A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus and Reagents
2.2. Preparation of rGO
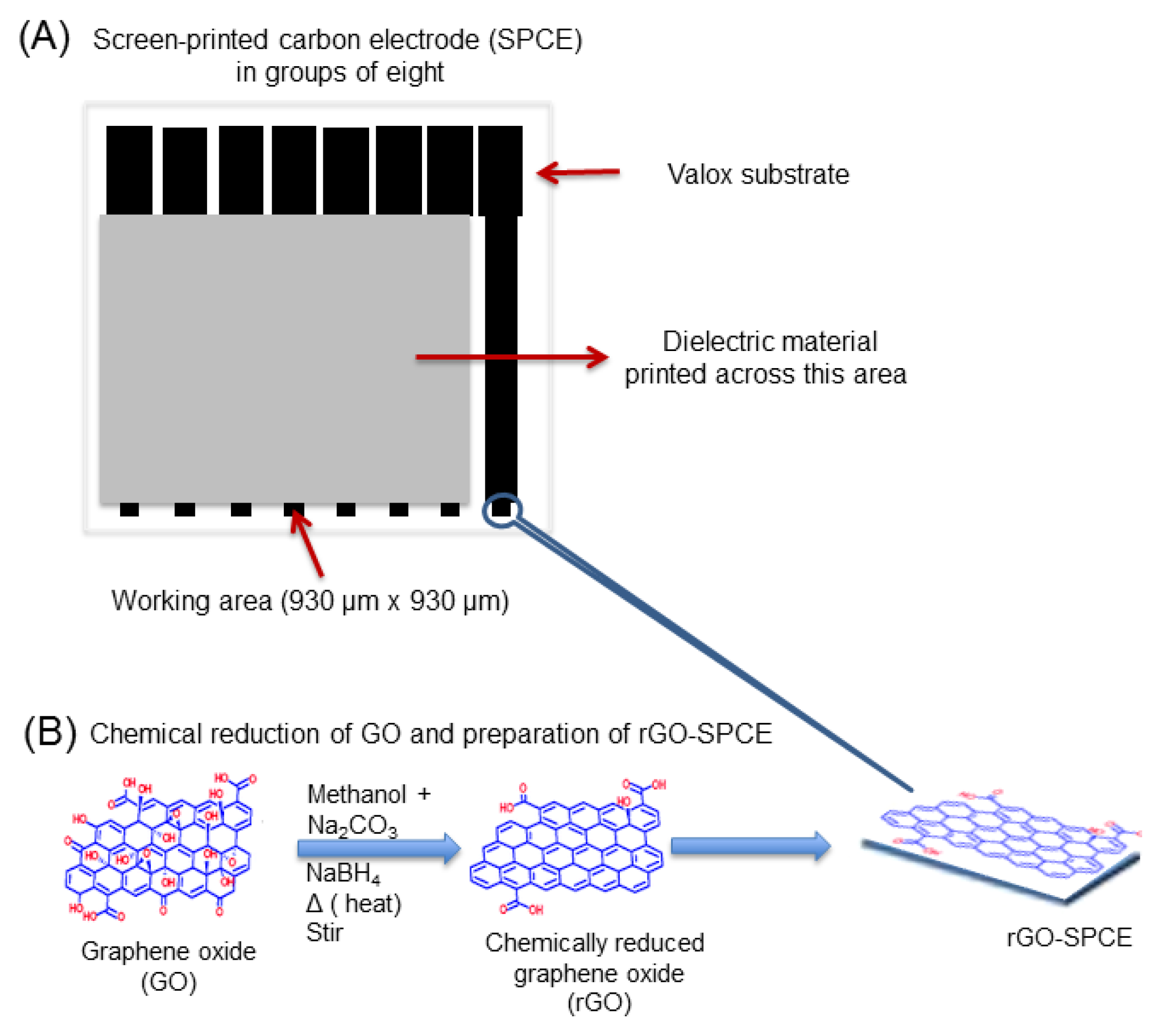
2.3. Fabrication of SPCE and rGO-SPCE
3. Results and Discussion
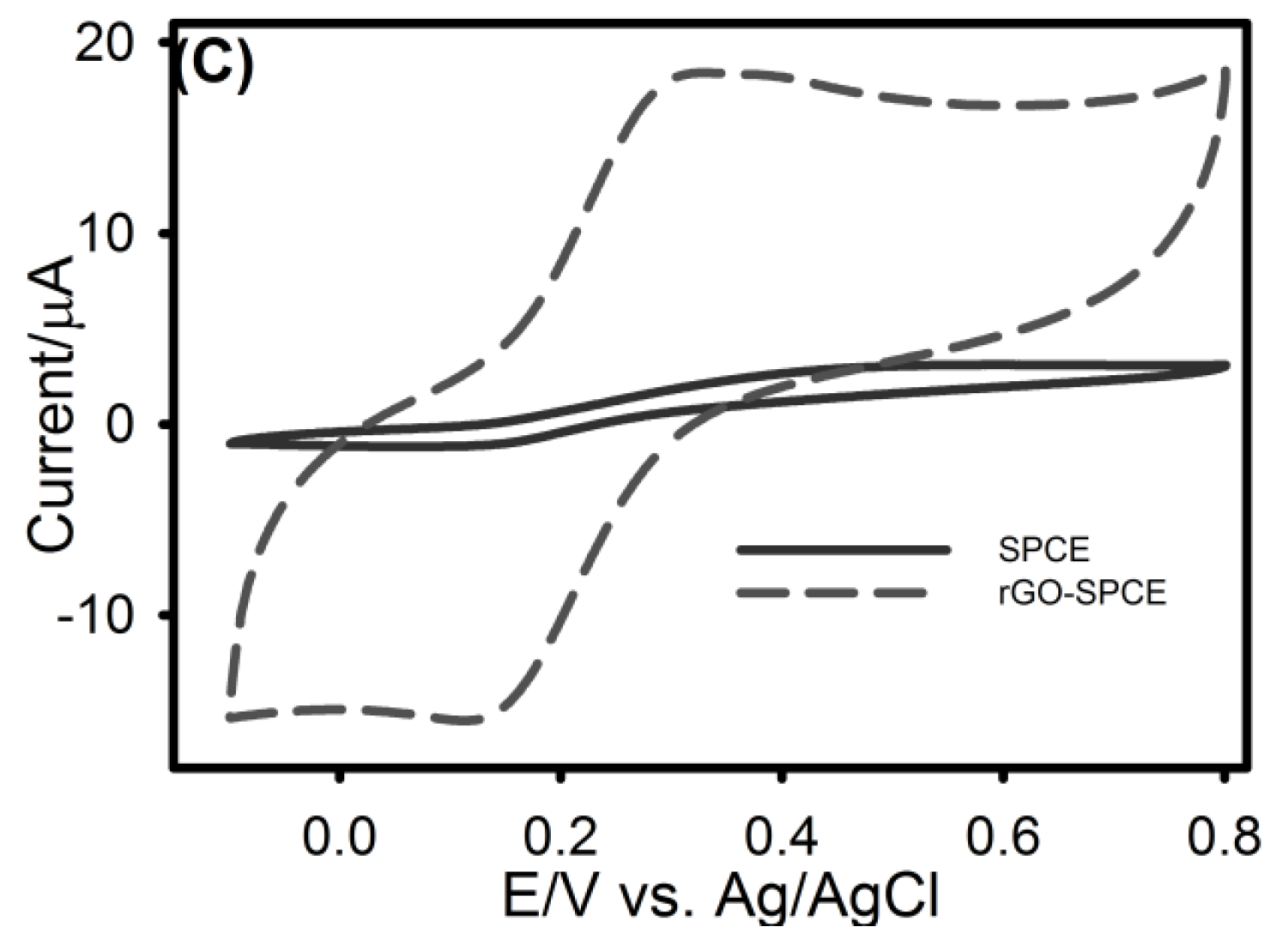
3.1. Characterization of rGO-SPCE
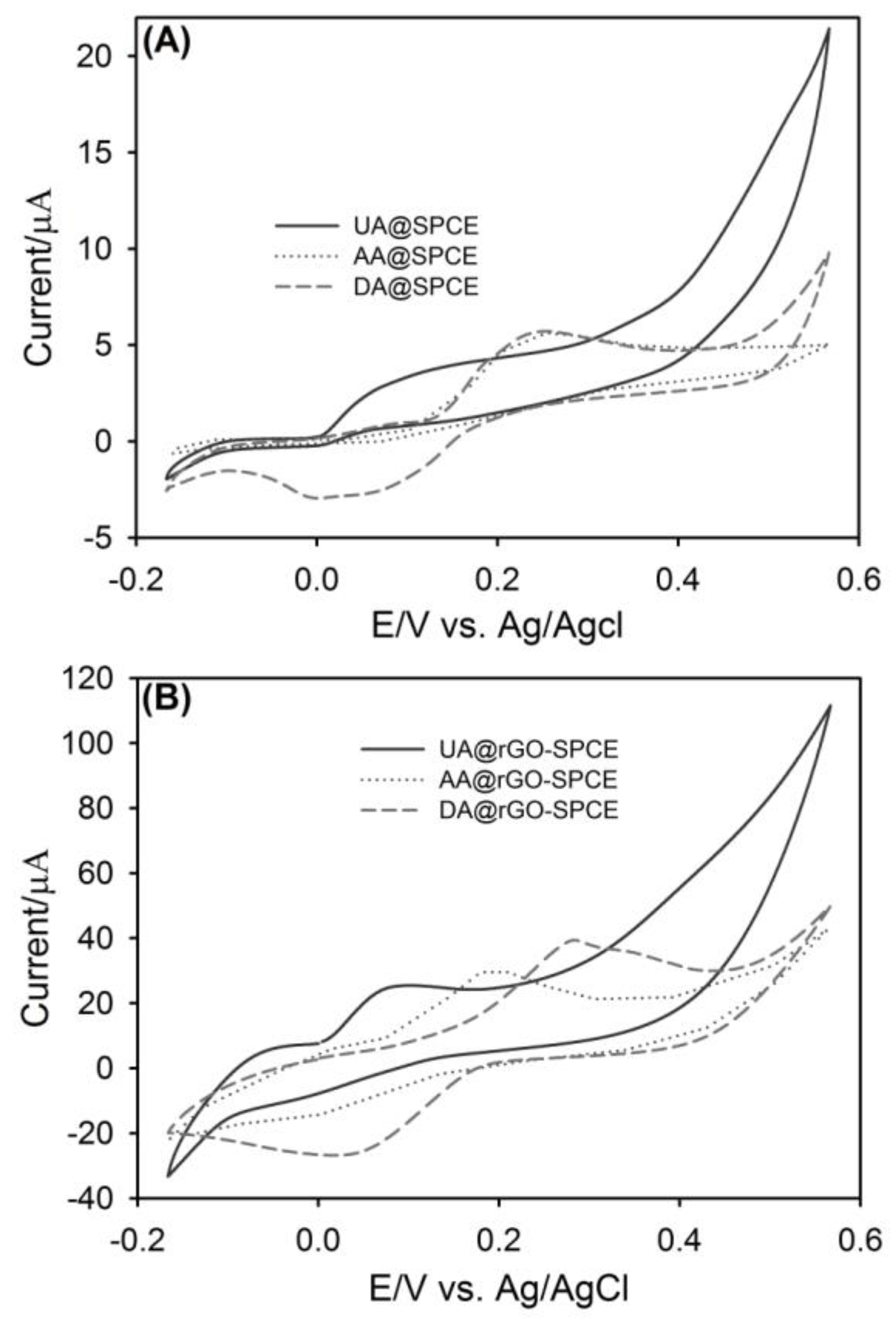
3.2. Cyclic Voltammetric Analysis of UA, AA and DA at SPCE and rGO-SPCE
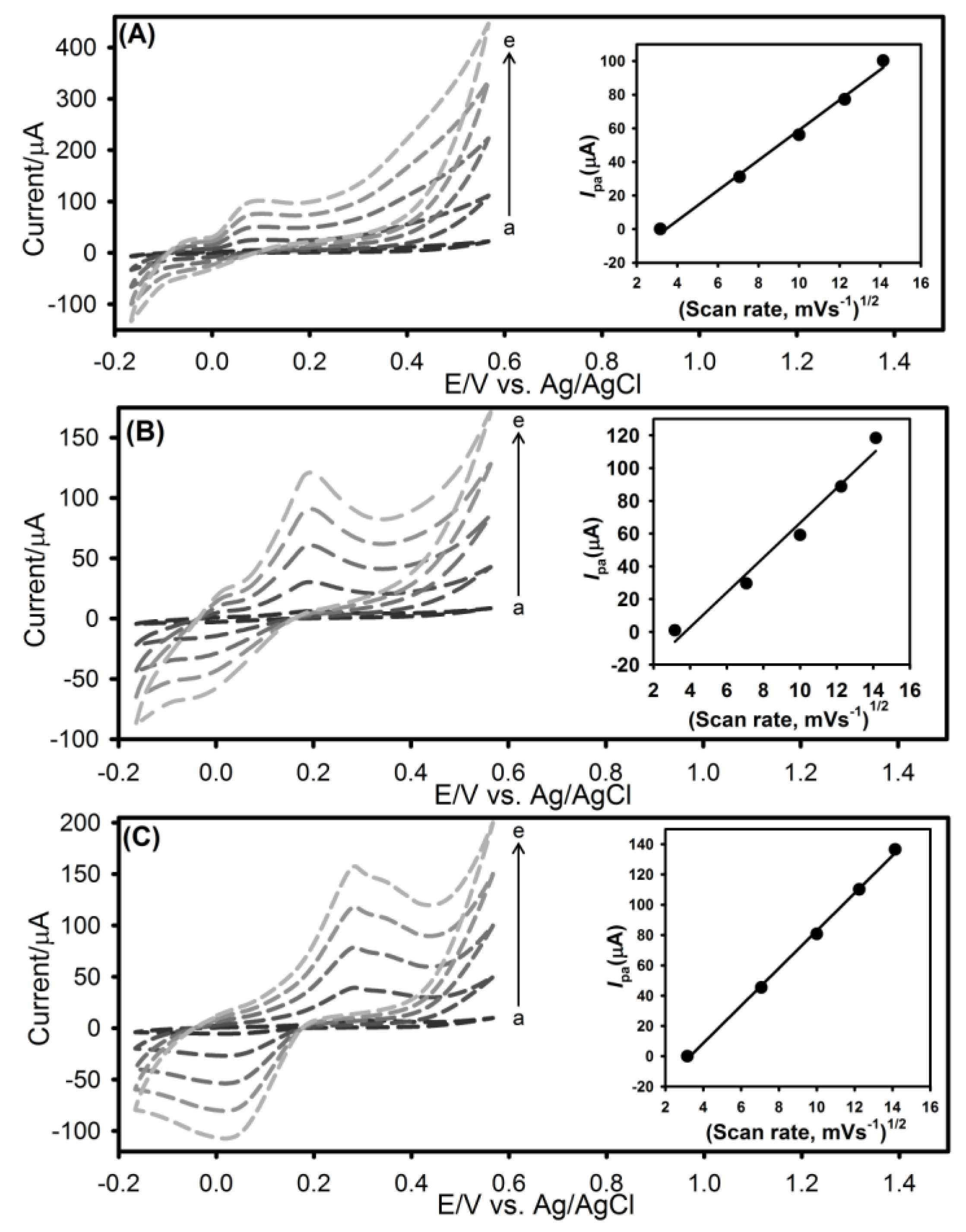
3.3. The Effect of Scan Rate on Voltammetric Behavior of UA, AA and DA at rGO-SPCE
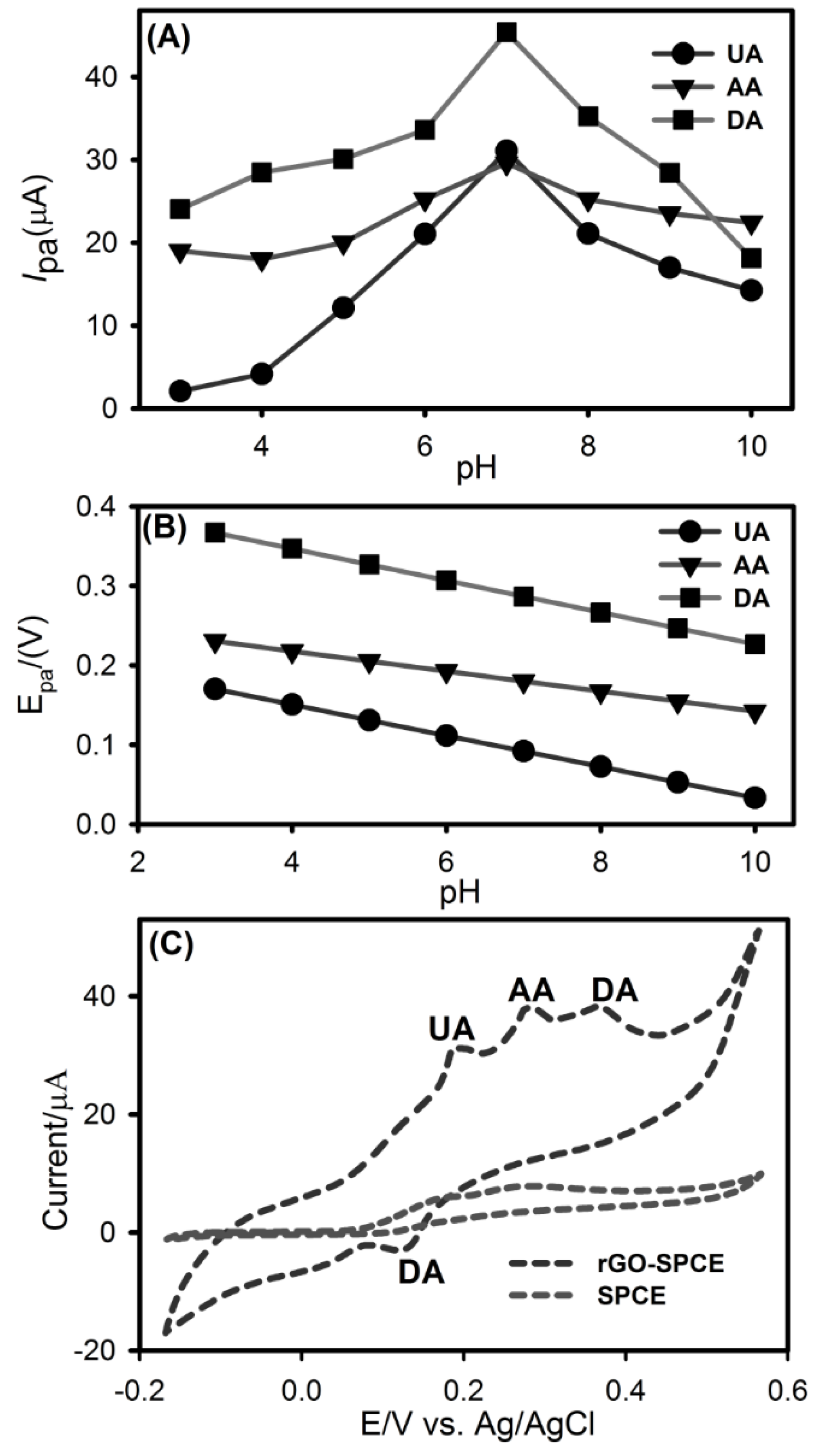
3.4. The Effect of pH on Voltammetric Behavior of UA, AA and DA at rGO-SPCE
3.5. CV Analysis of Ternary Mixture of UA, AA and DA at SPCE and rGO-SPCE
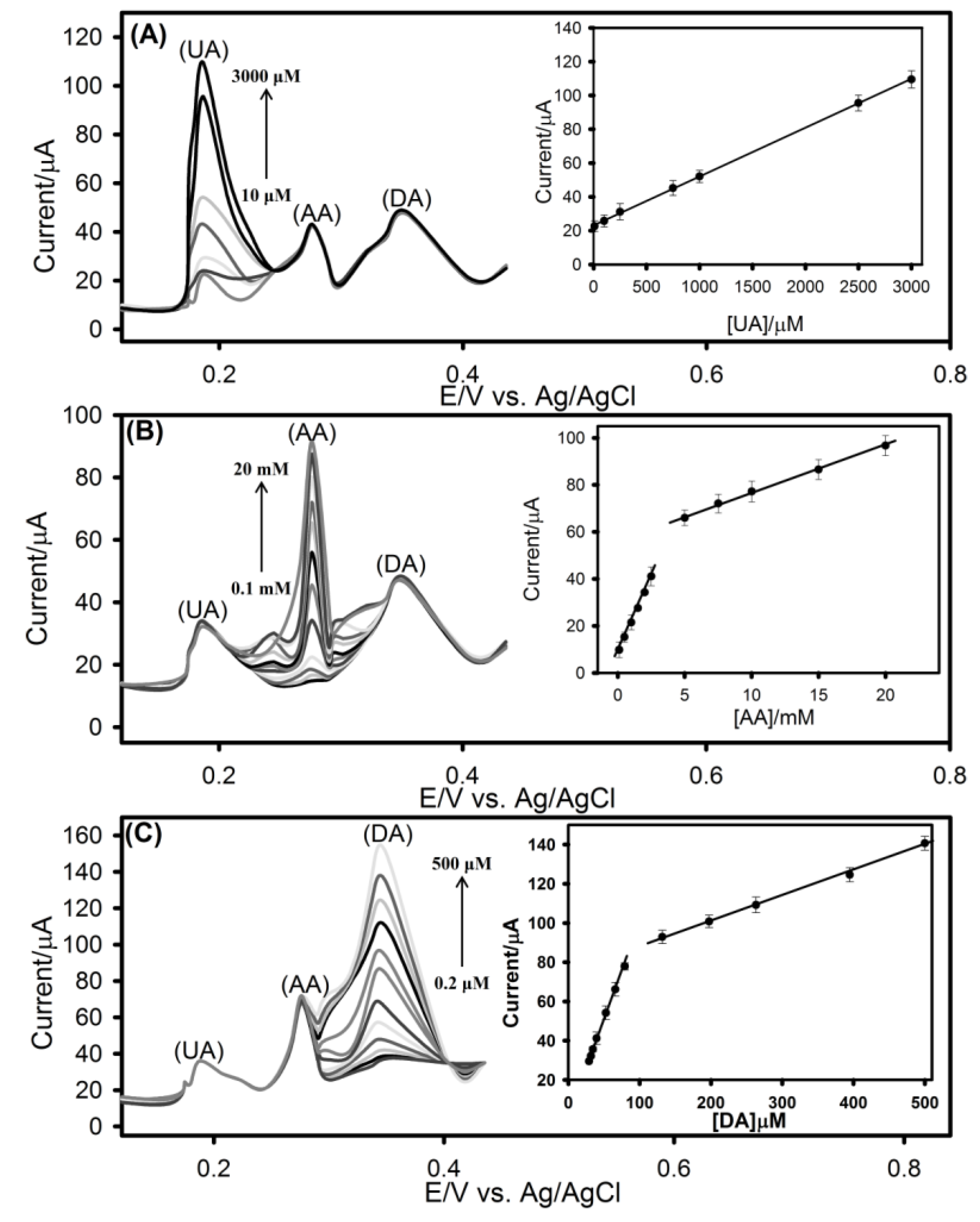
3.6. DPV Analysis of Ternary Mixtures of UA, AA and DA at rGO-SPCE
3.7. Reproducibility and Stability of rGO-SPCE, and Ternary Mixture Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of interest
References
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, J.G.A.; Magio, M.; Dixit, D.V.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Riches, P.L.; Wright, A.F.; Ralston, S.H. Recent insights into the pathogenesis of hyperuricemia and gout. Hum. Mol. Genet. 2009, 18, R177–R184. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favel, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2005, 80, 825–860. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S.J.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef]
- Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, R.; Hahn, M.K.; Blakely, R.D. Good riddance to dopamine: Roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem. Int. 2014, 73, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Camardese, G.; Giuda, D.; Nicola, M.; Cocciolillo, F.; Giordano, A.; Janiri, L.; Guglielmo, R. Imaging studies on dopamine transporter and depression: A review of literature and suggestions for future research. J. Psychiatr. Res. 2014, 51, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Szantai, E.; Szilagyi, A.; Guttman, A.; Sasvari-Szekely, M.; Ronai, Z. Genotyping and hapotyping of the dopamine D4 receptor gene by capillary electrophoresis. J. Chromatogr. A 2004, 1053, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.S.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAc Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- González, R.R.; Fernández, R.F.; Vidal, J.L.M.; Frenich, A.G.; Pérez, M.L.G. Development and validation of an ultra-high performance liquid chromatography–tandem mass-spectrometry (UHPLC–MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. J. Neurosci. Methods 2011, 198, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Huang, H. Simultaneous determination of dopamine, uric acid and ascorbic acid at an ultrathin film modified gold electrode. Chem. Commun. 1998, 19, 2107–2108. [Google Scholar] [CrossRef]
- Welch, C.W.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Moradlou, M.; Tajabadi, F. Simultaneous determination of dopamine, ascorbic acid and uric acid using carbon ionic liquid electrode. Anal. Biochem. 2006, 359, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Taei, M.; Khayamian, T. Simultaneous determination of ascorbic acid, dopamine and uric acid by differential pulse voltammetry using tiron modified glassy carbon electrode. Int. J. Electrochem. Sci. 2010, 5, 116–130. [Google Scholar]
- Kalimuthu, P.; John, S.A. Simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine using a nanostructured polymer film modified electrode. Talanta 2010, 80, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Shakkthivel, P.; Chen, S. Simultaneous determination of ascorbic acid and dopamine in the presence of uric acid on ruthenium oxide modified electrode. Biosens. Bioelectron. 2007, 22, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Ardunini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-printed electrodes modified with carbon nanomaterials: A comparison among carbon black, carbon nanotubes and graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Reduction of graphene oxide with substituted borohydrides. J. Mater. Chem. A 2013, 1, 1892–1898. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Gold nanoparticle modified screen-printed carbon arrays for the simultaneous electrochemical analysis of lead and copper in tap water. Microchim. Acta 2016, 183, 2361–2368. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Fabrication and electrochemical characterization of polydopamine redox polymer modified screen-printed carbon electrode for the detection of guanine. Sens. Actuators B Chem. 2016, 233, 528–534. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Suni, I.I. Impedance methods for electrochemical sensors using nanomaterials. Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Mani, V.; Chen, S.M.; Huang, W.T.; Jen, J.F. Rapid microwave assisted synthesis of graphene nanosheets/polyethylenimine/gold nanoparticle composite and its application to the selective electrochemical determination of dopamine. Talanta 2014, 120, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Temocin, Z. Modification of glassy carbon electrode in basic medium by electrochemical treatment for simultaneous determination of dopamine, ascorbic acid and uric acid. Sens. Actuators B Chem. 2013, 176, 796–802. [Google Scholar] [CrossRef]
- Habibi, B.; Pournaghi-Azar, M.H. Simultaneous determination of ascorbic acid, dopamine and uric acid by use of a MWCNT modified carbon-ceramic electrode and differential pulse voltammetry. Electrochim. Acta 2010, 55, 5492–5498. [Google Scholar] [CrossRef]
- Komura, T.; Niu, G.Y.; Yamaguchi, T.; Asano, M.; Matsuda, A. Coupled electron-proton transport in electropolymerized methylene blue and the influences of its protonation level on the rate of electron exchange with nicotinamide adenine dinucleotide. Electroanalysis 2010, 16, 1791–1800. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H. Review of voltammetric methods for the study of electrode reactions. In Microelectrdodes: Theory and Applications; Montenegro, M.R.M., Quirós, A., Daschbach, J.L., Eds.; Springer: Rotterdam, The Netherlands, 1991; pp. 17–32. [Google Scholar]
- Yang, L.; Liu, D.; Huang, J.S.; You, T.Y. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, L.L. Electrochemical sensor for simultaneous determination of uric acid, xanthine, and hypoxanthine based on poly (bromocresol purple) modified glassy carbon electrode. Sens. Actuators B Chem. 2010, 150, 43–49. [Google Scholar] [CrossRef]
- Lian, Q.; He, Z.; He, Q.; Luo, A.; Yan, K.; Zhang, D.; Lu, X.; Zhou, X. Simultaneous determination of ascorbic acid, dopamine and uric acid based on trptophan functionalized graphene. Anal. Chim. Acta 2014, 823, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Y.; Wang, Z.H.; Zhang, Y.Z.; Zheng, Z.X.; Wang, C.M.; Du, Y.L.; Ye, W.C. Simultaneous electrochemical determination of uric acid, xanthine and hypoxanthine based on poly(L-arginine)/graphene composite film modified electrode. Talanta 2012, 93, 20–325. