Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection
Abstract
:1. Introduction
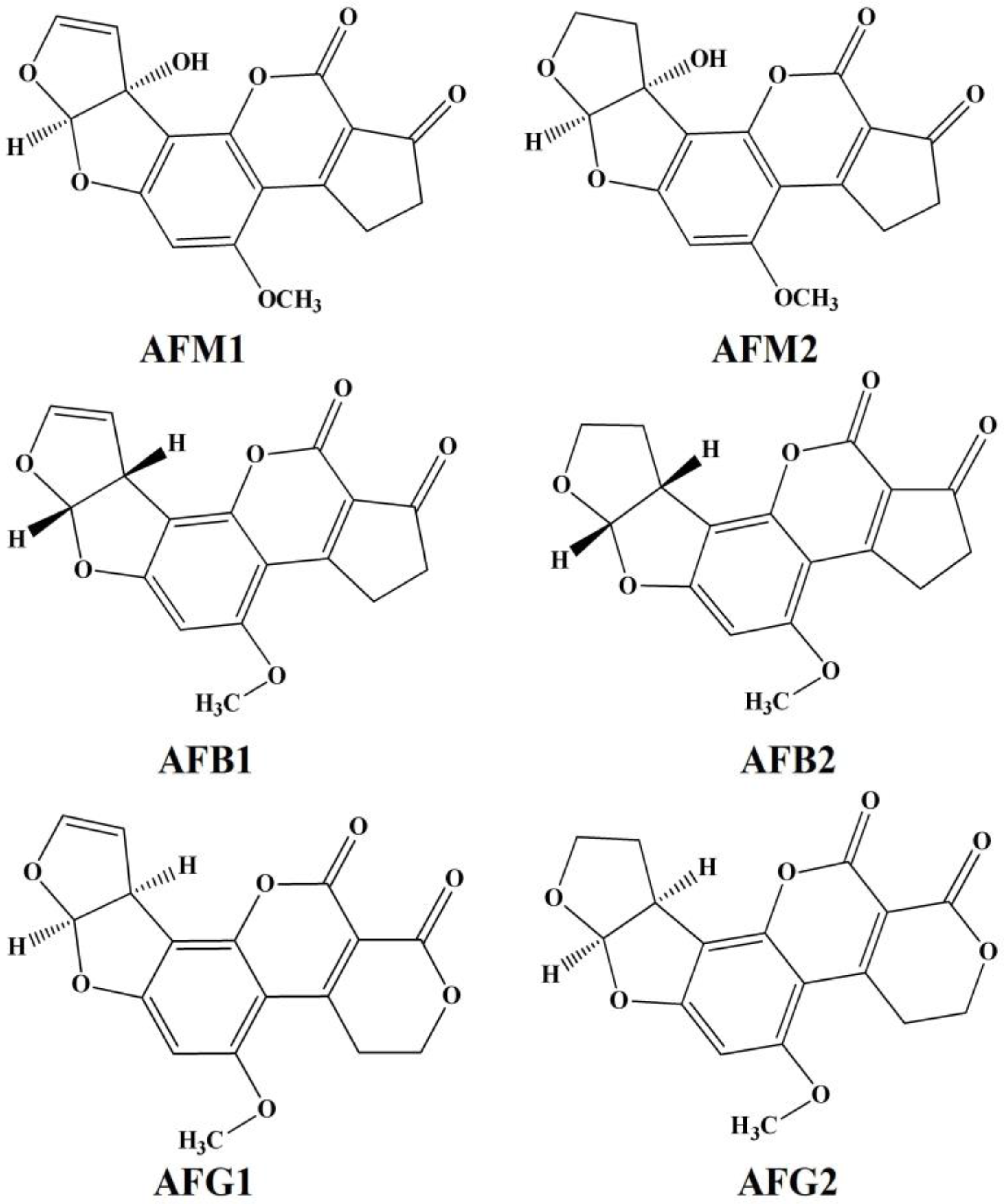
1.1. Toxicity of AFs
1.2. Monitoring of Aflatoxins (AFs)
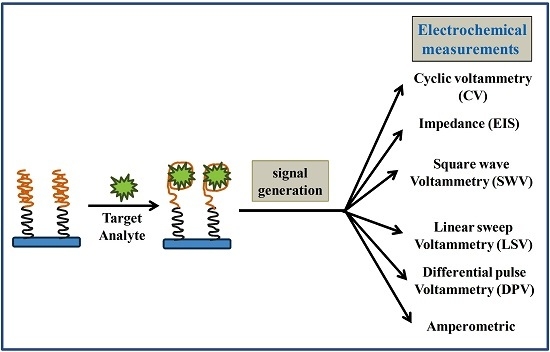
2. Biosensors (as an Alternative Tool)
Aptamer
3. Electrochemical Immunosensors for AFs Detections
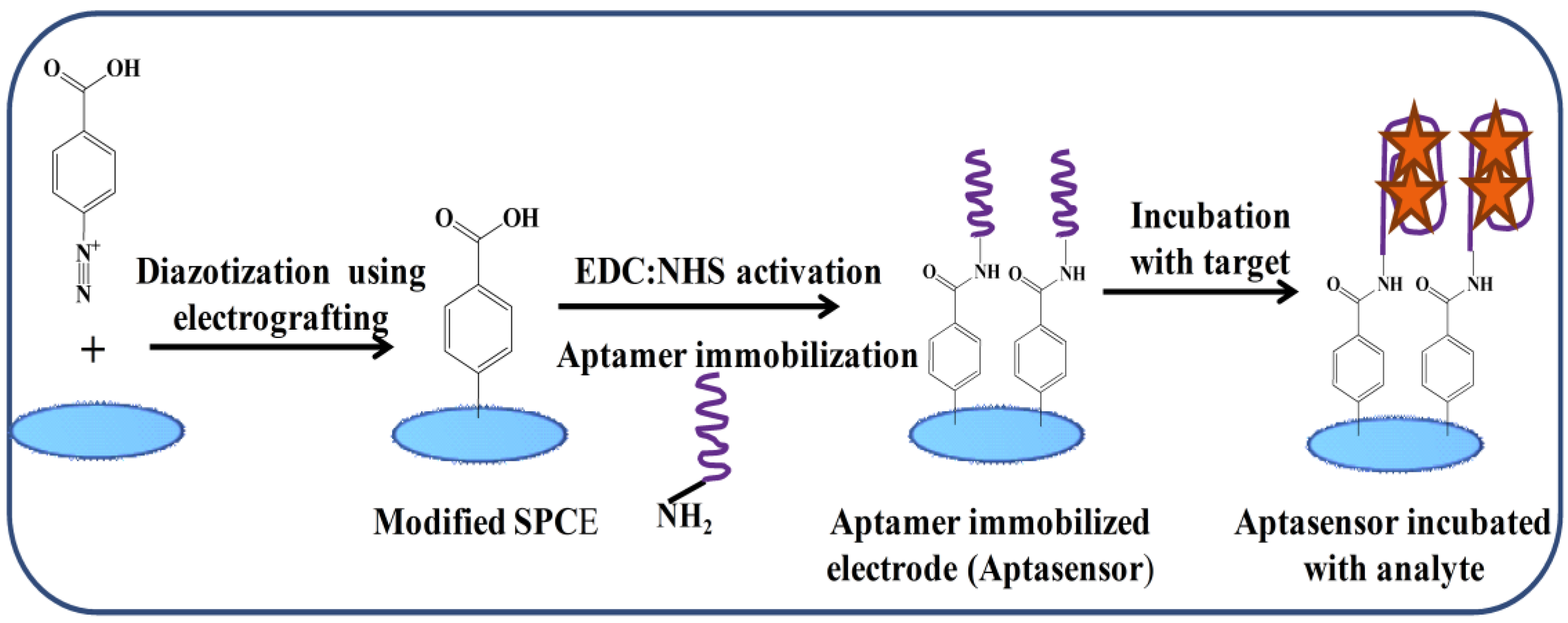
4. Aptamer-Based Sensing Strategies for Aflatoxins (AFs) Detection
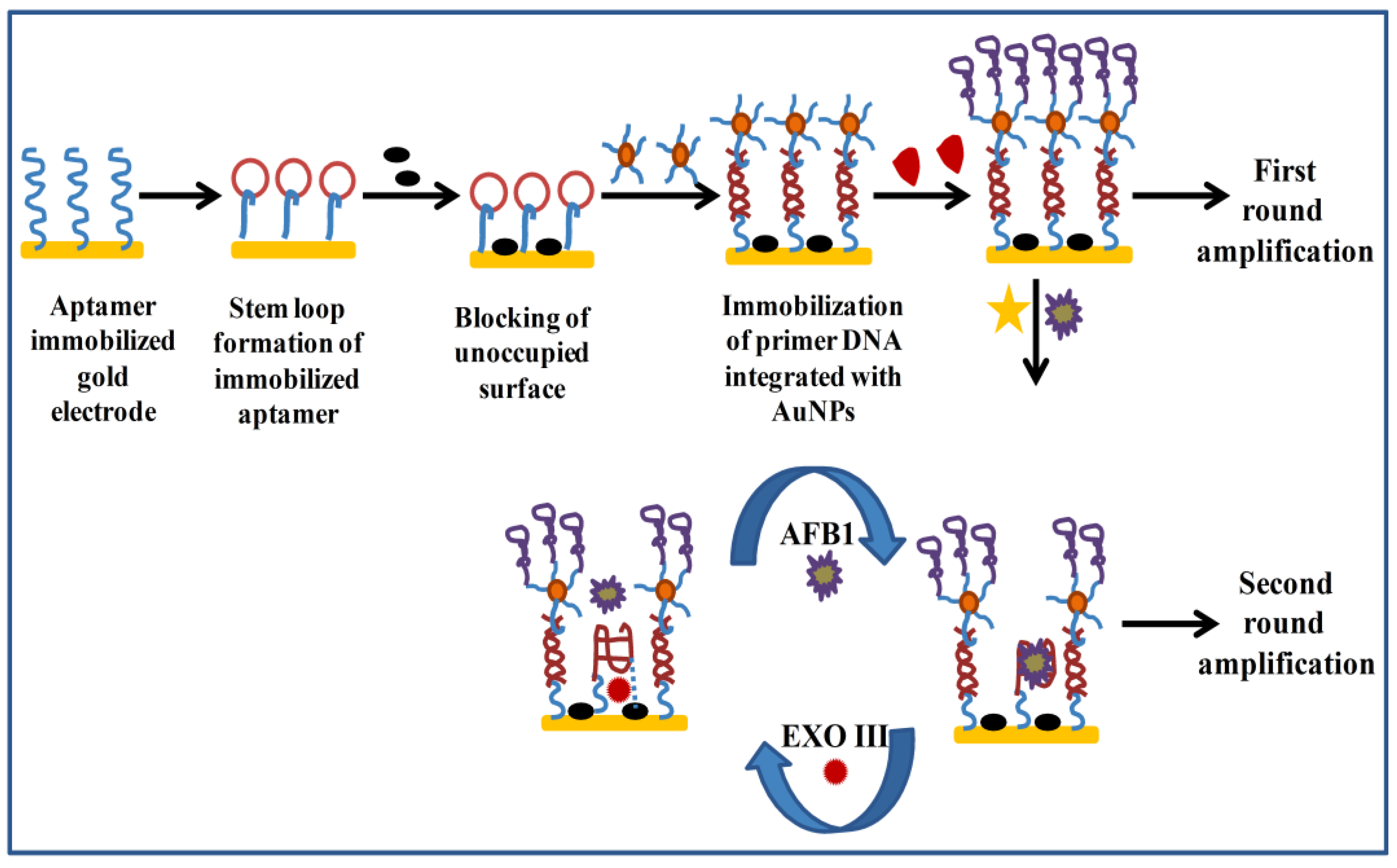
Electrochemical Aptasensors for AFs Detection
5. Safety Notes
6. Conclusions and Future Perspectives
Acknowledgements
Conflicts of Interest
References
- Guo, X.; Wen, F.; Zheng, N.; Luo, Q.; Wang, H.; Wang, H.; Li, S.; Wang, J. Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron. 2014, 56, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Malysheva, S.V.; Taevernier, L.; Diana Di Mavungu, J.; De Saeger, S.; De Spiegeleer, B. Human skin penetration of selected model mycotoxins. Toxicology 2012, 301, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hruska, Z.; Mavungu, J.D.D. Developments in detection and determination of aflatoxins. World Mycotoxin J. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Bakirci, I. A study on the occurrence of aflatoxin M1 in milk and milk products produced in van province of turkey. Food Control 2001, 12, 47–51. [Google Scholar] [CrossRef]
- Sharma, A.; Catanante, G.; Hayat, A.; Istamboulie, G.; Ben Rejeb, I.; Bhand, S.; Marty, J.L. Development of structure switching aptamer assay for detection of aflatoxin M1 in milk sample. Talanta 2016, 158, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.; Sachan, D. Dietary factors affecting aflatoxin B1 carcinogenicity. Malays. J. Nutr. 1997, 3, 161–197. [Google Scholar]
- Codex Committee on Food Additives and Contaminants. Comments Submitted on the Draft Maximum Level for Aflatoxin M1 in Milk, CL CX/FAC 01/20; Codex Alimentarious Commission Netherlands: Hague, The Netherlands, 2001; pp. 1–9. [Google Scholar]
- Stroka, J.; Anklam, E. New strategies for the screening and determination of aflatoxins and the detection of aflatoxin-producing moulds in food and feed. TrAC Trends Anal. Chem. 2002, 21, 90–95. [Google Scholar] [CrossRef]
- Badea, M.; Micheli, L.; Messia, M.C.; Candigliota, T.; Marconi, E.; Mottram, T.; Velasco-Garcia, M.; Moscone, D.; Palleschi, G. Aflatoxin M1 determination in raw milk using a flow-injection immunoassay system. Anal. Chim. Acta 2004, 520, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Wild, C.P.; Turner, P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; Germano, P.M. Aflatoxins: Current concepts on mechanisms of toxicity and their involvement in the etiology of hepatocellular carcinoma. Rev. Saude Publ. 1997, 31, 417–424. [Google Scholar]
