1. Introduction
Chronic kidney disease is the progressive failure of renal function over a period of years. In its end-stage, renal replacement therapies (RRT) such as hemodialysis (HD), peritoneal dialysis (PD), or kidney transplants are required to supplement the metabolic homeostatic functions of the kidney. The aging of the Canadian population is reflected in the demographic profile of new end-stage kidney diseases (ESKD) patients: 53% of those who initiated RRT in 2010 were aged 65 years and older, compared to 39% in 1991 [
1].
People with ESKD on HD experience multiple catabolic processes, including loss of albumin and amino acids during dialysis, metabolic derangements, and changes in skeletal muscle associated with conditions of muscle disuse [
2]. These changes result in muscle atrophy (loss of lean muscle mass). The presence of neurogenic (muscle atrophy or loss associated with nerve disorder), myogenic (damage intrinsic to the muscle), and mixed (neurogenic and myogenic) changes intrinsic to the skeletal muscle in people with ESKD on HD [
3] may further compromise the integrity of the motor-unit complex and contribute to muscle atrophy [
4]. As a result, such changes can lead to overall decreases in gait and mobility [
5].
People with ESKD on HD are also known to have renal osteo-dystrophy [
6] predisposing them to increased risk for falls [
7,
8] and long bone fractures [
9]. This incidence of falls in people on HD is higher than their non-uremic community-dwelling counterparts; for the community-dwelling older adults, the fall rate ranges from 0.32 to 0.70 fall/person-year [
10,
11] whereas the same amongst people requiring HD ranges from 1.18 [
7] to 1.6 [
8] falls per person-year. Since falls commonly predict morbidity, mortality and perhaps need for institutional care, it is important to initiate appropriate interventions in a timely manner to prevent falls and related consequences [
11].
The deleterious consequences of falls are not restricted to wounds, fractures, hospitalization, or death. Post-fall anxiety syndrome and fear of falling again result in a loss of self-confidence and self-restriction of activity creating a vicious circle leading to reduced exercise and muscle mass. Falls also are a leading cause of admission to nursing homes. Finally, the cost inherent to falls is substantial: they account for 6% of all medical expenditures in non-uremic patients 65 years or older [
10].
Walking speed (WS) or gait speed (GS) is a well-recognized prognostic factor in the geriatric population. It provides information on patient outcomes and hospitalization risk [
12]. It has been considered a reliable and sensitive outcome to measure functional abilities, and ability to predict future health status and quality of life. Fritz and Lusardi [
13] argue that GS can be used as a functional “vital sign” to help determine risk for falls in people on HD [
14], and outcomes such as functional status [
15,
16], discharge location [
17], and the need for rehabilitation in geriatric population [
18]. The clinical findings of impaired GS may alert the health care professionals to initiate appropriate exercise interventions and/or introduce suitable gait-aids for that individual. Therefore, it is essential to understand the impact of HD on spatial (distance walked) or temporal (gait speed) characteristics of gait. The primary objective of this study was to determine the effects of HD on spatio-temporal gait parameters. The secondary objective was to review the effect of exercises on these parameters within the cluster of studies selected for this review.
2. Materials and Methods
2.1. Literature Search
Electronic databases (PubMed, Medline, Embase, EBSCO, and Scopus) were searched from their inception until May 2017, using different strategies that encompass the wide range of gait parameters that may be affected by HD. The following keywords were searched as MeSH or “mapped terms” and as text words: (chronic OR end-stage) AND (kidney OR renal) AND (disease OR failure) AND (dialysis OR hemodialysis OR haemodialysis) AND (walk OR walking OR ambulation OR ambulatory OR ambulate OR gait) and (speed OR velocity).
2.2. Inclusion Criteria
The inclusion criteria were as follows: (a) the paper was published in the English language; (b) participants on HD were adults; (c) one of the outcome measures measured spatial or temporal parameters of gait (d) full text articles was available.
