Fungi Active Microbial Metabolism Detection of Rhizopus sp. and Aspergillus sp. Section Nigri on Strawberry Using a Set of Chemical Sensors Based on Carbon Nanostructures
Abstract
:1. Introduction
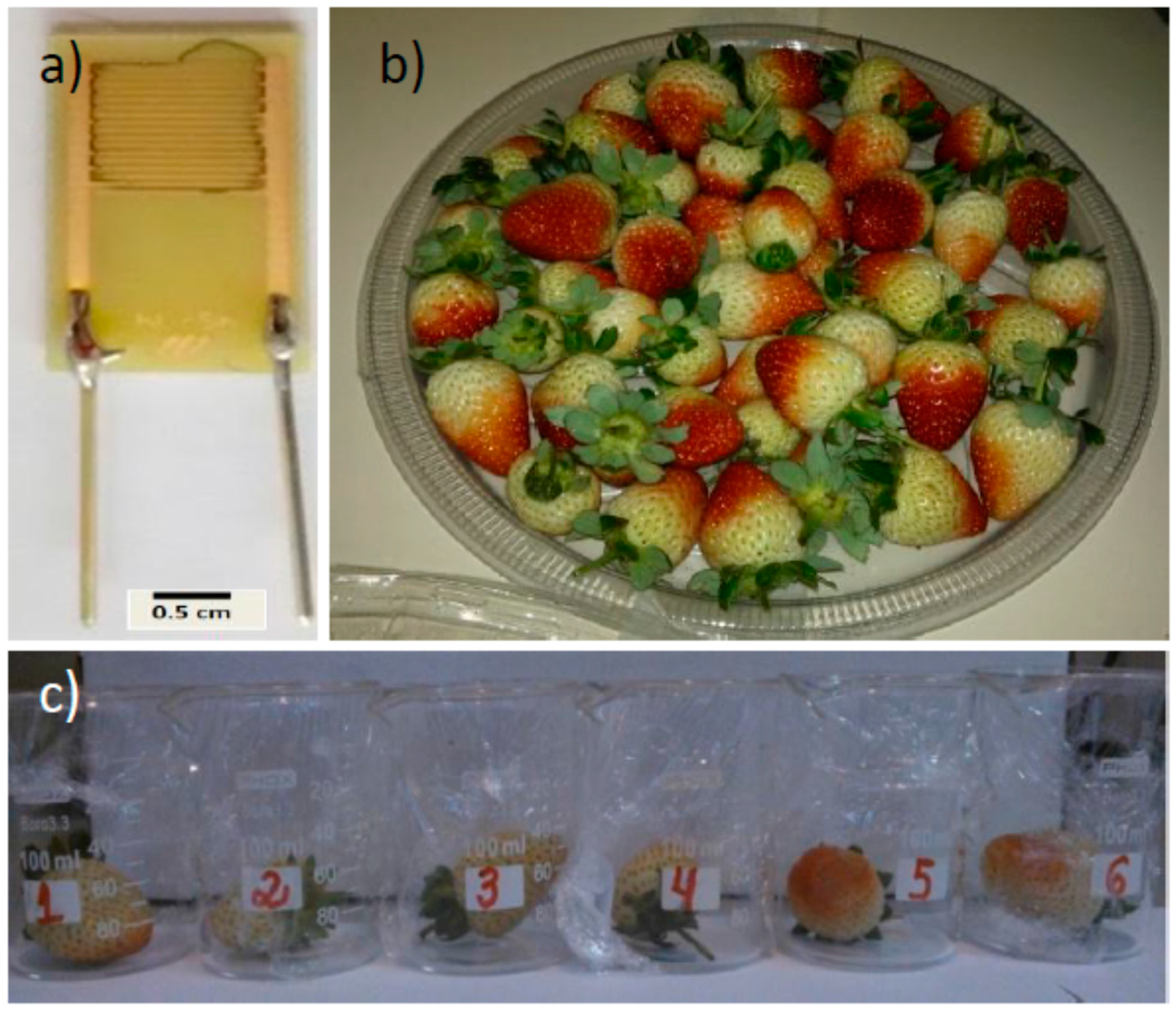
2. Materials and Methods
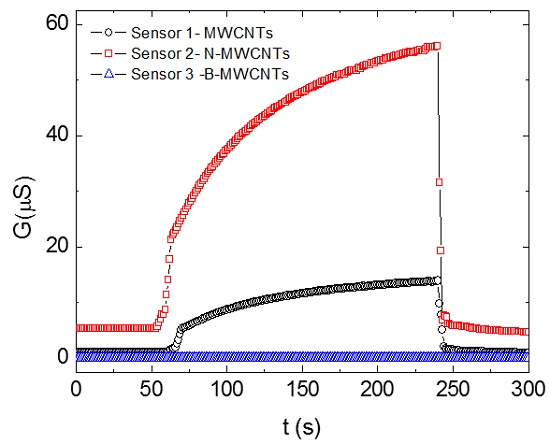
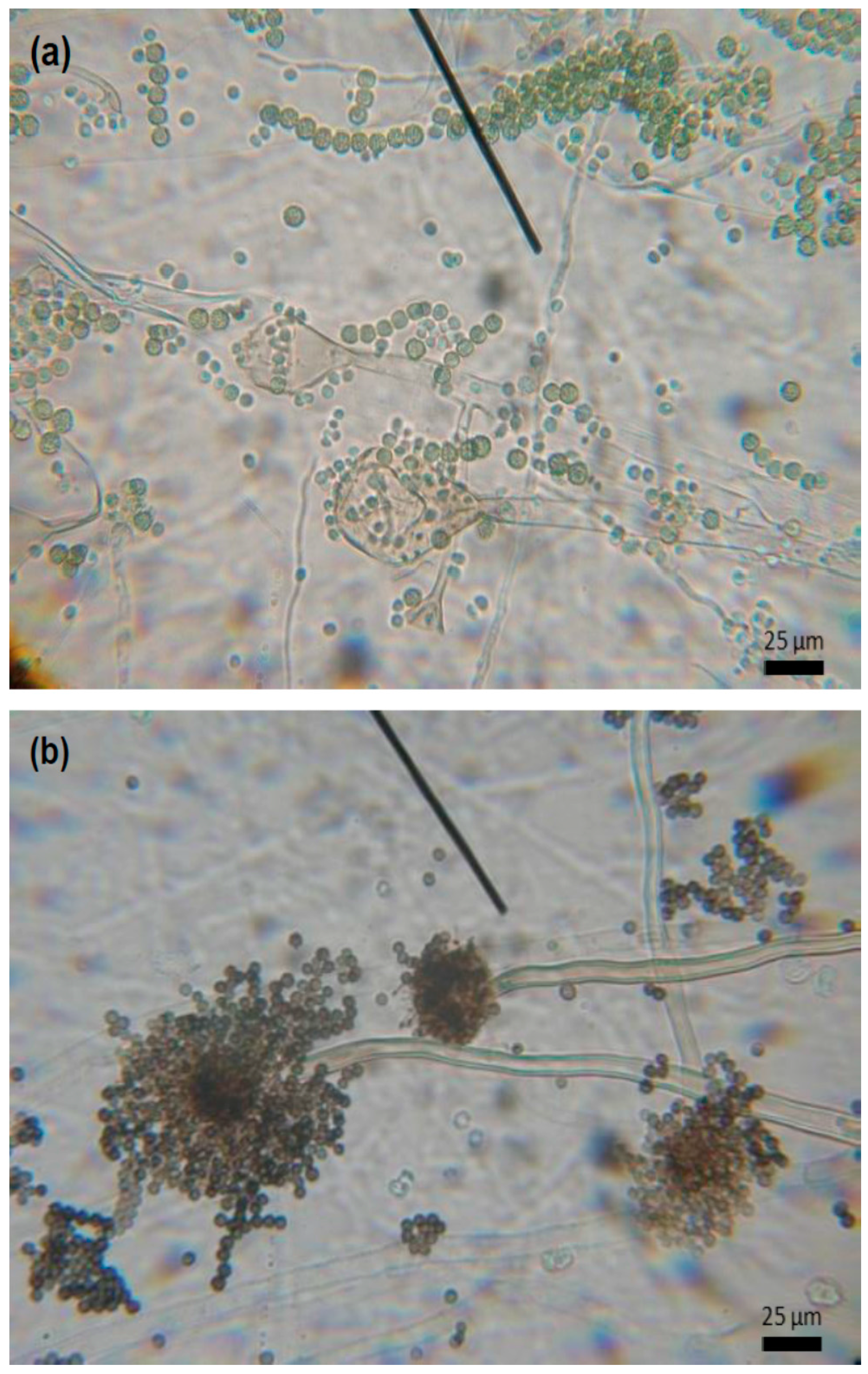
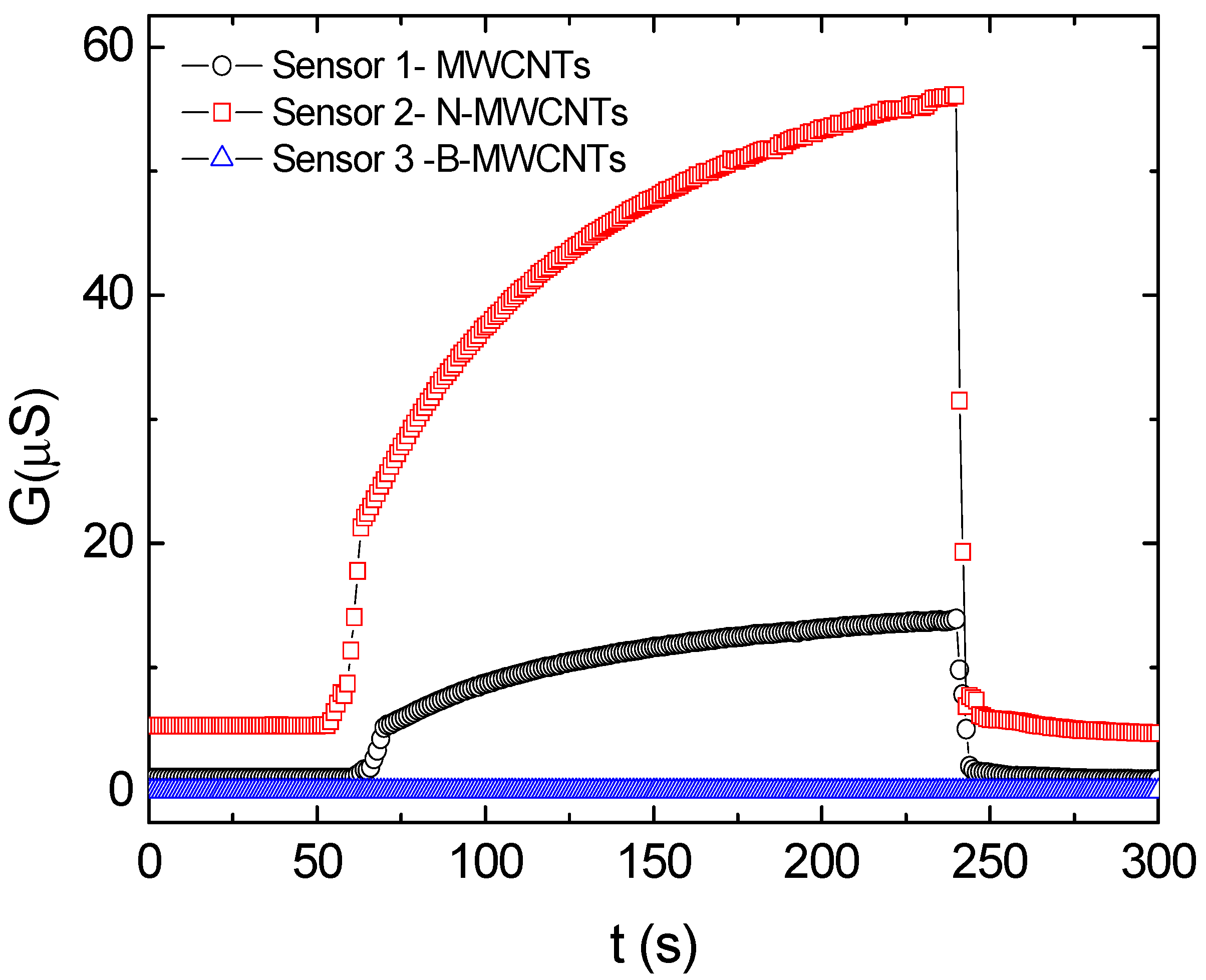
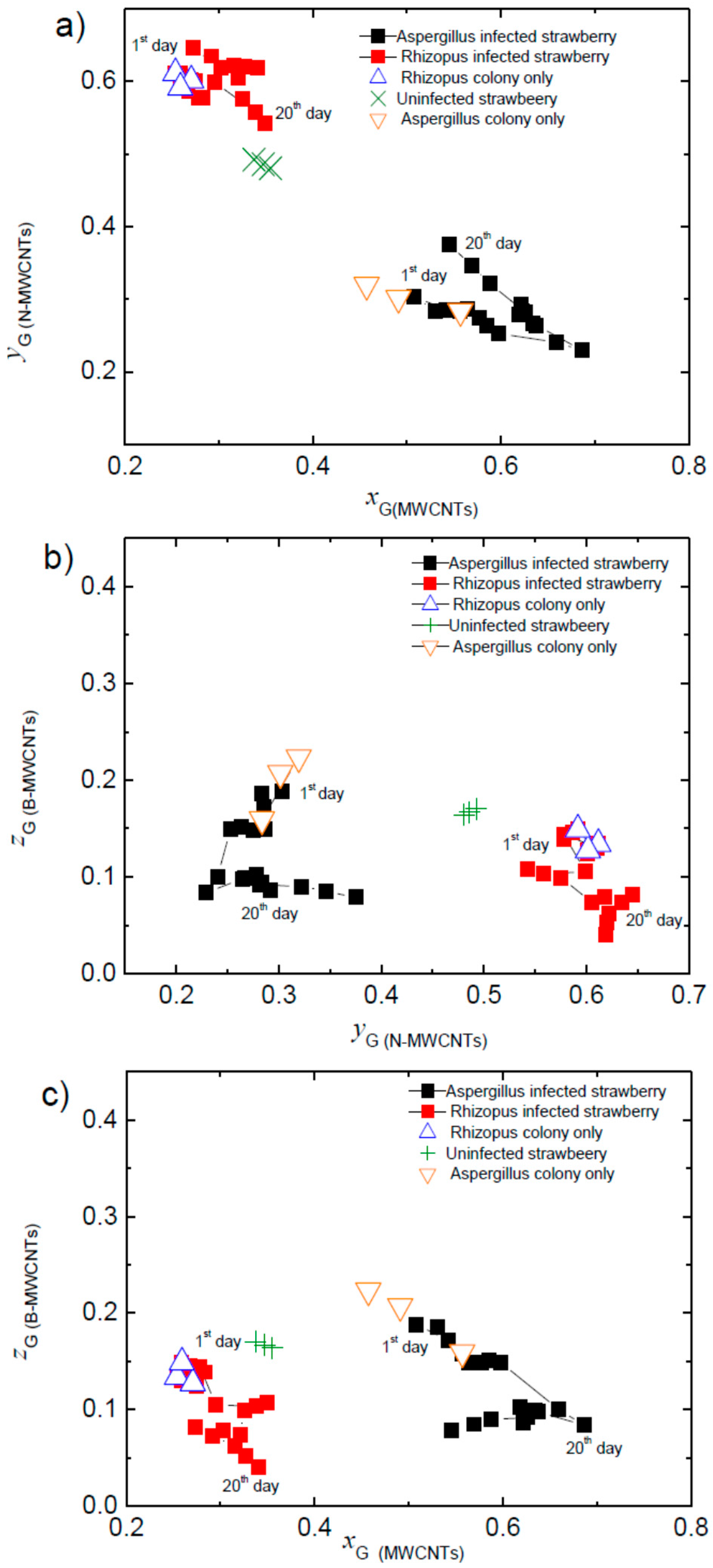
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| B-MWCNTs | B-doped multiwalled carbon nanotubes |
| BOD | Biochemical oxygen demand |
| CNSs | Carbon nanostructures |
| CTAB | Hexadecyltrimethylammonium bromide |
| ENIG | Electroless nickel immersion gold |
| MWCNTs | Multiwalled carbon nanotubes |
| N-MWCNTs | N-doped multiwalled carbon nanotubes |
| PET | Polyethylene terephthalate |
| PVA | Poly(vinyl alcohol) |
| VOC | Volatile organic compounds |
References
- Siqueira, H.H.; Vilas Boas, B.M.; Silva, J.D.; Nunes, E.E.; Lima, L.C.O.; Santana, M.T.A. Armazenamento de morango sob atmosfera modificada e refrigeração. Ciênc. Agrotec. 2009, 33, 1712–1715. [Google Scholar]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Brackmann, A.; Giehl, R.F.H.; Eisermann, A.C.; Weber, A.; Heldwein, A.B. Inibição da ação do etileno e temperatura de armazenamento no padrão de amadurecimento de tomates. Ciênc. Rural 2009, 39, 1688–1694. [Google Scholar] [CrossRef]
- Cunha, L.C., Jr.; Jacomino, A.P.; Ogassavara, F.O.; Trevisan, M.J.; Parisi, M.C. Armazenamento refrigerado de morango submetido a altas concentrações de CO2. Hortic. Bras. 2012, 30, 688–694. [Google Scholar] [CrossRef]