- Levy, D.D.; Groopman, J.D.; Lim, S.E.; Seidman, M.M.; Kraemer, K.H. Sequence specificity of aflatoxin B1-induced mutations in a plasmid replicated in xeroderma pigmentosum and DNA repair proficient human cells. Cancer Res. 1992, 52, 5668–5673. [Google Scholar] [PubMed]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Commission Regulation (EU) No 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins; OJ L 50; Official Journal of the European Union: Brusseles, Belgium, 2010; pp. 8–126. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EU) No 1058/2012 of 12 November 2012 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Aflatoxins in Dried Figs Text with EEA Relevance; OJ L 313; Official Journal of the European Union: Brusseles, Belgium, 2012; pp. 14–15. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of the European Union: Brusseles, Belgium, 2006; pp. L364/5–L364/24. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EC) No. 401/2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; Official Journal of the European Union: Brusseles, Belgium, 2006; Volume L70, p. 1234. [Google Scholar]
- Dorsm, G.C.; Caldas, S.C.; Feddern, V.; Bemvenuti, R.; Hackbart, H.C.D.S.; De Souza, M.M.; Oliveira, M.D.S.; Buffon, J.G.; Primel, E.G.; Furlong, E.B. Aflatoxins: Contamination, Analysis and Control, Aflatoxins-Biochemistry and Molecular Biology; Guevara-Gonzalez, R.G., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Soares, L.M.; Rodriguez-Amaya, D.B. Survey of aflatoxins, ochratoxin a, zearalenone, and sterigmatocystin in some brazilian foods by using multi-toxin thin-layer chromatographic method. J Assoc. Off. Anal. Chem. 1989, 72, 22–26. [Google Scholar] [PubMed]
- Stroka, J.; Otterdijk, R.V.; Anklam, E. Immunoaffinity column clean-up prior to thin-layer chromatography for the determination of aflatoxins in various food matrices. J. Chromatogr. A 2000, 904, 251–256. [Google Scholar] [CrossRef]
- Stroka, J.; Anklam, E. Development of a simplified densitometer for the determination of aflatoxins by thin-layer chromatography. J. Chromatogr. A 2000, 904, 263–268. [Google Scholar] [CrossRef]
- Rodríguez Velasco, M.L.; Calonge Delso, M.M.; Ordónez Escudero, D. ELISA and HPLC determination of the occurrence of aflatoxin M1 in raw cow’s milk. Food Addit. Contam. 2003, 20, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A 2008, 25, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Vahl, M.; Jørgensen, K. Determination of aflatoxins in food using LC/MS/MS. Zeitschrift für Lebensmitteluntersuchung und Forschung A 1998, 206, 243–245. (In German) [Google Scholar] [CrossRef]