2.3. Exclusion Criteria
The exclusion criteria were the following: (a) study participants on peritoneal dialysis or any other form of renal replacement therapy other than HD; (b) animal studies or trials; (c) case studies or literature reviews.
2.4. Outcome Measures
Outcome measures such as GS, timed up-and-go test (TUG) [
19], six minute walk test (6MWT) [
20], and the intermittent shuttle walk test (ISWT) [
21], were considered as appropriate outcome measures for inclusion in this review. All gait speed measurements that were reported in formats other than meters per second (m/s) were adjusted to meet this standardized unit of GS as m/s.
2.5. Data Collection and Analysis
Following the primary search, the primary authors independently reviewed the titles, abstracts and full texts to establish the inclusion of the study for this review. Discussions in case of discrepancy were adequate for resolution. All articles were included by consensus.
Required data for calculations of cumulative means and the 95% confidence interval (CI) around the mean were extracted using a standardized form, from the included studies by one author and reviewed by the second author for accuracy. In studies with experimental designs baseline values of the outcome measures in various groups was included for estimation of effect of HD.
2.6. Statistical Analyses
A statistical package, Comprehensive Meta-Analysis (Version 2.2.064, Biostat, Englewood, NJ, USA) software for meta-analysis of binary, continuous and diagnostic data, was used for computation of the cumulative means. The confidence interval at a 95% confidence limit was constructed around the point estimate of the cumulative mean.
The results reported were calculations using the random effects model to account for methodological differences amongst studies. The significance level for all statistical tests evaluating the effect of exercises or comparison with normal was set at p < 0.05.
4. Discussion
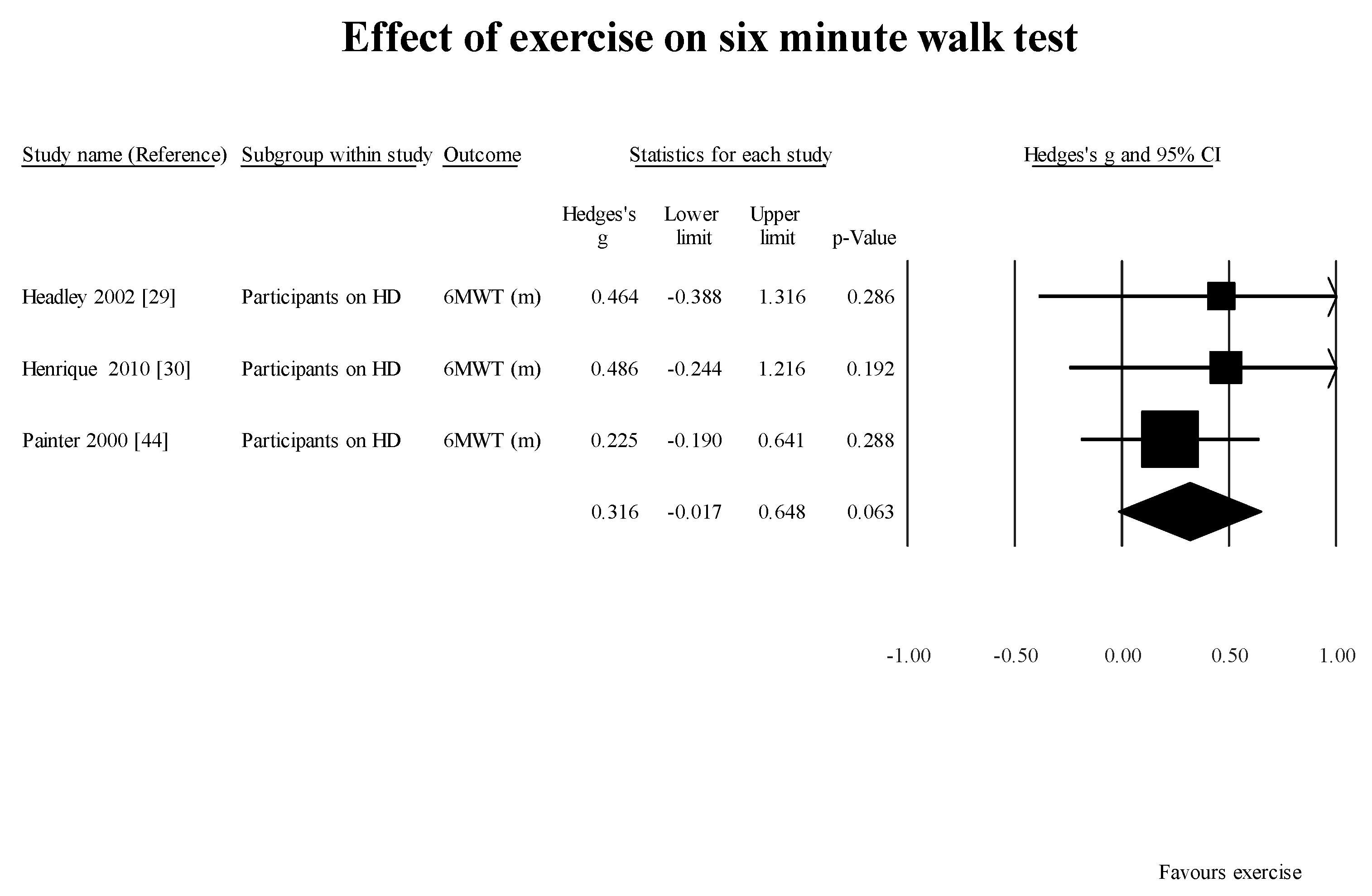
