Leveritt, M. General practitioners can offer effective nutrition care to patients with lifestyle-related chronic disease. J. Prim. Health Care 2013, 5, 59–69. [Google Scholar] [PubMed]
- Kim, C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabetic Med. 2014, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Chen, F.; Wang, W.; Zhao, J.; Teng, Y.; Wu, M.; Teng, H.; Zhang, X.; Qi, H.; Liu, X.; et al. Secular trends of low birthweight and macrosomia and related maternal factors in Beijing, China: A longitudinal trend analysis. BMC Pregnancy Childbirth 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.F.; Hedderson, M.M.; Feng, J.; Davenport, E.R.; Gunderson, E.P.; Ferrara, A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet. Gynecol. 2011, 117, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.W.; Chan, J.C.N.; Tam, W.H.; Hanson, M.A.; Gluckman, P.D. Gestational diabetes, maternal obesity, and the NCD burden. Clin. Obstet. Gynecol. 2013, 56, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.K.; Kjos, S.L.; Xiang, A.; Buchanan, T.A. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996, 347, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, S.J.; Schwartz, M.W. Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Harwell, T.S.; Vanderwood, K.K.; Hall, T.O.; Butcher, M.K.; Helgerson, S.D. Factors associated with achieving a weight loss goal among participants in an adapted Diabetes Prevention Program. Prim Care Diabetes 2011, 5, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.E.; Wang, J.; Sevick, M.A. Self-monitoring in weight loss: A systematic review of the literature. J. Am. Diet. Assoc. 2011, 111, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yank, V.; Xiao, L.; Lavori, P.W.; Wilson, S.R.; Rosas, L.G.; Stafford, R.S. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: A randomized trial. JAMA Intern. Med. 2013, 173, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Park, B.K.; Park, S.; Kim, S.-H. A comparative study of eating habits and food intake in women with gestational diabetes according to early postpartum glucose tolerance status. Diabetes Metab. J. 2011, 35, 354–363. [Google Scholar] [PubMed]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. HEalthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch. Intern. Med. 2012, 172, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384. [Google Scholar] [PubMed]
- Kahn, R.; Davidson, M.B. The reality of type 2 diabetes prevention. Diabetes Care 2014, 37, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Bowers, K.; Chavarro, J.; Vaag, A.; Grunnet, L.G.; Strøm, M.; Mills, J.; Liu, A.; Kiely, M.; et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: A prospective cohort study. JAMA Intern. Med. 2014, 174, 1047–1055. [Google Scholar] [CrossRef]
- Much, D.; Beyerlein, A.; Roßbauer, M.; Hummel, S.; Ziegler, A.-G. Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol. Metab. 2014, 3, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.-G.; Wallner, M.; Kaiser, I.; Rossbauer, M.; Harsunen, M.H.; Lachmann, L.; Maier, J.; Winkler, C.; Hummel, S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes 2012, 61, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Castonguay, S.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J. Relationship between lactation duration and insulin and glucose response among women with prior gestational diabetes. Eur. J. Endocrinol. 2013, 168, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Jacobs, D.R.; Chiang, V.; Lewis, C.E.; Feng, J.; Quesenberry, C.P.; Sidney, S. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-year prospective study in CARDIA (coronary artery risk development in young adults). Diabetes 2010, 59, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Cordero, L.; Gabbe, S.G.; Landon, M.B.; Nankervis, C.A. Breastfeeding initiation in women with gestational diabetes mellitus. J. Neonatal Perinatal Med. 2013, 6, 303–310. [Google Scholar] [PubMed]
- Wojcicki, J.M. Maternal prepregnancy body mass index and initiation and duration of breastfeeding: A review of the literature. J. Womens Health (Larchmt) 2011, 20, 341–347. [Google Scholar] [CrossRef]
- Kaiser, B.; Razurel, C.; Jeannot, E. Impact of health beliefs, social support and self-efficacy on physical activity and dietary habits during the post-partum period after gestational diabetes mellitus: Study protocol. BMC Pregnancy Childbirth 2013, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Heatley, E.; Middleton, P.; Hague, W.; Crowther, C. The DIAMIND study: Postpartum SMS reminders to women who have had gestational diabetes mellitus to test for type 2 diabetes: A randomised controlled trial-study protocol. BMC Pregnancy Childbirth 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, S.; Davis-Lameloise, N.; Janus, E.; Wildey, C.; Versace, V.; Hagger, V.; Asproloupos, D.; O’Reilly, S.; Phillips, P.; Ackland, M.; et al. Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) post-natal intervention: An update to the study protocol for a randomized controlled trial. Trials 2014, 15, 259. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.; Davis-Lameloise, N.; Janus, E.; Wildey, C.; Versace, V.; Hagger, V.; Asproloupos, D.; O'Reilly, S.; Phillips, P.; Ackland, M.; et al. Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) post-natal intervention: study protocol for a randomized controlled trial. Trials 2013, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Fortmann, A.; Dharkar-Surber, S.; Euyoque, J.; Ruiz, M.; Schultz, J.; Gallo, L. Dulce Mothers: An intervention to reduce diabetes and cardiovascular risk in Latinas after gestational diabetes. Behav. Med. Pract. Policy Res. 2014, 4, 18–25. [Google Scholar] [CrossRef]
- Hu, G.; Tian, H.; Zhang, F.; Liu, H.; Zhang, C.; Zhang, S.; Wang, L.; Liu, G.; Yu, Z.; Yang, X.; et al. Tianjin Gestational Diabetes Mellitus Prevention Program: Study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res. Clin. Pract. 2012, 98, 508–517. [Google Scholar] [PubMed]
- Ferrara, A.; Hedderson, M.; Albright, C.; Brown, S.; Ehrlich, S.; Caan, B.; Sternfeld, B.; Gordon, N.; Schmittdiel, J.; Gunderson, E.; et al. A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: Design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BMC Pregnancy Childbirth 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Neal, M.; Hall, E.; Schwartz, T.; Verbiest, S.; Bonuck, K.; Goodnight, W.; Brody, S.; Dorman, K.; Menard, M.; et al. Rationale, design, and methodology for the optimizing outcomes in women with gestational diabetes mellitus and their infants study. BMC Pregnancy Childbirth 2013, 13, 184. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Marcus, B.; Rosal, M.; Tucker, K.; Hartman, S.; Pekow, P.; Braun, B.; Moore Simas, T.; Solomon, C.; Manson, J.; et al. Estudio Parto: Postpartum diabetes prevention program for hispanic women with abnormal glucose tolerance in pregnancy: A randomised controlled trial—Study protocol. BMC Pregnancy Childbirth 2014, 14, 100. [Google Scholar] [CrossRef]
- Rono, K.; Stach-Lempinen, B.; Klemetti, M.; Kaaja, R.; Poyhonen-Alho, M.; Eriksson, J.; Koivusalo, S.; RADIEL Group. Prevention of gestational diabetes through lifestyle intervention: Study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Barimani, M.; Oxelmark, L.; Johansson, S.E.; Hylander, I. Support and continuity during the first 2 weeks postpartum. Scand. J. Caring Sci. 2014. [Google Scholar] [CrossRef]
- Martin, A.; Horowitz, C.; Balbierz, A.; Howell, E.A. Views of women and clinicians on postpartum preparation and recovery. Matern Child Health J. 2014, 18, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.A.; van der Ploeg, H.P.; Grzegrzulka, R.; Timperley, J.G. Lmplementing lifestyle change through phone-based motivational interviewing in rural-based women with previous gestational diabetes mellitus. Health Promot. J. Aust. 2012, 23, 5–9. [Google Scholar]
- Ehrlich, S.F.; Hedderson, M.M.; Quesenberry, C.P., Jr.; Feng, J.; Brown, S.D.; Crites, Y.; Ferrara, A. Post-partum weight loss and glucose metabolism in women with gestational diabetes: The DEBI Study. Diabet. Med. J. Br. Diabetic Assoc. 2014, 31, 862–867. [Google Scholar] [CrossRef]
- Ferrara, A.; Hedderson, M.M.; Albright, C.L.; Ehrlich, S.F.; Quesenberry, C.P., Jr.; Peng, T.; Feng, J.; Ching, J.; Crites, Y. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: A feasibility randomized control trial. Diabetes Care 2011, 34, 1519–1525. [Google Scholar] [CrossRef]
- Schmittdiel, J.A.; Brown, S.D.; Neugebauer, R.; Adams, S.R.; Adams, A.S.; Wiley, D. Health-plan and employer-based wellness programs to reduce diabetes risk: The Kaiser Permanente Northern California NEXT-D Study. Prev. Chronic Dis. 2013, 10, E15. [Google Scholar] [CrossRef]
- Infanti, J.; Dunne, F.; O’Dea, A.; Gillespie, P.; Gibson, I.; Glynn, L.; Noctor, E.; Newell, J.; McGuire, B. An evaluation of Croí MyAction community lifestyle modification programme compared to standard care to reduce progression to diabetes/pre-diabetes in women with prior gestational diabetes mellitus (GDM): Study protocol for a randomised controlled trial. Trials 2013, 14, 121. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O'Reilly, S.L. Prevention of Diabetes after Gestational Diabetes: Better Translation of Nutrition and Lifestyle Messages Needed. Healthcare 2014, 2, 468-491. https://doi.org/10.3390/healthcare2040468
O'Reilly SL. Prevention of Diabetes after Gestational Diabetes: Better Translation of Nutrition and Lifestyle Messages Needed. Healthcare. 2014; 2(4):468-491. https://doi.org/10.3390/healthcare2040468
Chicago/Turabian StyleO'Reilly, Sharleen L. 2014. "Prevention of Diabetes after Gestational Diabetes: Better Translation of Nutrition and Lifestyle Messages Needed" Healthcare 2, no. 4: 468-491. https://doi.org/10.3390/healthcare2040468