- Monbaliu, S.; Van Poucke, C.; Detavernier, C.L.; Dumoulin, F.; Van De Velde, M.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin lc-ms/ms method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.M.; de Medeiros, F.D.; de Oliveira, E.J.; Macedo, R.O. Development and validation of a method for the quantitative determination of aflatoxin contaminants in maytenus ilicifolia by HPLC with fluorescence detection. Phytochem. Anal. 2005, 16, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Navas, S.A.; Sabino, M.; Rodriguez-Amaya, D.B. Aflatoxin M1 and ochratoxin a in a human milk bank in the city of São Paulo, Brazil. Food Addit. Contam. 2005, 22, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Iha, M.H.; Barbosa, C.B.; Favaro, R.M.; Trucksess, M.W. Chromatographic method for the determination of aflatoxin M1 in cheese, yogurt, and dairy beverages. J. AOAC Int. 2011, 94, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takino, M.; Sugita-Konishi, Y.; Tanaka, T. Development of a liquid chromatography/time-of-flight mass spectrometric method for the simultaneous determination of trichothecenes, zearalenone and aflatoxins in foodstuffs. Rapid Commun. Mass Spectrom. 2006, 20, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2338. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical aptamer-based biosensors: Recent advances and perspectives. Int. J. Electrochem. 2011, 2011, 863196. [Google Scholar] [CrossRef]
- Sassolas, A.; Catanante, G.; Hayat, A.; Marty, J.-L. Development of an efficient protein phosphatase-based colorimetric test for okadaic acid detection. Anal. Chim. Acta 2011, 702, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Sharma, A.; Bhand, S. Ultrasensitive detection of streptomycin using flow injection analysis-electrochemical quartz crystal nanobalance (FIA-EQCN) biosensor. Biosens. Bioelectron. 2015, 67, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chu, X.; Shen, G.-L.; Yu, R.-Q. A signal-amplified electrochemical immunosensor for aflatoxin B1 determination in rice. Anal. Biochem. 2009, 387, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [PubMed]
- Zhou, W.; Huang, P.-J.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.N.; de Prada, P.; Landry, D.W. Fluorescent sensors based on aptamer self-assembly. J. Am. Chem. Soc. 2000, 122, 11547–11548. [Google Scholar] [CrossRef]