The results of this systematic review confirm the earlier findings of reduced GS (normal walking) and distance walked in six minutes by participants on HD in the literature. The mean dialysis vintage of the participants included in the studies reporting dialysis vintage was 53 months. This indicates that within a period of 53 months, people on HD have a significantly slower gait speed when compared to an age-matched population without ESKD. One of the studies [
47] selected in this review did not report the dialysis vintage of the participants in the study. According to Roshanravan et al. [
48], GS measured with a 4-m walk test in participants with CKD stages 2 to 4 was about 30% lower than predicted. Hence, at this time it is unclear whether the participants who progressed to requiring HD continued to decline after the treatment was started. Further longitudinal studies are required to elucidate the effect of HD on GS as a function of time.
We evaluated the effect of whole body fluid loss following HD on tibialis anterior (TA) strength and water content [
49]. Overall, a significant reduction (
p < 0.05) in peak strength by 1.54 Nm (95% CI: 0.05, 3.02), and extra cellular fluid (ECF) (measured using transverse relaxation times on magnetic resonance spectroscopy (T
2) shortened by 2.38 ms; 95% CI: 1.04, 3.71) of TA were observed between before- and after-HD measurements. Based on these findings, we recommended that deteriorating muscle functions such as gait and mobility should be added as a symptom of chronic dehydration, requiring further assessment of dry weight of a HD patient. This is important, as reduction in muscle strength has been associated with whole-body fluid loss [
50], and Edwards et al. [
51] suggest that a weaker muscle, unable to meet functional demands such as standing or walking made upon it, leads to further muscle weakness.
Although we set out to evaluate the effect of HD on GS, we did not find any longitudinal study reporting the time frame following HD when measurable reductions in GS and/or mobility occur. However, we recommend that gait assessments be conducted at three-month intervals as Johansen and colleagues [
28] have demonstrated a decrement in the Human Activity Profile (HAP) Questionnaire (measure of self reported physical activity) adjusted activity score (HAP–AAS) scores in HD participants at a follow-up data collection after three months.