- Kopjar, M.; Piližota, V.; Hribar, J.; Simčič, M.; Zlatič, E.; Tiban, N.N. Influence of trehalose addition and storage conditions on the quality of strawberry cream filling. J. Food Eng. 2008, 87, 341–350. [Google Scholar] [CrossRef]
- Mohsen, F.; Hossein, K.; Rohollah, S.; Mojtaba, R.; Javad, H. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Parikkab, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- Amiri, A.; Chai, W.; Schnabel, G. Effect of nutrient status, pH, temperature and water potential on germination and growth of Rhizopus Stolonifer and Gilbertella Persicaria. J. Plant Pathol. 2011, 93, 603–612. [Google Scholar]
- Borges, C.D.; Mendonça, C.R.B.; Zambiazi, R.C.; Silva, E.M.P.; Paiva, F.F. Conservação de morangos com revestimentos à base de goma xantana e óleo essencial de sálvia = Strawberries conservation with coatings based on xanthan gum and sage essential. Biosci. J. 2013, 29, 1071–1083. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Jensen, B.; Knudsen, I.M.B.; Andersen, B.; Nielsen, K.F.; Thrane, U.; Jensen, D.F.; Larsen, J. Characterization of microbial communities and fungal metabolites on field grown strawberries from organic and conventional production. Int. J. Food Microbiol. 2013, 160, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Duru, M.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Nemec, S. Response of Three Root Rot Fungi to Strawberry Phenolics and the Relation of Phenolics to Disease Resistance. Mycopathologia 1976, 59, 37–40. [Google Scholar] [CrossRef]