- Bunka, D.H.J.; Stockley, P.G. Aptamers come of age—At last. Nat. Rev. Microbiol. 2006, 4, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hayat, A.; Mishra, R.K.; Catanante, G.; Shahid, S.A.; Bhand, S.; Marty, J.L. Design of a fluorescence aptaswitch based on the aptamer modulated nano-surface impact on the fluorescence particles. RSC Adv. 2016, 6, 65579–65587. [Google Scholar] [CrossRef]
- McKeague, M.; McConnell, E.M.; Cruz-Toledo, J.; Bernard, E.D.; Pach, A.; Mastronardi, E.; Zhang, X.; Beking, M.; Francis, T.; Giamberardino, A.; et al. Analysis of in vitro aptamer selection parameters. J. Mol. Evol. 2015, 81, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Mairal, T.; Ozalp, V.C.; Lozano Sanchez, P.; Mir, M.; Katakis, I.; O’Sullivan, C.K. Aptamers: Molecular tools for analytical applications. Anal. Bioanal. Chem. 2008, 390, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, R.; Xu, J.; Xu, D.; Chen, H. Characterizing the interaction between aptamers and human IgE by use of surface plasmon resonance. Anal. Bioanal. Chem. 2008, 390, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Ammida, N.H.S.; Micheli, L.; Palleschi, G. Electrochemical immunosensor for determination of aflatoxin B1 in barley. Anal. Chim. Acta 2004, 520, 159–164. [Google Scholar] [CrossRef]
- Micheli, L.; Grecco, R.; Badea, M.; Moscone, D.; Palleschi, G. An electrochemical immunosensor for aflatoxin m1 determination in milk using screen-printed electrodes. Biosens. Bioelectron. 2005, 21, 588–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.O.; Tothill, I.E. Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosens. Bioelectron. 2009, 24, 2452–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanungo, L.; Pal, S.; Bhand, S. Miniaturised hybrid immunoassay for high sensitivity analysis of aflatoxin M1 in milk. Biosens. Bioelectron. 2011, 26, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Paniel, N.; Radoi, A.; Marty, J.-L. Development of an electrochemical biosensor for the detection of aflatoxin M1 in milk. Sensors 2010, 10, 9439–9448. [Google Scholar] [CrossRef] [PubMed]
- Vig, A.; Radoi, A.; Muñoz-Berbel, X.; Gyemant, G.; Marty, J.-L. Impedimetric aflatoxin M1 immunosensor based on colloidal gold and silver electrodeposition. Sens. Actuators B Chem. 2009, 138, 214–220. [Google Scholar] [CrossRef]
- Bacher, G.; Pal, S.; Kanungo, L.; Bhand, S. A label-free silver wire based impedimetric immunosensor for detection of aflatoxin M1 in milk. Sens. Actuators B Chem. 2012, 168, 223–230. [Google Scholar] [CrossRef]
