Hierarchical Self-Assembly of Amino Acid Derivatives into Enzyme-Responsive Luminescent Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
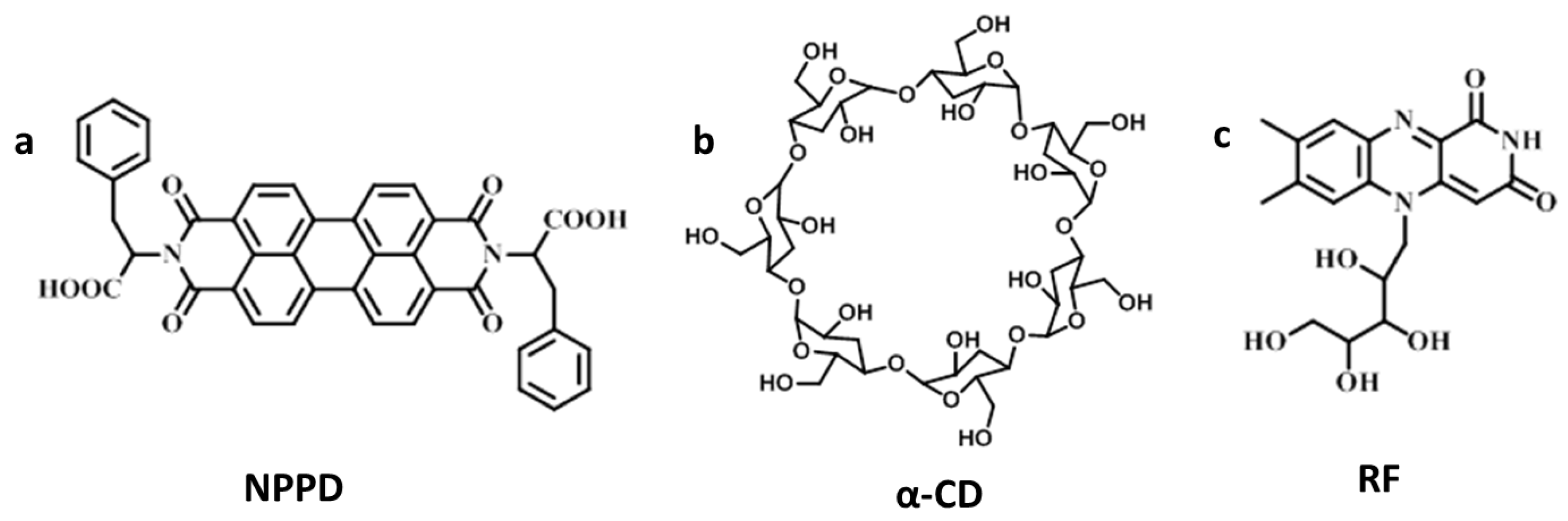
2.2. Synthesis of NPPD
2.3. Characterization
2.4. Gelation Preparation
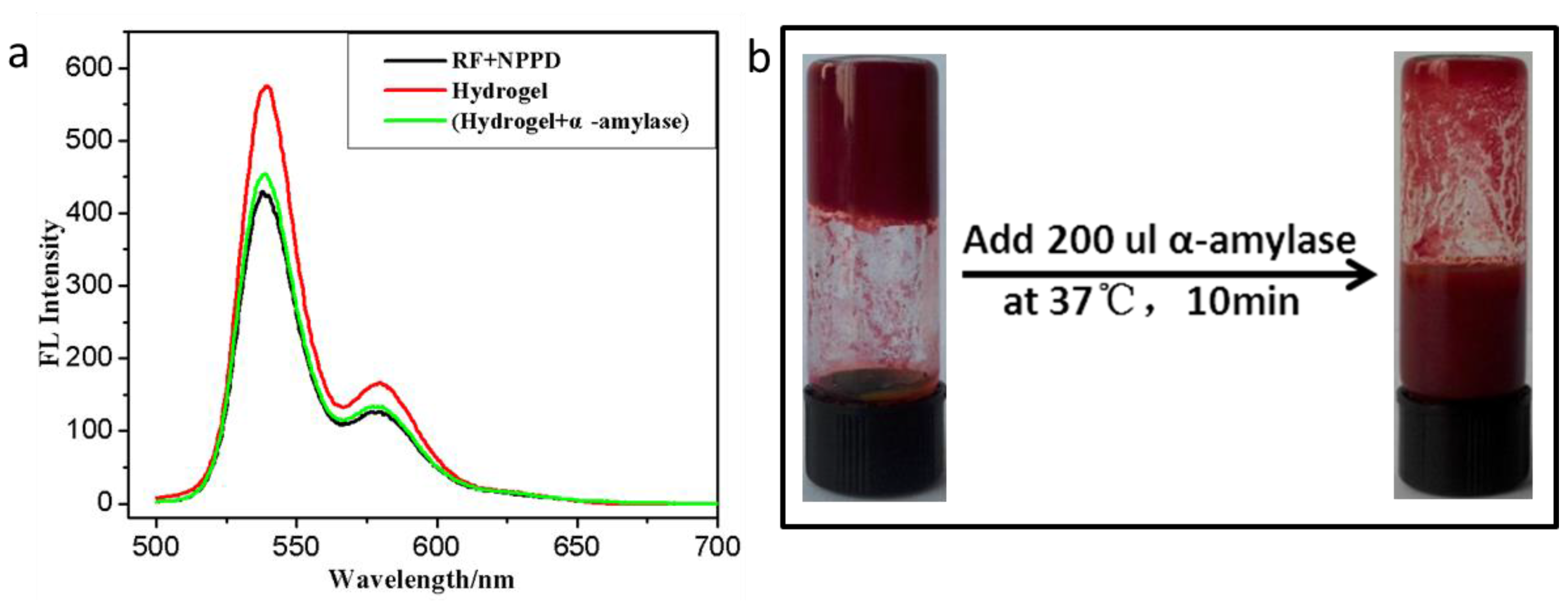
2.5. α-Amylase Responsive Test
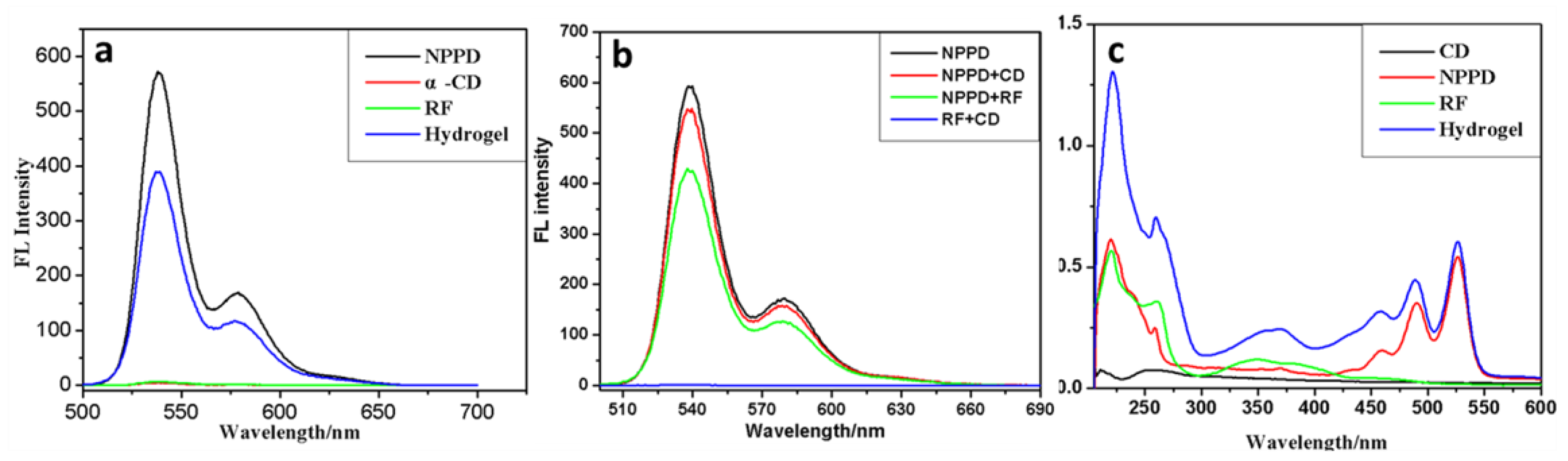
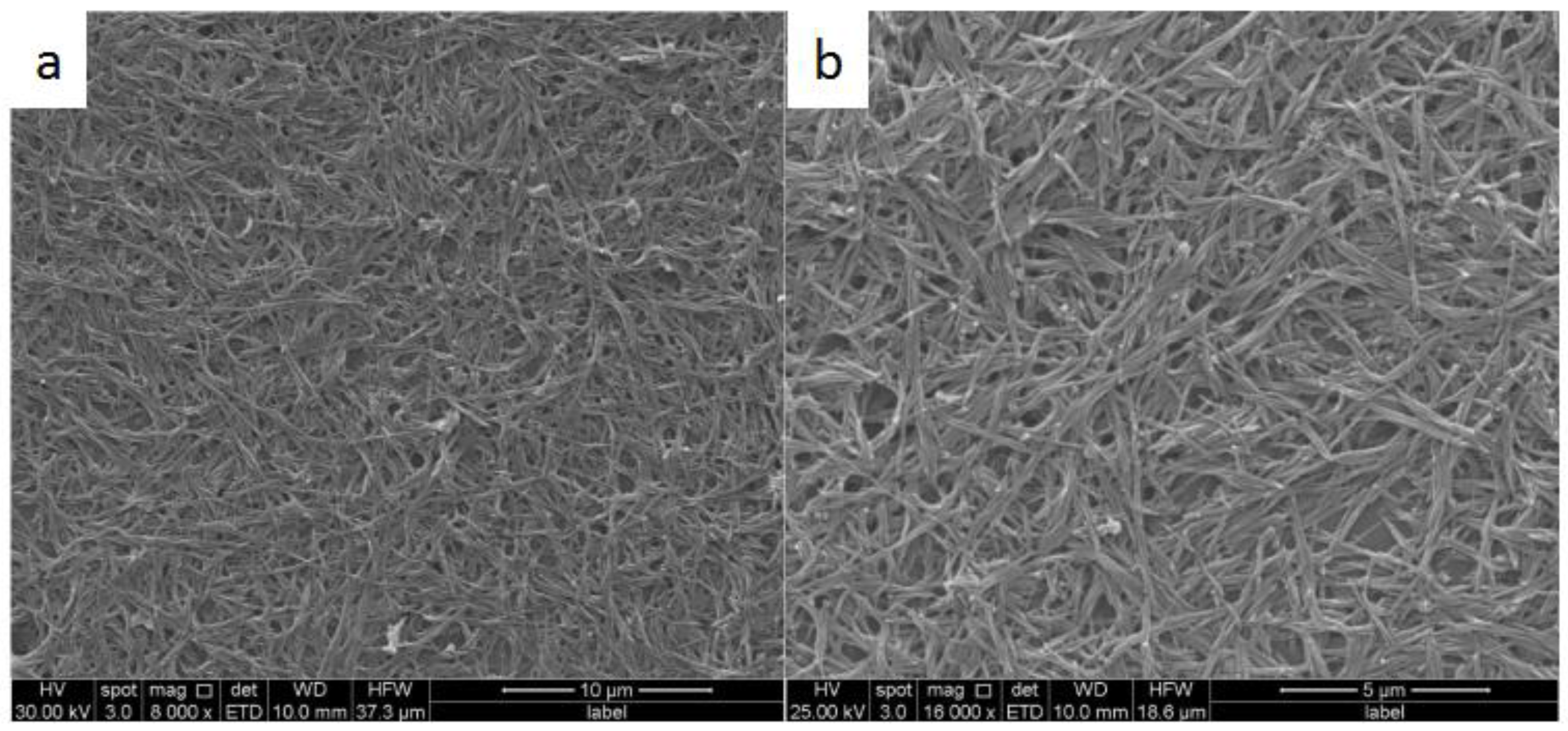
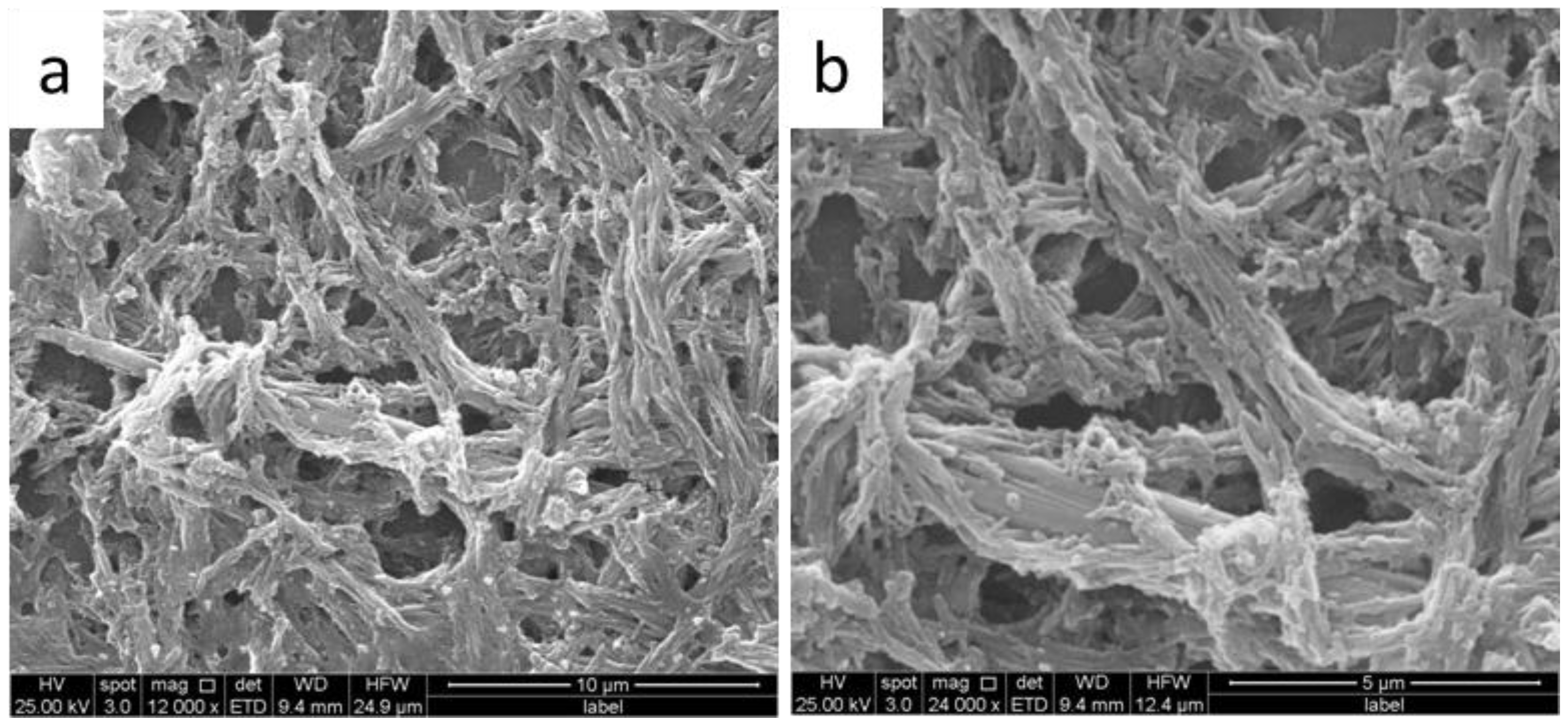
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, H.L.; Wang, Y.H.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Cheng, L.X.; Huang, P.; Peng, Y.; Wu, Y.; Li, X.; Li, X.K.; Fan, X.L. A Smart pH-Responsive Three Components Luminescent Hydrogel. J. Funct. Biomater. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Okano, T. (Eds.) Stimuli-Responsive Hydrogels and Their Application to Functional Materials; Springer: New York, NY, USA, 2010.
