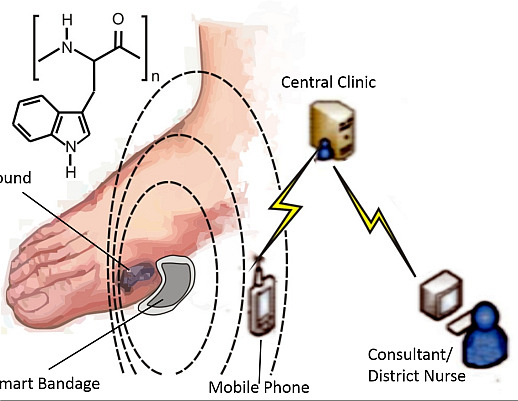
Molecular Wiring in Smart Dressings: Opening a New Route to Monitoring Wound pH
Abstract
:1. Introduction
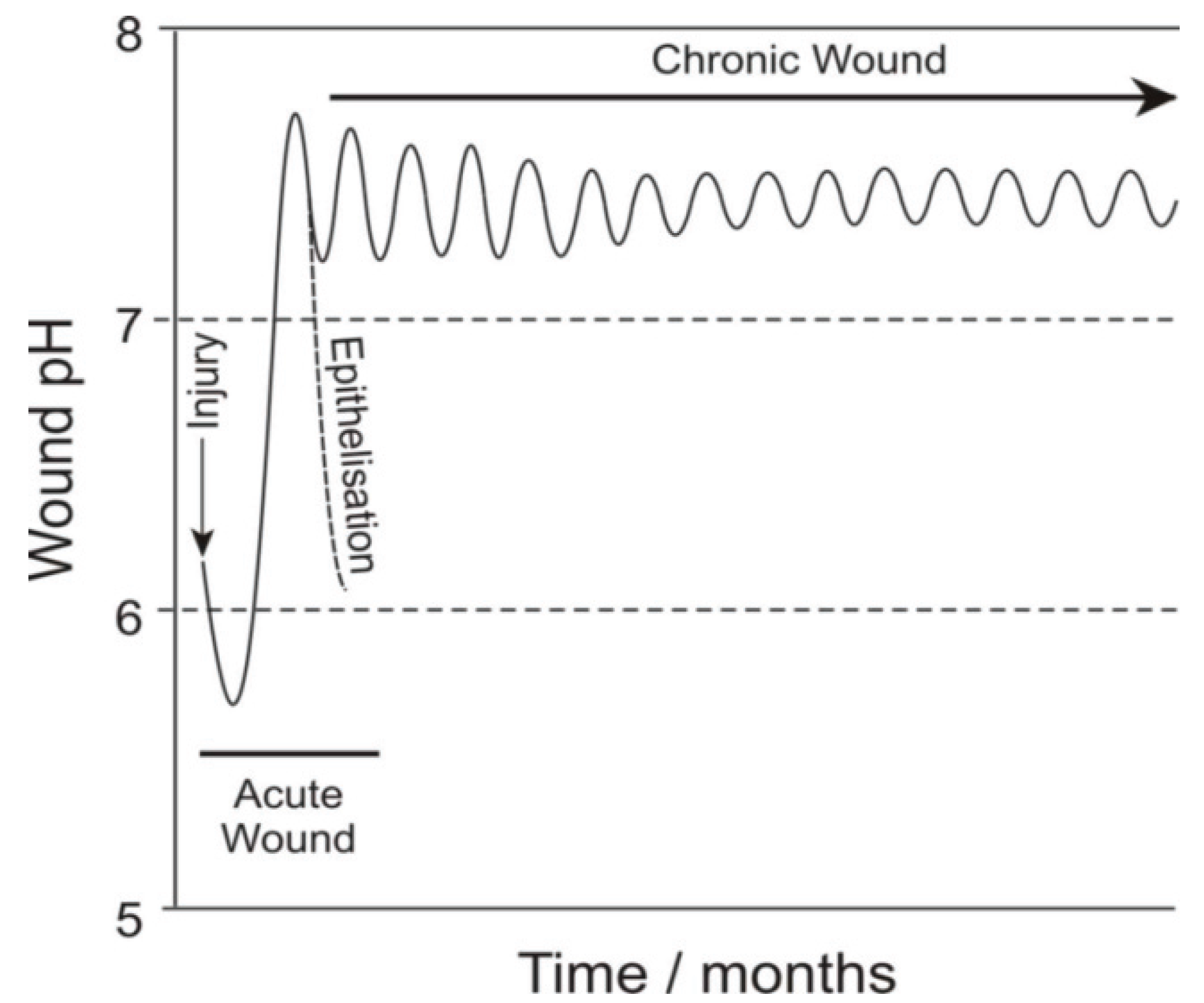
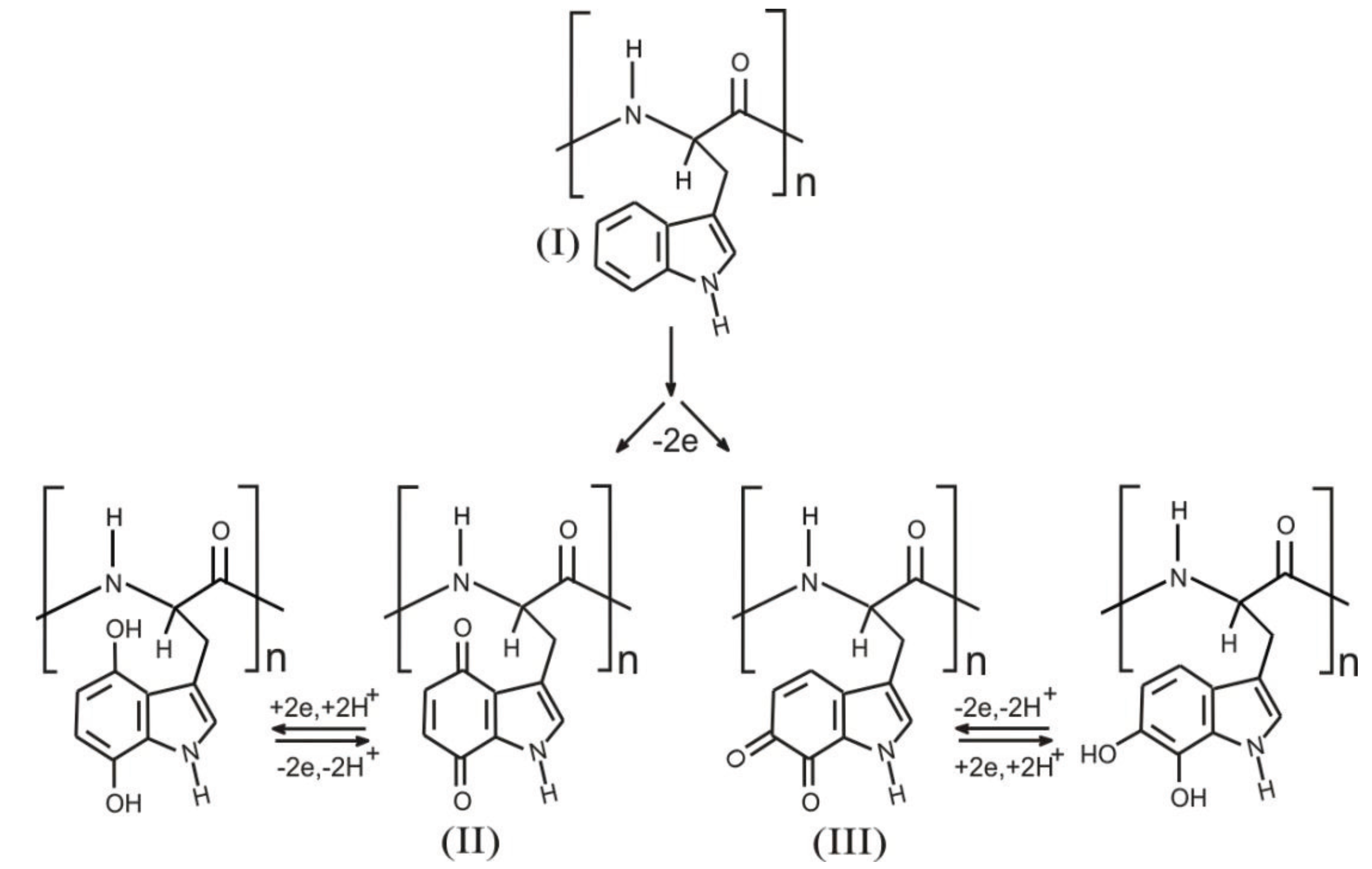
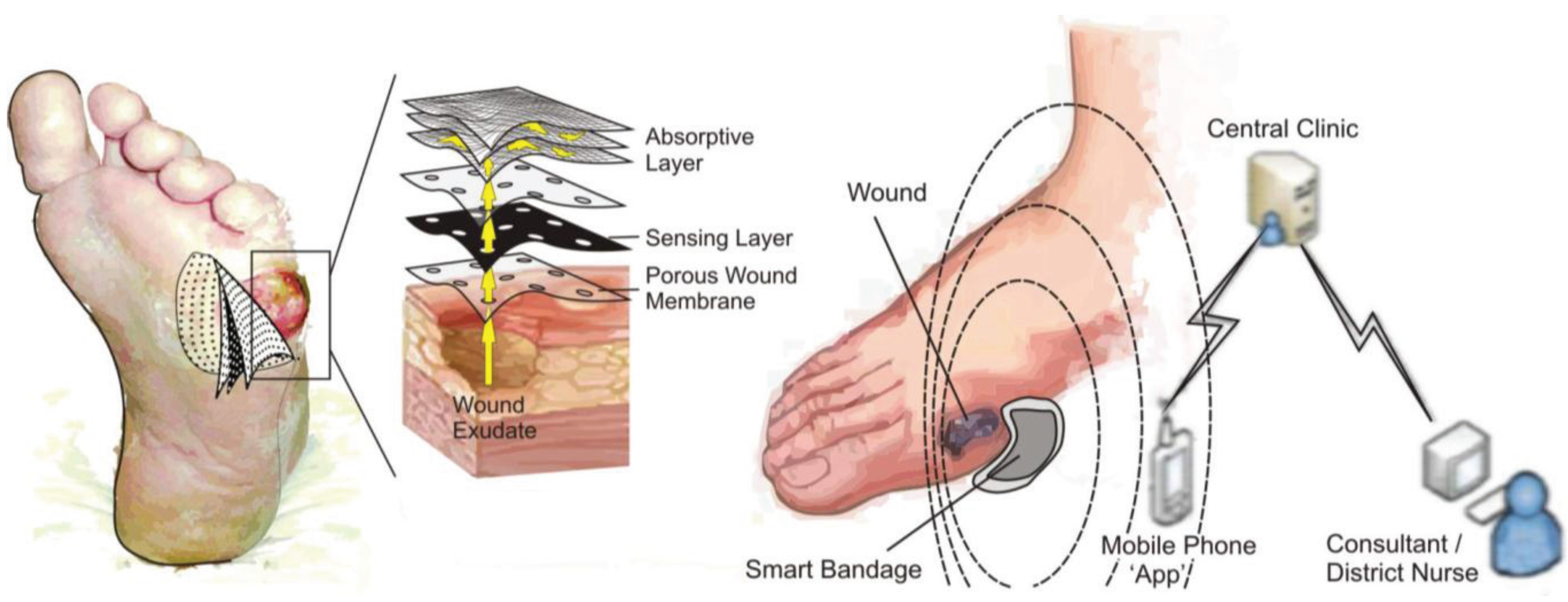
2. Experimental Section
3. Results and Discussion
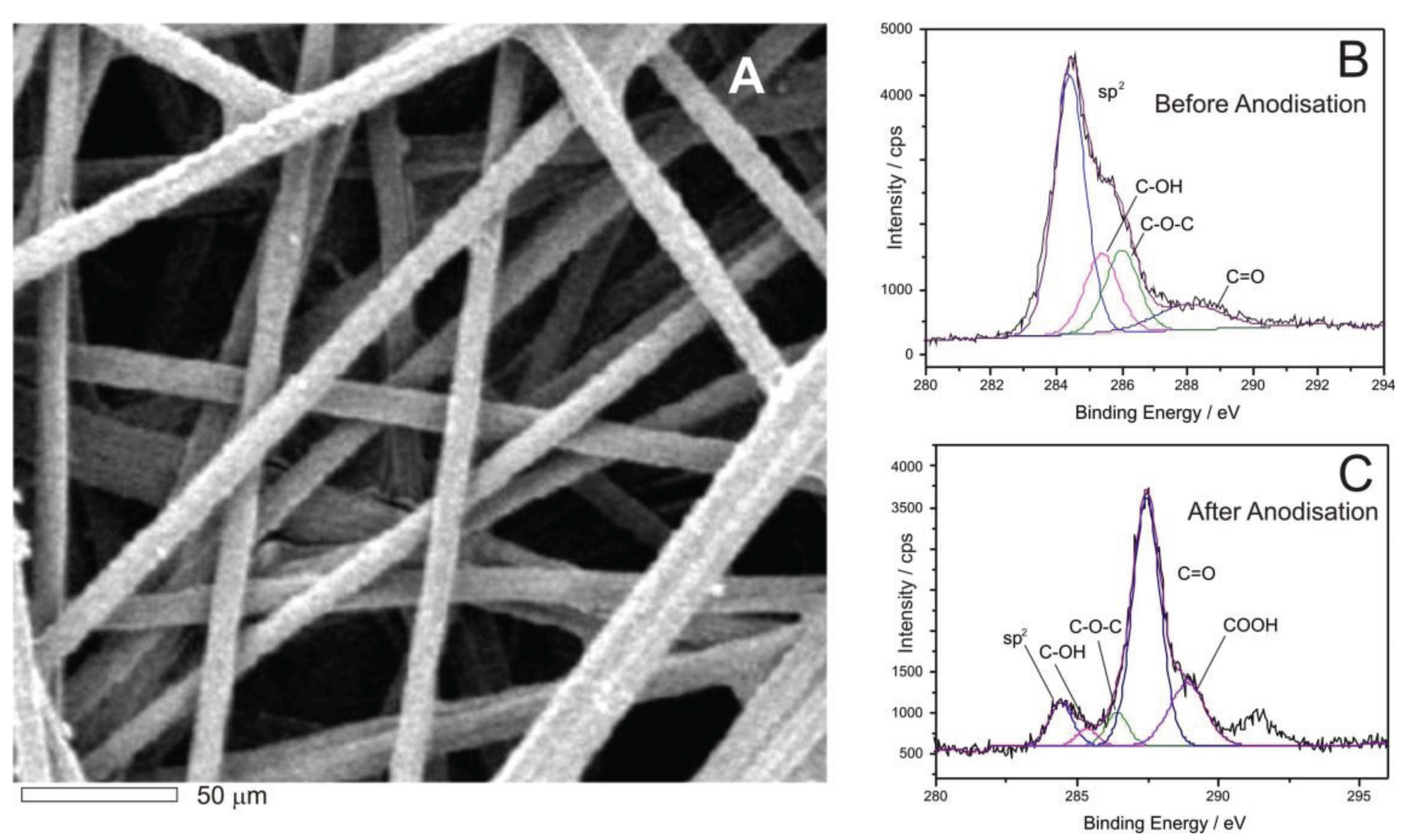
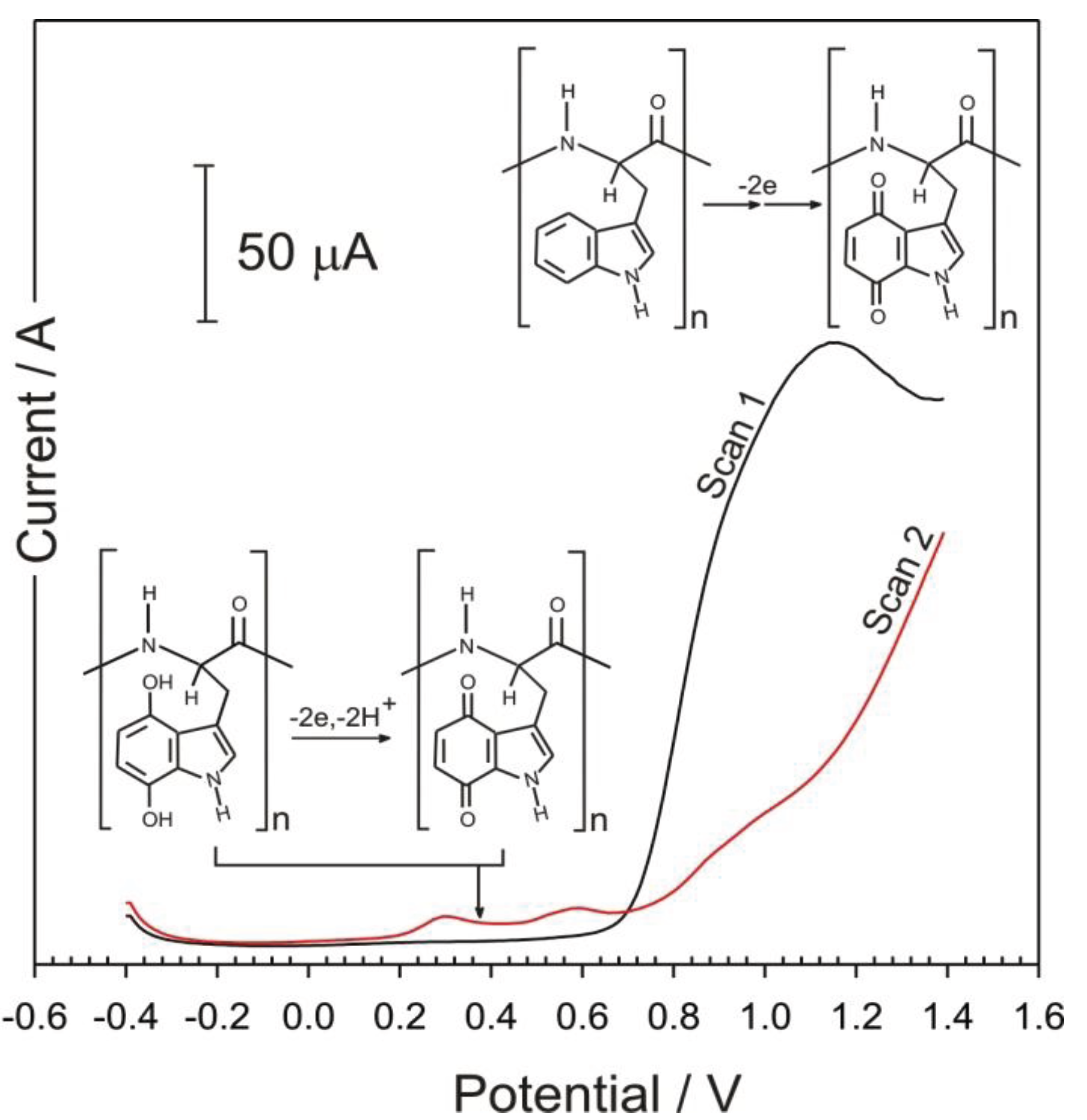
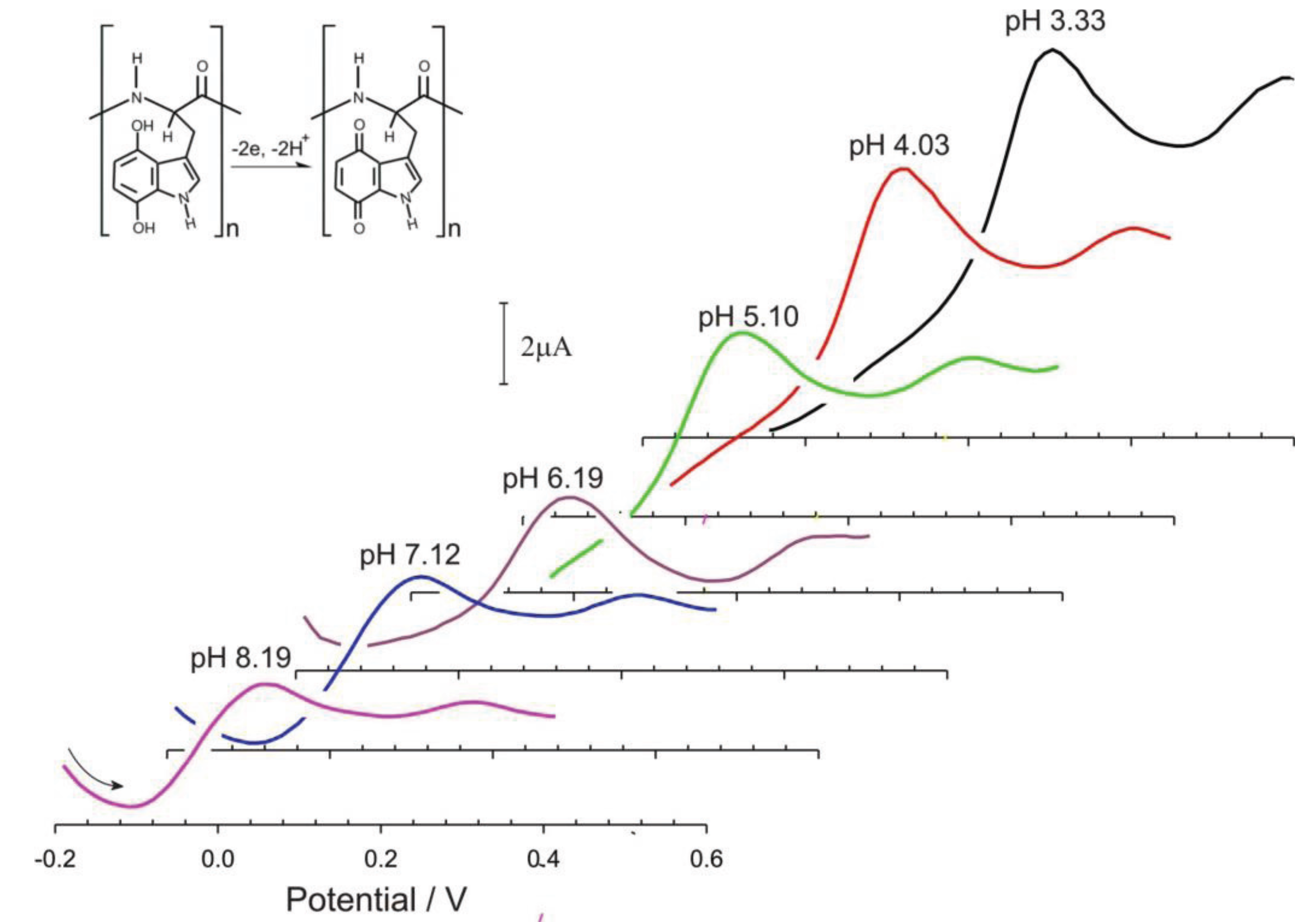
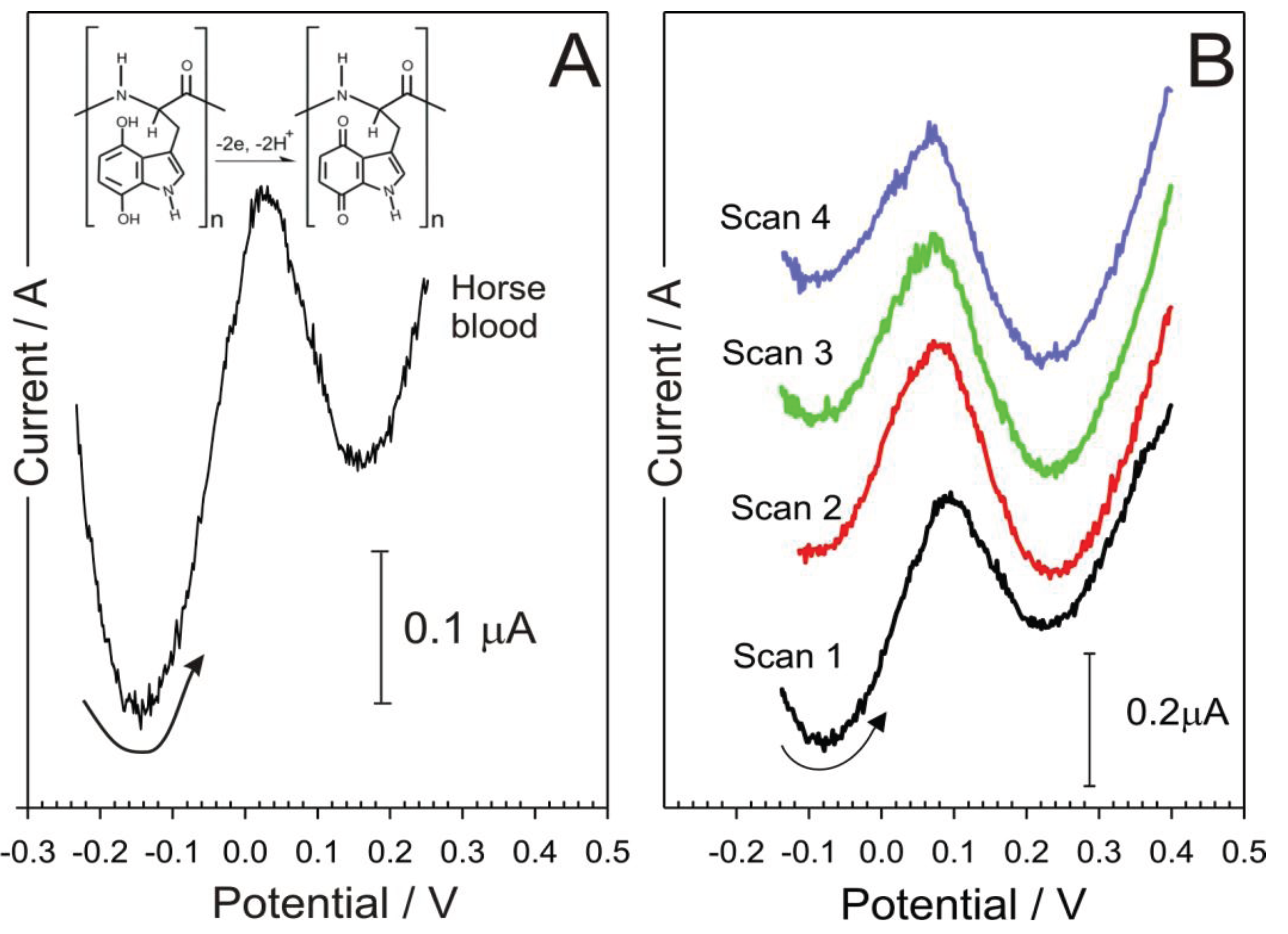
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas, global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabetes Med. 2012, 29, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Fejfarová, V.; Jirkovská, A.; Petkov, V.; Bouc̆ek, P.; Skibová, J. Comparison of microbial findings and resistance to antibiotics between transplant patients, patients on hemodialysis, and other patients with the diabetic foot. J. Diabetes Complicat. 2004, 18, 108–112. [Google Scholar] [CrossRef]