- Parker, C.O.; Lanyon, Y.H.; Manning, M.; Arrigan, D.W.; Tothill, I.E. Electrochemical immunochip sensor for aflatoxin M1 detection. Anal. Chem. 2009, 81, 5291–5298. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, L.; Sadeghi, O.; Banitaba, M.H.; Shahrjerdi, A.; Davarani, S.S.H. A non-enzymatic nanomagnetic electro-immunosensor for determination of aflatoxin b1 as a model antigen. Sens. Actuators B Chem. 2013, 177, 1122–1127. [Google Scholar] [CrossRef]
- Owino, J.; Arotiba, O.; Hendricks, N.; Songa, E.; Jahed, N.; Waryo, T.; Ngece, R.; Baker, P.; Iwuoha, E. Electrochemical immunosensor based on polythionine/gold nanoparticles for the determination of aflatoxin B1. Sensors 2008, 8, 8262–8274. [Google Scholar] [CrossRef] [PubMed]
- Zaijun, L.; Zhongyun, W.; Xiulan, S.; Yinjun, F.; Peipei, C. A sensitive and highly stable electrochemical impedance immunosensor based on the formation of silica gel-ionic liquid biocompatible film on the glassy carbon electrode for the determination of aflatoxin B1 in bee pollen. Talanta 2010, 80, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.-L.; Qi, Q.-A.; Dong, Z.-L.; Liang, K.Z. An electrochemical enzyme immunoassay for aflatoxin B1 based on bio-electrocatalytic reaction with room-temperature ionic liquid and nanoparticle-modified electrodes. Sens. Instrum. Food Qual. Saf. 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.-R.; Wang, W.-C.; Xue, J.; Huang, Y.-L.; Yang, X.-X.; Tan, B.; Zhou, X.-P.; Shao, C.; Ding, S.-J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Zhou, J.; Xiong, P.; Hu, F.; Cao, Y.; Li, T.; Jiang, Q. An ultrasensitive electrochemiluminescent immunoassay for aflatoxin M1 in milk, based on extraction by magnetic graphene and detection by antibody-labeled cdte quantumn dots-carbon nanotubes nanocomposite. Toxins 2013, 5, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, W.; Xiong, Y.; Xu, Y.; Li, M.C. Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetric immunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens. Bioelectron. 2015, 63, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Le, C.L.; Cruz-Aguado, J.; Penner, G.A. DNA Ligands for Aflatoxin and Zearalenone. US Patent No. US20120225494 A1, 6 September 2012. [Google Scholar]