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Panidyan, T.; Satpati, B.; Srivastava, R. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloid. Surface B 2013, 11, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, M.; Tang, W.R.; Zhu, Y.; Wang, L.W.; Wang, Q.J.; He, P.G.; Fang, Y.Z. Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid suing a palladium nanoparticles/graphene/chitosan modified electrode. J. Electroanal. Chem. 2013, 695, 10–16. [Google Scholar] [CrossRef]
- Li, S.J.; Qian, C.; Wang, K.; Hua, B.Y.; Wang, F.B.; Sheng, Z.H.; Xia, X.H. Application of thermally reduced graphene oxide modified electrode in simultaneous determination of dihydroxybenzene isomers. Sens. Actuators B Chem. 2012, 174, 441–448. [Google Scholar] [CrossRef]
- Ping, J.F.; Wang, J.W.Y.X.; Ying, Y.B. Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens. Bioelectron. 2012, 34, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.J.; Wang, X.Y.; Zhang, G.H.; Wang, C. Simultaneous determination of dopamine, ascorbic acid and uric acid using helical carbon nanotubes modified electrode. Sens. Actuators B Chem. 2012, 161, 1139–1143. [Google Scholar] [CrossRef]
- Wang, C.F.; Xu, P.P.; Zhuo, K.L. Ionic liquid functionalized graphene-based electrochemical biosensor for simultaneous determination of dopamine and uric acid in the presence of ascorbic acid. Electroanalysis 2014, 26, 191–198. [Google Scholar] [CrossRef]
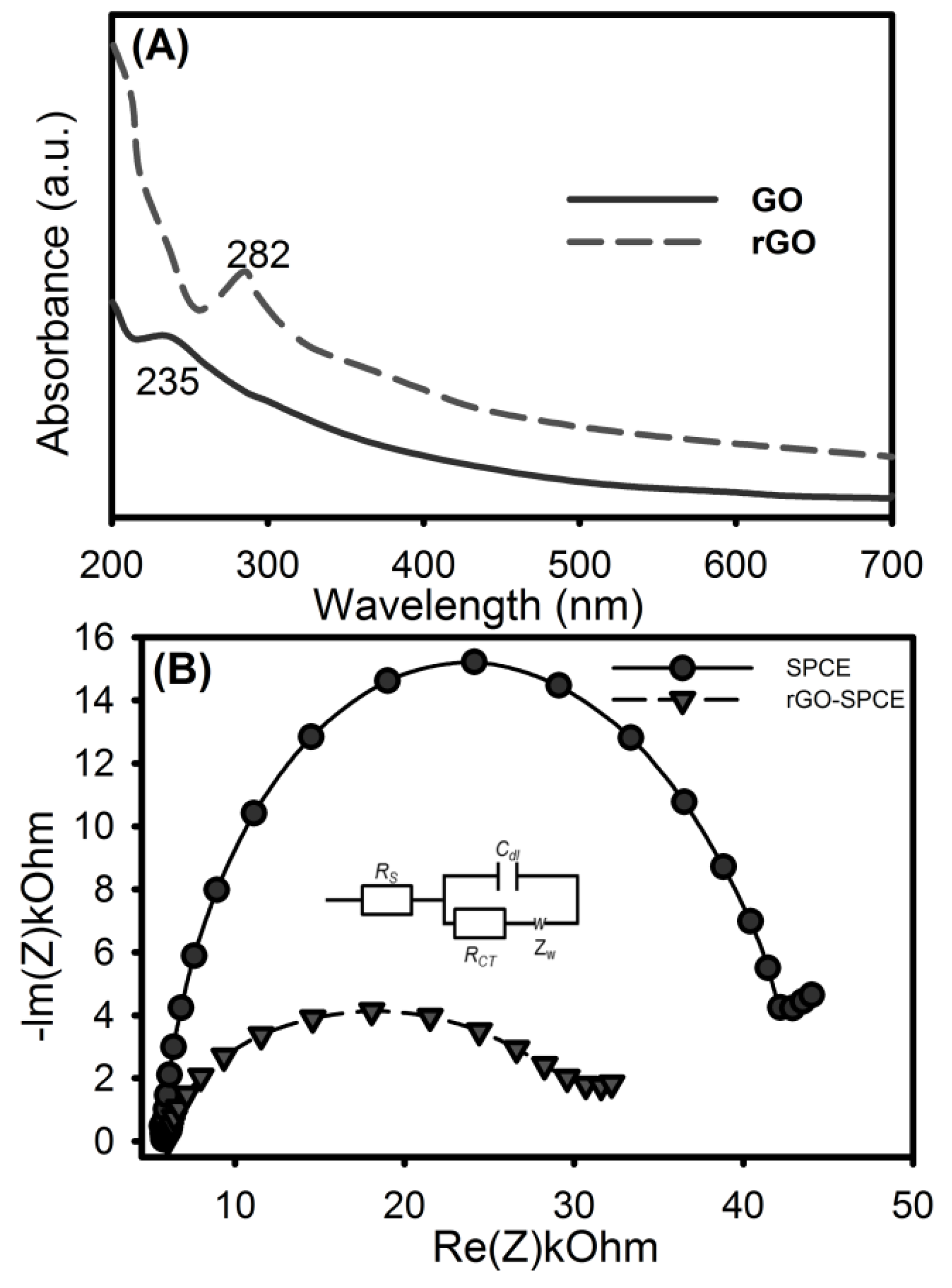
| Parameter | SPCE | rGO-SPCE |
|---|---|---|
| Rs (Ω) | 2998 | 2997 |
| Cdl (μF) | 1.4986 | 7.071 |
| RCT (Ω) | 16049 | 5004 |
| Zw (Ω·s−½) | 2181 | 1983 |
| Electrode Material | Limit of Detection (μM) | Linear Range (μM) | Reference | ||||
|---|---|---|---|---|---|---|---|
| UA | AA | DA | UA | AA | DA | ||
| ERGO-GCE | 0.5 | 300.0 | 0.5 | 0.5–60.0 | 500.0–2000.0 | 0.5–60.0 | [31] |
| GCE | 4.7 | 23.38 | 2.67 | 5.0–70.0 | 25,000.0–300,000.0 | 3.0–30.0 | [32] |
| Trp-GR | 1.24 | 10.09 | 0.29 | 10.0–1000.0 | 200.0–3400.0, | 0.5–110.0 | [33] |
| 3400.0–12,900.0 | |||||||
| PdNPs-GR-CS-GCE | 0.17 | 20.0 | 0.1 | 0.5.0–200.0 | 100.0–400.0 | 0.50–15.0, | [34] |
| 20.00–200.00 | |||||||
| AgNPs-rGO-GCE | 8.2 | 9.6 | 5.4 | 10.0–800.0 | 10.0–800.0 | 10.00–800.00 | [35] |
| MWCNT-GCE | 0.42 | 7.71 | 0.31 | 0.55–90.0 | 15.0–800.0 | 0.50–100.00 | [36] |
| Nitrogen doped GR | 0.045 | 2.2 | 0.25 | 0.1–20.0 | 5.0–1300.0 | 0.50–170.00 | [29] |
| poly(Tyr)-MWCNTs-COOH-GCE | 0.3 | 2.0 | 0.02 | 1.0–350.0 | 50–1000.0 | 0.10–30.00 | [37] |
| Screen printed graphene electrode | 0.2 | 0.95 | 0.12 | 0.8–2500.0 | 4.0–4500.0 | 0.50–2000.00 | [38] |