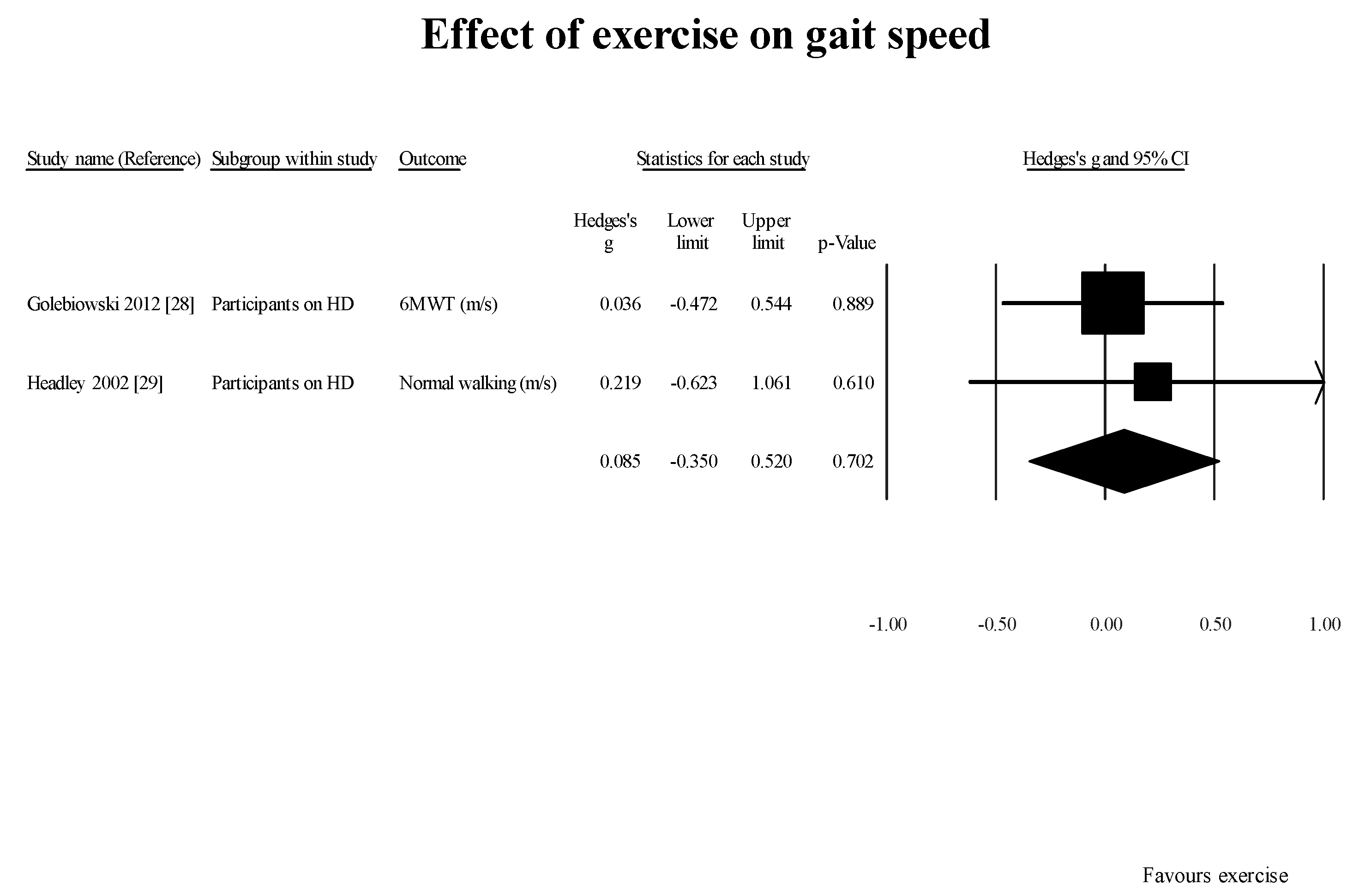
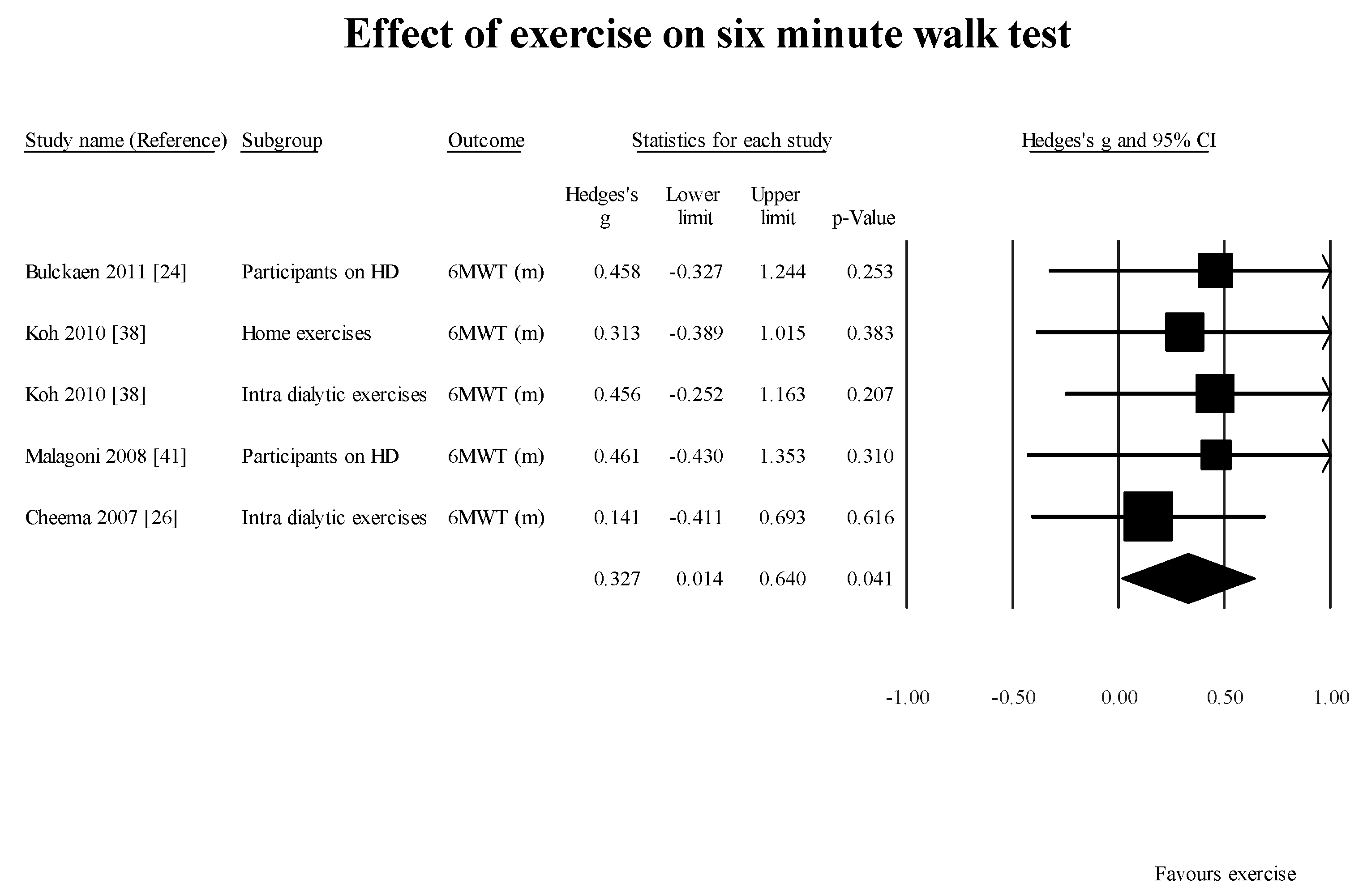
Two [
46,
47] of the six case-control studies evaluating the effect of exercise established a significant increment in GS. These findings are in alignment with what has been reported in the literature [
52,
53]. However, adherence and uptake of these exercises is limited [
54]. Hence, it is important to identify people on HD with deteriorating GS or self-reported physical function. These findings can lead to initiating multi-disciplinary intervention to optimize patient’s functional abilities, reduce the risk for falls and related co-morbidities.