- Ma, X.; Wang, W.; Chen, X.; Xia, Y.; Wu, S.; Duan, N.; Wang, Z. Selection, identification, and application of aflatoxin B1 aptamer. Eur. Food Res. Technol. 2014, 238, 919–925. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Chen, X.; Xia, Y.; Duan, N.; Wu, S.; Wang, Z. Selection, characterization and application of aptamers targeted to aflatoxin B2. Food Control 2015, 47, 545–551. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C 2013, 33, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Pandey, A.K.; Rajput, Y.S.; Sharma, R. Selection of aptamers for aflatoxin M1 and their characterization. J. Mol. Recognit. 2014, 27, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Song, S.; Zhang, J.; Pan, D.; Wang, L.; Fan, C. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J. Am. Chem. Soc. 2007, 129, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Xiao, Y.; Plaxco, K.W. High specificity, electrochemical sandwich assays based on single aptamer sequences and suitable for the direct detection of small-molecule targets in blood and other complex matrices. J. Am. Chem. Soc. 2009, 131, 6944–6945. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.M.; Jackson, S.R.; Haselton, F.R.; Wright, D.W. Design, synthesis, and characterization of nucleic-acid-functionalized gold surfaces for biomarker detection. Langmuir 2012, 28, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Sharma, A.; Hayat, A.; Catanante, G.; Gobi, K.V.; Gurban, A.M.; Marty, J.L. Tetramethyl-6-carboxyrhodamine quenching-based aptasensing platform for aflatoxin B1: Analytical performance comparison of two aptamers. Anal. Biochem. 2016, 508, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sassolas, A.; Marty, J.-L.; Radi, A.-E. Highly sensitive ochratoxin A impedimetric aptasensor based on the immobilization of azido-aptamer onto electrografted binary film via click chemistry. Talanta 2013, 103, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Siontorou, C.G.; Nikolelis, D.P.; Miernik, A.; Krull, U.J. Rapid methods for detection of aflatoxin M1 based on electrochemical transduction by self-assembled metal-supported bilayer lipid membranes (s-BLMs) and on interferences with transduction of DNA hybridization. Electrochim. Acta 1998, 43, 3611–3617. [Google Scholar] [CrossRef]
- Dinçkaya, E.; Kınık, Ö.; Sezgintürk, M.K.; Altuğ, Ç.; Akkoca, A. Development of an impedimetric aflatoxin M1 biosensor based on a DNA probe and gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using pamam dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
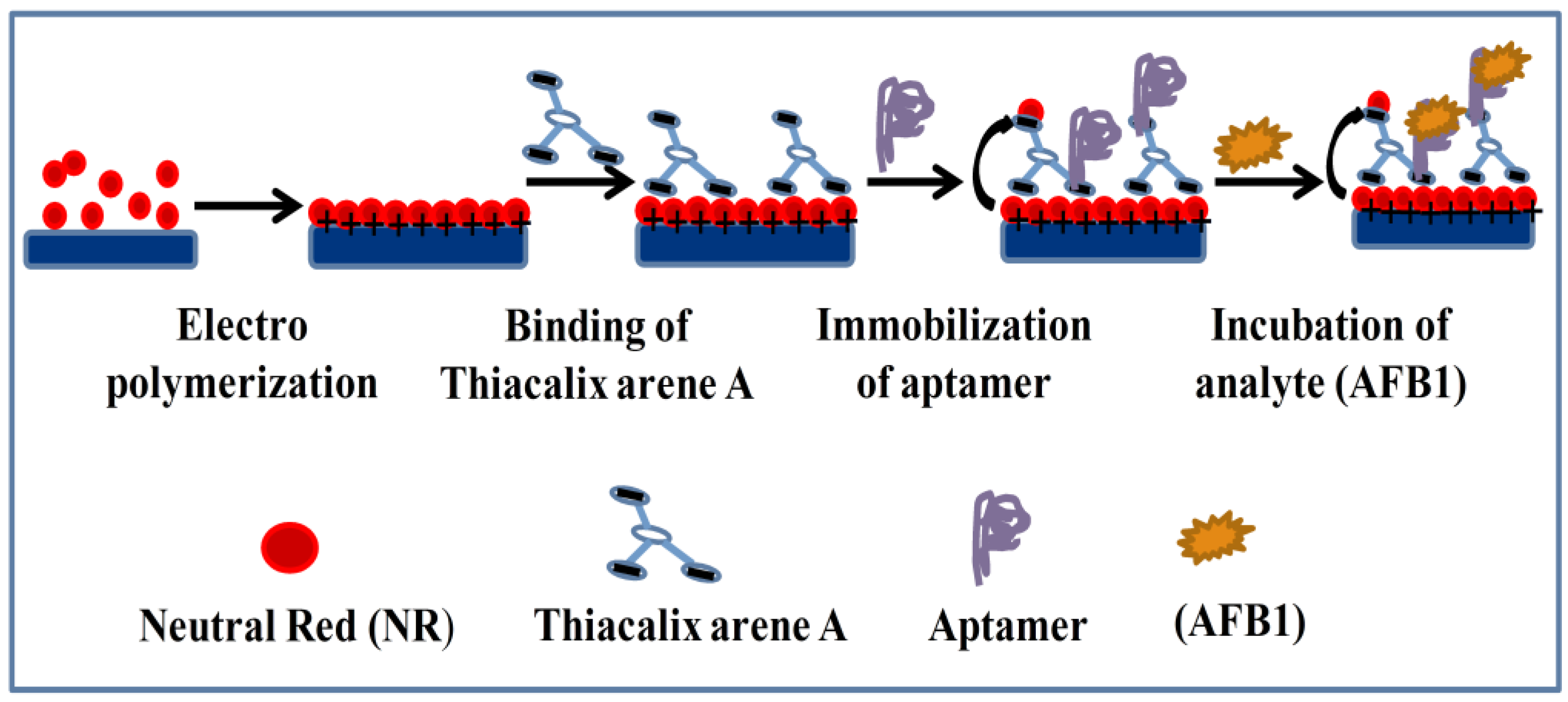