- Niemi, M.; Vestberg, M. Inoculation of commercially grown strawberry with VA mycorrhizal fungi. Plant Soil 1992, 144, 133–142. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Bangerth, F.K.; Song, J.; Streif, J. Physiological Impacts of Fruit Ripening and Storage Conditions on Aroma Volatile Formation in Apple and Strawberry Fruit: A Review. Hortscience 2012, 47, 4–10. [Google Scholar]
- Reddy Bhaskara, M.V.; Angers, P.; Gosselin, J.A. Characterization and use of essential oil from Thymus vulgaris against Botrytis cinerea and Rhizopus stolonifer in strawberry fruits. Phytochemistry 1998, 47, 1515–1520. [Google Scholar] [CrossRef]
- Rosado-May, F.J.; Werner, M.R.; Gliessman, S.R.; Webb, R. Incidence of strawberry root fungi in conventional and organic production systems. Appl. Soil Ecol. 1994, 1, 261–267. [Google Scholar] [CrossRef]
- Siefkes-Boer, H.J.; Boyd-Wilson, K.S.; Petley, M.; Walter, M. Influence of Cold-Storage Temperatures on Strawberry Leak Caused by Rhizopus Spp. Plant Dis. 2009, 62, 243–249. [Google Scholar]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Han, C.; Lederer, C.; McDaniel, M.; Zhao, Y. Sensory evaluation of fresh strawberries (Fragaria ananassa) coated with chitosan-based edible coatings. J. Food Sci. 2005, 70, S172–S178. [Google Scholar] [CrossRef]
- Calegaro, J.M.; Pezzi, E.; Bender, R.J. Utilização de atmosfera modificada na conservação de morangos em pós-colheita. Pesqui. Agropecu. Bras. 2002, 37, 1049–1055. [Google Scholar] [CrossRef]