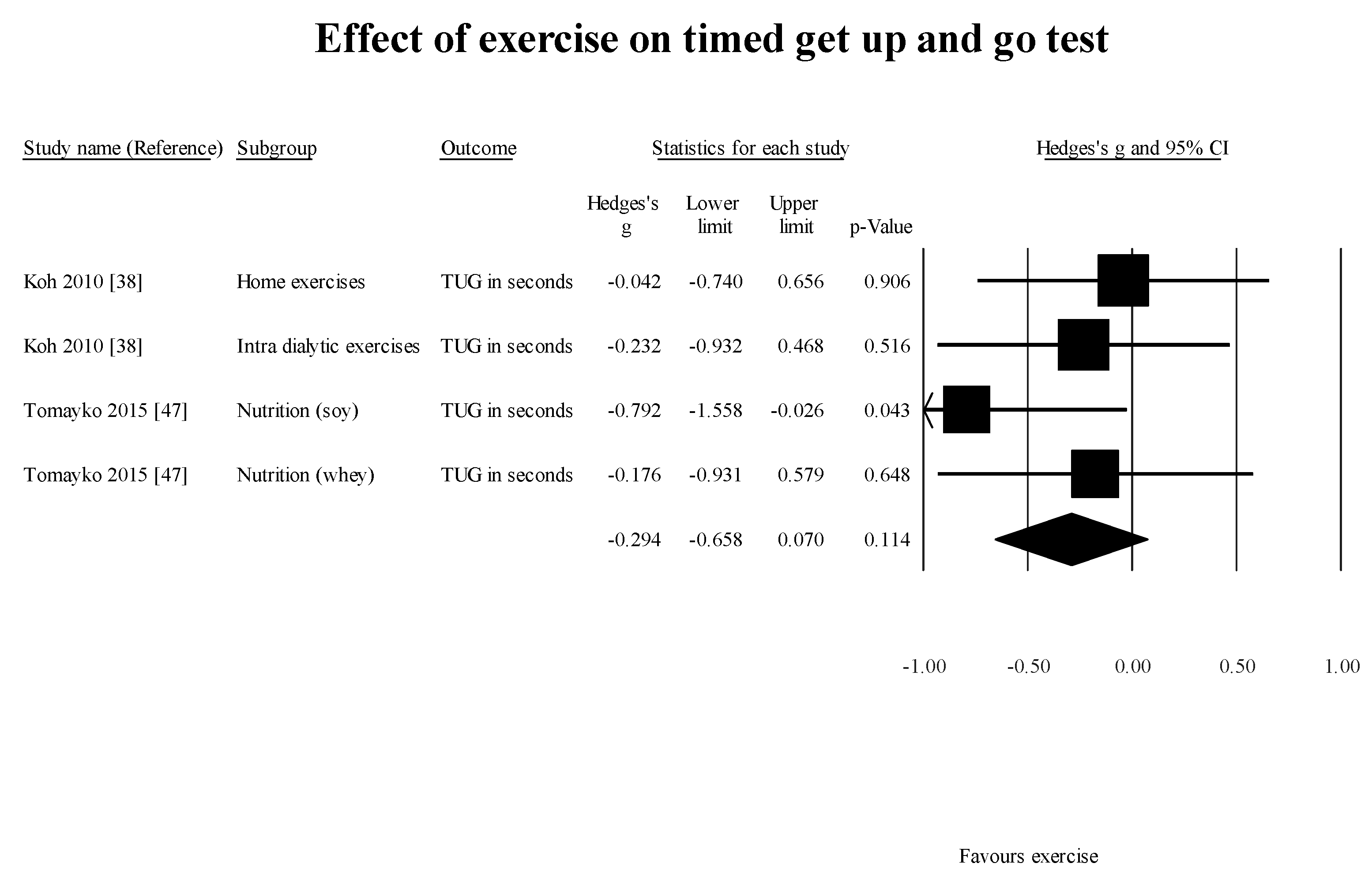
Another factor requiring further investigation is whether supervised exercise is superior to home-based exercise for improving GS among these patients. All the case-control studies included in this review did not conclusively determine if home-based exercise program or supervised exercise programs are superior. Tomayako et al. [
47] indicated that a supervised exercise program resulted in improved gait speed, whereas Koh et al. [
38] suggested that home-based exercises were as effective as supervised exercise programs.
