Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management
Abstract
:1. Introduction
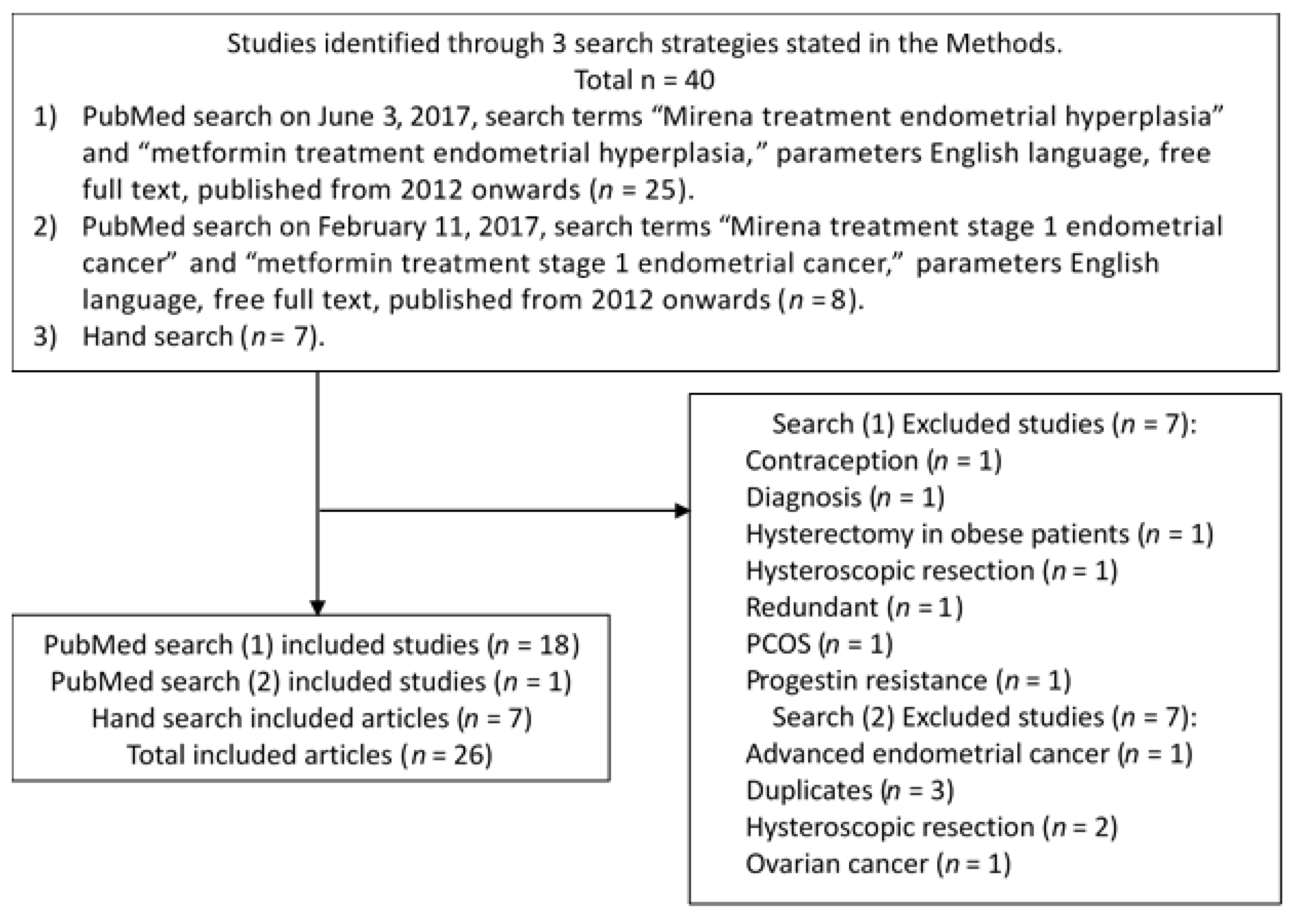
2. Materials and Methods
3. Results
3.1. Intrauterine Progestins
3.1.1. Progestins’ Mechanism of Action
3.1.2. Early Stage Endometrial Cancer Treatment: 52 mg-, 60 mg-, and 60 mg-frameless-IUDs
3.1.3. Endometrial Hyperplasia Treatment: 52 mg-, 60 mg-, and 60 mg-frameless-IUDs
3.1.4. Prophylaxis for Tamoxifen-Induced Endometrial Lesions: 52 mg-IUD/10–20 mcg-LNG-14t
3.1.5. Limitations: 52 mg-IUD/10–20 mcg-LNG-14t
3.1.6. Benefits: 52 mg-, 60 mg-, and Frameless-IUDs
3.2. Metformin for Endometrial Hyperplasia Treatment
3.2.1. Metformin: Mechanism of Action
3.2.2. Metformin: Single Agent
3.2.3. Metformin with Cyproterone/Ethinyl Estradiol 2 mg/35 mcg
3.2.4. Metformin with Megestrol Acetate versus Single Agent Megestrol Acetate
3.2.5. Metformin: Limitations
4. Future Research
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. The Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, Z.; Park, J.Y.; Guo, D.; Liao, H.; Yi, X.; Zheng, Y.; Zhang, D.; Chambers, S.K.; et al. Mechanism of progestin resistance in endometrial precancer/cancer through Nrf2-AKR1C1 pathway. Oncotarget 2016, 7, 10363–10372. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group. Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, U.; Quereshi, S.; Srivastava, P.; Goel, M. Clinicopathological profile of endometrial hyperplasia and endometrial carcinoma. Glob. J. Res. Anal. 2016, 5, 3. [Google Scholar]
- Clement, N.S.; Oliver, T.R.; Shiwani, H.; Saner, J.R.; Mulvaney, C.A.; Atiomo, W. Metformin for endometrial hyperplasia: A Cochrane protocol. BMJ Open 2016, 6, e013385. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.L.; Seong, S.J. Clinical applications of levonorgestrel-releasing intrauterine system to gynecologic diseases. Obstet. Gynecol. Sci. 2013, 56, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Y.R.; Lin, J.F.; Feng, Y.; Billig, H.; Shao, R. Combination of Diane-35 and metformin to treat early endometrial carcinoma in PCOS women with insulin resistance. J. Cancer 2014, 5, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Nevadunsky, N.S.; Van Arsdale, A.; Strickler, H.D.; Moadel, A.; Kaur, G.; Frimer, M.; Conroy, E.; Goldberg, G.L.; Einstein, M.H. Metformin use and endometrial cancer survival. Gynecol. Oncol. 2014, 132, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Schmandt, R.; Celestino, J.; McCampbell, A.; Yates, M.S.; Urbauer, D.L.; Broaddus, R.; Loose, D.S.; Shipley, G.L.; Lu, K.H. CGRRF1 as a novel biomarker of tissue response to metformin in the context of obesity. Gynecol. Oncol. 2014, 133, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, V.N.; Kitson, S.; McVey, R.; Roberts, C.; Pemberton, P.; Gilmour, K.; Ali, S.; Renehan, A.G.; Kitchener, H.C.; Crosbie, E.J. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br. J. Cancer 2016, 114, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Westin, S.N.; Broaddus, R.R.; Schmeler, K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet. Gynecol. 2012, 119, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, A.D.; Melli, M.S.; Foroughi, M.; Ghojazadeh, M.; Bidadi, S. Antiproliferative effect of metformin on the endometrium–a clinical trial. Asian Pac. J. Cancer Prev. 2014, 15, 10067–10070. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Seong, S.J. Conservative treatment for atypical endometrial hyperplasia: What is the most effective therapeutic method? J. Gynecol. Oncol. 2014, 25, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Wildemeersch, D.; Andrade, A.; Goldstuck, N.D.; Hasskamp, T.; Jackers, G. Intrauterine levonorgestrel delivery with frameless fibrous delivery system: Review of clinical experience. Int. J. Womens Health 2017, 21, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wildemeersch, D.; Andrade, A.; Goldstuck, N. Femilis® 60 Levonorgestrel-Releasing Intrauterine System-A Review of 10 Years of Clinical Experience. Clin. Med. Insights Reprod. Health 2016, 10, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Wang, C.; Zhang, Z.; Gu, C.; Ning, C.; Luo, X.; Zhou, Q.; Chen, X. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J. Gynecol. Oncol. 2014, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Orbo, A.; Vereide, A.; Arnes, M.; Pettersen, I.; Straume, B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: A national multicentre randomised trial. BJOG 2014, 121, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ørbo, A.; Arnes, M.; Vereide, A.B.; Straume, B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. BJOG 2016, 123, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Behnamfar, F.; Ghahiri, A.; Tavakoli, M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J. Res. Med. Sci. 2014, 19, 686–690. [Google Scholar] [PubMed]
- Abu Hashim, H.; Zayed, A.; Ghayaty, E.; El Rakhawy, M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: A randomized controlled trial. J. Gynecol. Oncol. 2013, 24, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Janssens, D.; Verbeeck, G.; Wildemeersch, D. Use of a frameless LNG-IUS as conservative treatment for a pre-malignant uterine polyp in a premenopausal woman—A case report. Facts Views Vis. Obgyn 2015, 7, 257–260. [Google Scholar] [PubMed]
- Fu, Y.; Zhuang, Z. Long-term effects of levonorgestrel-releasing intrauterine system on tamoxifen-treated breast cancer patients: A meta-analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6419–6429. [Google Scholar] [PubMed]