- Schleibinger, H.; Laussmann, D.; Bornehag, C.G.; Eis, D.; Rueden, H. Microbial volatile organic compounds in the air of moldy and mold-free indoor environments. Indoor Air 2008, 18, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kurze, S.; Bahl, H.; Dahl, R.; Berg, G. Biological Control of Fungal Strawberry Diseases by Serratia plymuthica HRO-C48. Plant Dis. 2001, 85, 529–534. [Google Scholar] [CrossRef]
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensor 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Da, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Kuske, M.; Romain, A.; Nicolas, J. Microbial volatile organic compounds as indicators of fungi—Can an electronic nose detect fungi in indoor environments. Build. Environ. 2005, 40, 824–831. [Google Scholar] [CrossRef]
- Greenshields, M.W.; Meruvia, M.S.; Hümmelgen, I.A.; Coville, N.J.; Mhlanga, S.D.; Ceragioli, H.J.; Quispe, J.C.; Baranauskas, V. AC-conductance and capacitance measurements for ethanol vapor detection using carbon nanotube-polyvinyl alcohol composite based devices. J. Nanosci. Nanotechnol. 2011, 11, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Mamo, M.A.; Coville, N.J.; Spina, A.P.; Rosso, D.F.; Latocheski, E.C.; Destro, J.G.; Pimentel, I.C.; Hümmelgen, I.A. Electronic Detection of Drechslera sp. Fungi in Charentais Melon (Cucumis melo Naudin) Using Carbon-Nanostructure-Based Sensors. J. Agric. Food Chem. 2012, 60, 10420–10425. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Hümmelgen, I.A.; Mamo, M.A.; Saikjee, A.; Mhalanga, S.D.; Otterlo van, W.A.; Coville, N.J. Composites of polyvinyl alcohol and carbon (coils, undoped and nitrogen doped multiwalled carbon nanotubes) as ethanol, methanol and toluene vapor sensors. J. Nanosci. Nanotechnol. 2011, 11, 10211–10218. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Mamo, M.A.; Coville, N.J.; Pimentel, I.C.; Destro, J.G.; Porsani, M.V.; Bozza, A.; Hümmelgen, I.A. Tristimulus mathematical treatment application for monitoring fungi infestation evolution in melon using the electrical response of carbon nanostructure-polymer composite based sensors. Sens. Actuators B Chem. 2013, 188, 378–384. [Google Scholar] [CrossRef]
- Cunha, B.B.; Greenshields, M.W.; Mamo, M.; Coville, N.J.; Hümmelgen, I.A. A surfactant dispersed N-doped carbon sphere-poly(vinyl alcohol) composite as relative humidity sensor. J. Mater. Sci. Mater. Electron. 2015, 26, 198–4201. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Mamo, M.A.; Coville, N.J.; Hümmelgen, I.A. Hydrostatic pressure sensors based on carbon spheres dispersed in polyvinyl alcohol prepared using hexadecyltrimethylammonium bromide as surfactant and water as solvent. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Duan, W.H.; Wang, Q.; Collins, F. Dispersion of carbon nanotubes with SDS surfactants: A study from a binding energy perspective. Chem. Sci. 2011, 2, 1407–1413. [Google Scholar] [CrossRef]