- Foster, A.V.M.; Edmonds, M.E. Diabetic Foot Care: Case Studies in Clinical Management; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yates, C.; May, K.; Hale, T.; Allard, B.; Rowlings, N.; Freeman, A.; Harrison, J.; McCann, J.; Wraight, P. Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcer. Diabetes Care 2009, 32, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLister, A.; Phair, J.; Cundell, J.; Davis, J. Electrochemical approaches to the development of smart bandages: A mini-review. Electrochem. Commun. 2014, 40, 96–99. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Hemmink, G.J.M.; Weusten, B.L.A.M.; Oors, J.; Bredenoord, A.J.; Timmer, R.; Smout, A.J.P.M. Ambulatory oesophageal pH monitoring: A comparison between antimony, ISFET, and glass pH electrodes. Eur. J. Gasteronenterol. Hepatol. 2010, 22, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Taouil, A.; Lallemand, F.; Meolt, J.M.; Husson, J.; Hihn, J.Y.; Lakard, B. Effect of polypyrrole modified electrode functionalization on potentiometric pH responses. Synth. Met. 2010, 160, 1073–1080. [Google Scholar] [CrossRef]
- Carrol, S.; Baldwin, R.P. Self calibrating microfabricated iridium oxide pH electrode array for remote monitoring. Anal. Chem. 2010, 82, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Herlem, G.; Zeggari, R.; Rauch, J.Y.; Monney, S.; Anzola, F.T.; Guillaume, Y.; Andre, C.; Gharbhi, T. One-pot electrosynthesis of polyglycine like thin film of platinum electrodes as transducer for solid state pH measurements. Talanta 2010, 82, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.E.; del Campo, F.J.; Abramova, N.; Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Arcos-Martinez, M.J.; Brivio, M.; Snakenborg, D.; Geschke, O.; Kutter, J.P. Disposable miniaturised screen printed pH and reference electrodes for potentiometric systems. Electroanalysis 2011, 23, 115–121. [Google Scholar] [CrossRef]
- Inoue, T.; Baba, T.; Yuchi, A. Responses of Metalloporphyrin-based ion selective electrodes to pH. Electroanalysis 2011, 23, 536–542. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Zhang, J.; Xu, Z. A novel pH potentiometric sensor based on electrochemically synthesized polybisphenol A films at an ITO electrode. Sens. Actuators B 2011, 155, 730–736. [Google Scholar] [CrossRef]