| HCNTs-GCE | 1.5 | 0.92 | 0.8 | 6.7–65.0 | 7.5–180.0 | 2.50–105.00 | [39] |
| IL-GR-GCE | 0.323 | – | 0.679 | 1.0–600.0 | – | 1.00–400.00 | [40] |
| rGO-SPCE | 0.35 | 50 | 0.1 | 10.0–3000.0 | 100.0–2500.0, | 0.20–80.00, | This study |
| 5000.0–20000.0 | 120.0–500.0 | ||||||
| Sample | Amount Added (μM) | Amount Recovered (μM) | Mean Recovery (%) |
|---|---|---|---|
| UA | - | - | - |
| Repeat 1 | 50 | 47.9 | |
| Repeat 2 | 50 | 49.4 | |
| Repeat 3 | 50 | 49.8 | |
| Mean | - | 49.033 | 98.07 |
| AA | - | - | - |
| Repeat 1 | 500 | 497.3 | |
| Repeat 2 | 500 | 499.7 | |
| Repeat 3 | 500 | 499.5 | |
| Mean | - | 499.833 | 99.97 |
| DA | - | - | |
| Repeat 1 | 50 | 49.4 | |
| Repeat 2 | 50 | 48.8 | |
| Repeat 3 | 50 | 49.9 | |
| Mean | - | 49.367 | 98.73 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanyong, P.; Rawlinson, S.; Davis, J. A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine. Chemosensors 2016, 4, 25. https://doi.org/10.3390/chemosensors4040025
Kanyong P, Rawlinson S, Davis J. A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine. Chemosensors. 2016; 4(4):25. https://doi.org/10.3390/chemosensors4040025
Chicago/Turabian StyleKanyong, Prosper, Sean Rawlinson, and James Davis. 2016. "A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine" Chemosensors 4, no. 4: 25. https://doi.org/10.3390/chemosensors4040025