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Sitdikov, R.; Stoikov, I.; Nikolelis, D.; Hianik, T. Electrochemical aptasensor based on polycarboxylic macrocycle modified with neutral red for aflatoxin B1 detection. Electroanalysis 2014, 26, 2100–2109. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Vengatajalabathy Gobi, K.; Marty, J.L. Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Zheng, W.; Teng, J.; Cheng, L.; Ye, Y.; Pan, D.; Wu, J.; Xue, F.; Liu, G.; Chen, W. Hetero-enzyme-based two-round signal amplification strategy for trace detection of aflatoxin B1 using an electrochemical aptasensor. Biosens. Bioelectron. 2016, 80, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chu, H.; Mei, Z.; Deng, Y.; Xue, F.; Zheng, L.; Chen, W. Ultrasensitive one-step rapid detection of ochratoxin a by the folding-based electrochemical aptasensor. Anal. Chim. Acta 2012, 753, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Ivanov, A.; Konovalova, O.; Hianik, T. Molecularly imprinted polymerized methylene green as a platform for electrochemical sensing of aptamer–thrombin interactions. Electroanalysis 2009, 21, 1272–1277. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, K.; Ni, X.; Su, J.; Chen, Z. Electrochemical detection of thrombin based on aptamer and ferrocenylhexanethiol loaded silica nanocapsules. Biosens. Bioelectron. 2011, 26, 3536–3541. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Jeong, H.; Chung, B.H. Label-free electrochemical detection of human α-thrombin in blood serum using ferrocene-coated gold nanoparticles. Biosens. Bioelectron. 2011, 28, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Chen, C.; Zhu, L.; Ai, S. An electrochemical signal ‘off-on’ sensing platform for microrna detection. Analyst 2012, 137, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Dragacci, S.; Grosso, F. Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxinm1 in liquid milk: Collaborative study. J. AOAC Int. 2001, 84, 437–443. [Google Scholar] [PubMed]
- Kanungo, L.; Bhand, S. Fluorimetric immunoassay for multianalysis of aflatoxins. J. Anal. Methods Chem. 2013, 2013, 584964. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Maximum Permissible Level | |||
|---|---|---|---|---|
| EU | USFDA | CAC | FSSAI | |
| Milk and Milk based products | 25 pg/mL (AFM1) | 500 pg/mL (AFM1) | 500 pg/mL (AFM1) | 500 pg/mL (AFM1) |
| 50 pg/mL (AFM1) | ||||
| Nuts and dried food | 5 µg/kg (AFB1) | 20 µg/kg-Total | Not specified | 30 µg/kg |
| 10 µg/kg-Total | ||||
| Groundnuts & dried fruits and their processed products | 2 µg/kg (AFB1) | 20 µg/kg-Total | 15 µg/kg-Total | 30 µg/kg |
| 4 µg/kg-Total | ||||
| Cereals | 2 µg/kg (AFB1) | 20 µg/kg-Total | Not specified | 30 µg/kg |
| 4 µg/kg-Total | ||||
| Analyte | Method | LOD | Matrix | Reference |
|---|---|---|---|---|
| AFB1 | ELISA with DPV | 30 pg/mL | Barley | [49] |