- Betelu, S.; Polychronopoulou, K.; Rebholz, C.; Ignatiadis, I. Novel CeO2 based screen printed potentiometric electrodes for pH monitoring. Talanta 2011, 87, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Cui, T. Carbon nanotube thin film pH electrode for potentiometric enzymatic acetylcholine biosensing. Microelectron. Eng. 2012, 93, 39–42. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Kats, E.; Kalantar-Zadeh, K.; Breedon, M.; Miura, N. Influence of thickness of submicron Cu2O doped RuO2 electrode on sensing performance of planar electrochemical pH sensors. Mater. Lett. 2012, 75, 165–168. [Google Scholar] [CrossRef]
- Kim, S.; Rim, T.; Kim, K.; Lee, U.; Baek, E.; Lee, H.; Baek, C.K.; Meyyappan, M.; Deen, M.J.; Lee, J.S. Silicon nanowire ion sensitive field effect transistor with integrated Ag/AgCl electrode: pH sensing and noise characteristics. Analyst 2011, 136, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Cherchour, N.; Deslouis, C.; Messaoudi, B.; Pailleret, A. pH Sensing in aqueous solutions using a MnO2 thin film electrodeposited on a glassy carbon electrode. Electrochim. Acta 2011, 56, 9746–9755. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, M.; Wang, J.; Chen, G. A pH sensor based on TiO2 nanotube array modified Ti electrode. Electrochim. Acta 2010, 55, 5647–5651. [Google Scholar] [CrossRef]