- Howard, W. Dispersing carbon nanotubes using surfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 364–371. [Google Scholar]
- Dölle, S.; Lechner, B.D.; Park, J.H.; Shymura, S.; Lagerwall, J.P.F.; Scalia, G. Utilizing the Krafft Phenomenon to Generate Ideal Micelle-Free Surfactant-Stabilized Nanoparticle Suspensions. Angew. Chem. Int. Ed. 2012, 51, 32547. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenshields, M.W.C.C.; Cunha, B.B.; Coville, N.J.; Pimentel, I.C.; Zawadneak, M.A.C.; Dobrovolski, S.; Souza, M.T.; Hümmelgen, I.A. Fungi Active Microbial Metabolism Detection of Rhizopus sp. and Aspergillus sp. Section Nigri on Strawberry Using a Set of Chemical Sensors Based on Carbon Nanostructures. Chemosensors 2016, 4, 19. https://doi.org/10.3390/chemosensors4030019
Greenshields MWCC, Cunha BB, Coville NJ, Pimentel IC, Zawadneak MAC, Dobrovolski S, Souza MT, Hümmelgen IA. Fungi Active Microbial Metabolism Detection of Rhizopus sp. and Aspergillus sp. Section Nigri on Strawberry Using a Set of Chemical Sensors Based on Carbon Nanostructures. Chemosensors. 2016; 4(3):19. https://doi.org/10.3390/chemosensors4030019
Chicago/Turabian StyleGreenshields, Marcia W. C. C., Bruno B. Cunha, Neil J. Coville, Ida C. Pimentel, Maria A. C. Zawadneak, Steffani Dobrovolski, Mireli T. Souza, and Ivo A. Hümmelgen. 2016. "Fungi Active Microbial Metabolism Detection of Rhizopus sp. and Aspergillus sp. Section Nigri on Strawberry Using a Set of Chemical Sensors Based on Carbon Nanostructures" Chemosensors 4, no. 3: 19. https://doi.org/10.3390/chemosensors4030019