- Stanczyk, F.Z.; Hapgood, J.P.; Winer, S.; Mishell, D.R. Progestogens used in postmenopausal hormone therapy: Differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr. Rev. 2013, 34, 171–208. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Owen, G.I. Glucose transporters: Expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, A.L.; Quinn, M.; Gebski, V.; Armes, J.; Brennan, D.; Janda, M.; Obermair, A. Improving treatment for obese women with early stage cancer of the uterus: Rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp. Clin. Trials 2014, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
| Source | Population | BMI | Diagnosis | Method | Treatment | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age | (kg/m2) | 3-Months | 6-Months | 12-Months | Other | |||||
| [7] | 5 - women | - | EC | - | 52 mg-IUD/10–20 mcg-LNG-14t + MPA 500 mg daily | - | - | - | 10.2 months: 80% remission | |
| [8] | 5- 29 y.o. | - | Stage 1A EC | Curettage | Diane-35 + metformin × 6 months | - | 100% regression | - | - | |
| [11] | 28- 63.6 y.o. | 35 | Atypical EH, EC | Pipelle EMB | Metformin, 850 mg 2 × daily, × 20 days | - | - | - | 17.2% reduced Ki-67 expression | |
| [12] | 18 y.o. P0 | 47.7 | Grade 2 EAC | D&C | 5yr-IUD | - | - | - | 13-months: Disease-free | |
| [13] | 22- women | - | 8- Simple EH, 9- DPE, 3- CH 2- low grade EC | - | Metformin, 500 mg 2 × daily | 95.5% regression | - | - | - | |
| [15,16] | 21- 54 y.o. | - | 12- simple EH 8- atypical EH 1-moderately differentiated EAC | Pipelle EMB or D&C | 60 mg-LD-frameless-IUD/14 mcg-LNG × 3-years, then 60 mg-HD-frameless-IUD/20 mcg-LNG | - | - | - | 10-year remission: 100% | |
| [17] | 8 women | - | Atypical EH | D&C | Metformin 500 mg 3 × daily + megestrol 160 mg daily | 75% regression | - | - | - | |
| [18] | 53 women | - | 6- simple EH 41- CH, 6- ACH | Pipelle EMB | 52 mg-IUD/10–20 mcg-LNG-14t | - | 100% regression | - | - | |
| [19] | 53 women | - | EH | Pipelle EMB | 52 mg-IUD/10–20 mcg-LNG-14t × 6-months | - | - | - | 2-year relapse: 41% | |
| [20] | 28- 38.3 ± 5.1 y.o. | 26.5 ± 3.4 | EH | Pipelle EMB | 52 mg-IUD/10–20 mcg-LNG-14t | 89.3% | - | - | Progression: 0 | |
| [21] | 59- 45.2 ± 1.7 y.o. | 31.6 ± 2.8 | 5- simple EH 54-complex EH | Hysteroscopy D&C | 52 mg-IUD/10–20 mcg-LNG-14t | 67.88% regression | 79.7% regression | 88.1% regression | Hysterectomy rate: 22% | |
| [22] | 44 y.o. | - | Grade 3 EIN | Hysteroscopy D&C | 60 mg-IUD/14 mcg-LNG | - | - | - | 12-years: Endometrial atrophy | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwanodi, O. Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management. Healthcare 2017, 5, 30. https://doi.org/10.3390/healthcare5030030
Nwanodi O. Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management. Healthcare. 2017; 5(3):30. https://doi.org/10.3390/healthcare5030030
Chicago/Turabian StyleNwanodi, Oroma. 2017. "Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management" Healthcare 5, no. 3: 30. https://doi.org/10.3390/healthcare5030030