- Mignani, A.; Corticelli, C.; Tonelli, D.; Scavetta, E. A new pH sensor based on a glassy carbon electrode coated with a Co/Al layered double hydroxide. Electroanalysis 2011, 23, 1745–1751. [Google Scholar] [CrossRef]
- Hickman, J.J.; Ofer, D.; Laibinis, P.E.; Whitesides, G.M.; Wrighton, M.S. Molecular self assembly of 2-terminal voltammetric microsensors with internal references. Science 1991, 252, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Makos, A.; Omiatek, D.M.; Ewing, A.G.; Heien, M.L. Development and characterisation of a voltammetric carbon fiber microelectrode pH sensor. Langmuir 2010, 26, 10386–10391. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Batchelor-McAuley, C.; Compton, R.G. Calibrationless pH sensors based on nitrosophenyl and ferrocenyl co-modified screen printed electrodes. Sens. Actuators B 2011, 159, 251–255. [Google Scholar] [CrossRef]
- Park, W.; Kim, S. Triggerable single component two electrode voltammetric pH sensors using dyad molecules. Electrochem. Commun. 2013, 26, 109–112. [Google Scholar] [CrossRef]
- Streeter, I.; Leventis, H.C.; Wildgoose, G.G.; Pandurangappa, M.; Lawrence, N.S.; Jiang, L.; Jones, T.G.; Compton, R.G. A sensitive reagentless pH probe with a ca. 120 mV/pH unit response. J. Solid State Electrochem. 2004, 8, 718–721. [Google Scholar] [CrossRef]
- Heald, C.G.R.; Wildgoose, G.G.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Chemical derivatisation of multiwalled carbon nanotubes using diazonium salts. Chemphyschem 2004, 5, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, V.G.H.; Wang, W.X.; Yashina, A.S.; Lawrence, N.S. Anthraquinone-ferrocene film electrodes: Utility in pH and oxygen sensing. Electrochem. Commun. 2008, 12, 1831–1834. [Google Scholar] [CrossRef]
- Davis, J.; Molina, M.T.; Leach, C.P.; Cardosi, M.F. Plasma-polyplumbagin modified microfiber probes: A functional material approach to monitoring vascular access line contamination. ACS 2013, 8, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.A.; Xia, F.; Pan, M.; Mu, S.; Dale, S.E.C.; Tsang, S.C.; Hammett, F.W.; Bowen, C.R.; Marken, F. Voltammetric