Donat and Özcan [
55] compared the effectiveness of supervised group exercise and unsupervised home exercise programs on parameters related to risk of falls among older adults. Both groups showed significant improvements in balance, but the supervised group also showed significant improvement in strength and proprioception, which are important, factors in overall postural control. There are several reasonable hypotheses as to why supervised exercise interventions produce more favorable outcomes compared to home-based exercise programs. In a supervised exercise class, the patient will engage in social interaction with other participants, which will improve mood, attitude and motivation which can be depressed in patients with a chronic illness such as ESKD requiring maintenance HD [
56]. Especially for some elderly patients who may have become more socially isolated, this camaraderie can allow them to interact with others who share the same experiences in everyday life. During supervised exercise, patients will also have the advantage of receiving complete or semi-personalized attention in order to ensure that the intervention is being applied correctly in order to maximize results.
This finding is extremely relevant in today’s political environment. The Canadian provincial governments have decided to promote self-management as the main intervention for chronic disease in order to relieve some of the burden on the health care system. Using the Stanford program (USA) and the Expert Patient Programmed (UK) as models, the Ontario (Canada) government conducted a systematic review in 2008 in order to determine the efficacy of self-management strategies in chronic diseases [
57]. The intervention involved patient education and counseling in order to inform patient about their disease and how to manage the symptoms. The aim is that patients will then take on the responsibility of managing their disease under self-supervision after the initial education. However, our results, at least in the chronic disease of ESKD on HD, show that relying on the patient as the sole supervisor of their own health and wellness program does not produce favorable results, as evidenced by ongoing deterioration of GS and distance walked. We believe that self-management in combination with periods of supervised intervention may be more beneficial when new deficits of gait or new challenges arise particularly in patients with ESKD/HD. Access to appropriate health professionals in a timely manner will help address the gait and mobility impairments, reduce the risk of falls and related consequences and in turn save valuable dollars associated with hospitalizations and need for institutional care. Further research is required to elucidate the benefits of supervised versus home-based exercise programs leading to clinically meaningful changes in GS and distances walked.
Limitations
We have comprehensively reviewed the effect of chronic hemodialysis on physical function. We have not reviewed several factors that can impact the outcome of the studies. For example, the relative and absolute reliability of the outcome measures require consideration when used in studies utilizing repeated measures designs. We established that for a stable estimate of 6MWT, a minimum of two measures are required [
58]. None of the studies included in this review collected the measure on two occasions for stable baseline value of the measure. We did not find any study that evaluated the relative and absolute reliability of the GS, or TUG in the population of interest.
The effect of whole-body fluid loss following HD may impact the assessment of the functional outcome measure collected. Hence, it is important to report the time of data collection in relation to the participants’ HD treatment schedule; e.g., data was collected on non-dialysis day, or just prior to HD treatment. Most of the studies included here did not categorically indicate the time of data collection.
It was not the intent of this review to comprehensively evaluate the effect of exercise/nutrition on gait parameters. We have reviewed the studies reporting the benefits of exercise /nutrition within the cluster of studies selected for addressing the primary objective of looking at the effect of HD on spatio-temporal gait parameters.