| AFM1 | Amperomertic | 25 pg/mL | Milk | [50] |
| AFB1 | intermittent pulse amperometry (IPA) | 30 pg/mL | Corn | [52] |
| AFM1 | EIS | 15 ng/L | Milk | [55] |
| AFB1 | LSV | 0.06 ng/mL | Rice | [37] |
| AFM1 | EIS | 1 pg/mL | Milk | [56] |
| AFM1 | EIS | 8 ng/L | Milk | [57] |
| AFB1 | DPV | 0.07 ng/mL | - | [58] |
| AFM1 | DPV | 0.2 ng/mL | PBS | [59] |
| 0.7 ng/mL | - | [60] | ||
| AFB1 | EIS | 0.01 ng/mL | Bee pollen | [61] |
| AFB1 | Amperometric | 0.05 ng/mL | Human serum and grape samples | [62] |
| AFB1 | DPV | 3.5 pg/mL | Corn powder | [63] |
| AFM1 | Electrochemiluminescent (ECL) | 0.3 pg/mL | Milk | [64] |
| AFB1 | EIS | 0.5 pg/mL | Corn samples | [65] |
| Target | Aptamer Length | Base Pair Sequences (No.) | Sequences | Kd (nM) | Ref. |
|---|---|---|---|---|---|
| AFB1 | 50 | 16 | GTTGGGCACGTGTTGTCTCTCTGTGTCTCGTGCCCTTCGCTAGGCCCACA | N.R. | [66] |
| 80 | 26 | AGCAGCACAGAGGTCAGATGGTGCTATCATGCGCTCAATGGGAGACTTTAGCTGCCCCCACCTATGCGTGCTACCGTGAA | 11.29 ± 1.27 | [67] | |
| AFB2 | 80 | 26 | AGCAGCACAGAGGTCAGATGCTGACACCCTGGACCTTGGGATTCCGGAAGTTTTCCGGTACCTATGCGTGCTACCGTGAA | 9.83 ± 0.99 | [68] |
| AFM1 | 21 | 7 | ACTGCTAGAGATTTTCCACAT | N.R. | [69] |
| 72 | 24 | ATCCGTCACACCTGCTCTGACGCTGGGGTCGACCCGGAGAAATGCATTCCCCTGTGGTGTTGGCTCCCGTAT | 35.6 ± 2.6 | [70] |
| Target | Method Used | Limit of Detection (LOD) | Matrix | Ref. |
|---|---|---|---|---|
| AFM1 | Cyclic (CV) and square wave voltammetry (SWV) | 1.98 ng/L | Milk | [69] |
| AFM1 | Amperomertic | 0.5 nM | Milk | [76] |
| AFM1 | Electrochemical impedance spectroscopy (EIS) | N.R. | Milk | [77] |
| AFM1 | CV and SWV | 1.98 ng/L | Milk | [78] |
| AFB1 | CV and EIS | 0.40 ± 0.03 nM | peanuts-corn snacks | [79] |
| AFB1 | CV | 0.10 nM | peanuts, cashew nuts, white wine and soy sauce | [80] |
| EIS | 0.05 nM | |||
| AFB1 | EIS | 0.12 ng/mL (seqA) | Alcoholic beverages | [81] |
| 0.25 ng/mL (seqB) | ||||
| AFB1 | SWV | 0.6 × 10−4 ng/L | Corn | [82] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Goud, K.Y.; Hayat, A.; Bhand, S.; Marty, J.L. Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection. Chemosensors 2017, 5, 1. https://doi.org/10.3390/chemosensors5010001
Sharma A, Goud KY, Hayat A, Bhand S, Marty JL. Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection. Chemosensors. 2017; 5(1):1. https://doi.org/10.3390/chemosensors5010001
Chicago/Turabian StyleSharma, Atul, Kotagiri Yugender Goud, Akhtar Hayat, Sunil Bhand, and Jean Louis Marty. 2017. "Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection" Chemosensors 5, no. 1: 1. https://doi.org/10.3390/chemosensors5010001