probing of pH at carbon nanofiber-Nafion—Carbon nanofiber membrane electrode assemblies. Electrochim. Acta 2012, 62, 97–102. [Google Scholar] [CrossRef]
- Fierro, S.; Mitani, N.; Comninellis, C.; Einaga, Y. pH sensing using boron doped diamond electrodes. Phys. Chem. Chem. Phys. 2011, 13, 16795–16799. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Phair, J.; Cardosi, M.F.; Davis, J. Nanostructuring carbon fibre probes for use in central venous catheters. Anal. Chim. Acta 2014, 812, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Phair, J.; Newton, L.; McCormac, C.; Cardosi, M.F.; Leslie, R.; Davis, J. A disposable sensor for point of care wound pH monitoring. Analyst 2011, 136, 4692–4695. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Pathways of electrochemical oxidation of indolic compounds. Electroanalysis 2011, 23, 1337–1344. [Google Scholar] [CrossRef]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. CheapStat: An open-source, “Do-It-Yourself” potentiostat for analytical and educational applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef] [PubMed]
- Phair, J.; Joshi, M.; Benson, J.; McDonald, D.; Davis, J. Laser patterned carbon-polyethylene mesh electrodes for wound diagnostics. Mater. Chem. Phys. 2014, 143, 991–995. [Google Scholar] [CrossRef]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Anderson, A.; Phair, J.; Benson, J.; Meenan, B.J.; Davis, J. Investigating the use of endogenous quinoid moieties on carbon fibre as means of developing micro pH sensors. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 43, 533–537. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLister, A.; Davis, J. Molecular Wiring in Smart Dressings: Opening a New Route to Monitoring Wound pH. Healthcare 2015, 3, 466-477. https://doi.org/10.3390/healthcare3030466
McLister A, Davis J. Molecular Wiring in Smart Dressings: Opening a New Route to Monitoring Wound pH. Healthcare. 2015; 3(3):466-477. https://doi.org/10.3390/healthcare3030466
Chicago/Turabian StyleMcLister, Anna, and James Davis. 2015. "Molecular Wiring in Smart Dressings: Opening a New Route to Monitoring Wound pH" Healthcare 3, no. 3: 466-477. https://doi.org/10.3390/healthcare3030466