- Henderson, P.W.; Singh, S.P.; Krijgh, D.D.; Yamamoto, M.; Rafii, D.C.; Sung, J.J.; Rafii, S.; Rabbany, S.Y.; Spector, J.A. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regener. 2011, 19, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V. Enzyme-responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Zelzer, M.; Todd, S.J.; Hirst, A.R.; McDonald, T.O.; Ulijn, R.V. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2013, 1, 11–39. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Shi, S.; Wang, B.K.; Li, I.; Jalan, A.A.; Sarkar, B.; Wickremasinghe, N.C.; Hartgerink, J.D. Drug-triggered and cross-linked self-assembling nanofibrous hydrogels. J. Am. Chem. Soc. 2015, 137, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Habibia, N.; Kamalyb, N.; Memicc, A.; Shafieea, H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Bond, C.W.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanotechnol. Biol. Med. 2017, 13, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, Y.; Drechsler, M.; Huang, J. Enzyme-triggered model self-assembly in surfactant-cyclodextrin systems. Chem. Commun. 2012, 48, 7347–7349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C. Supramolecular amphiphiles. Chem. Soc. Rev. 2011, 40, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Zhang, X. Amphiphilic building blocks for self-assembly: From amphiphiles to supra-amphiphiles. Acc. Chem. Res. 2012, 45, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Zhang, X. Superamphiphiles as building blocks for supramolecular engineering: Towards functional materials and surfaces. Small 2011, 7, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, C.; Wang, Z.; Zhang, X. Bolaform superamphiphile based on a dynamic covalent bond and its self-assembly in water. Langmuir 2011, 27, 12375–12380. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, Y.; Liu, Y.; Wang, C.; Li, Z.; Zhang, X. Self-assembly of supra-amphiphiles based on dual charge-transfer interactions: From nanosheets to nanofibers. Langmuir 2012, 28, 10697–10702. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Ma, N.; Ren, H.; Xu, H.; Li, Z.; Wang, Z.; Zhang, X. Oxidation-responsive micelles based on a selenium-containing polymeric superamphiphile. Langmuir 2010, 26, 14414–14418. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, Y.; Wang, Z.; Zhang, X. Superamphiphiles based on charge transfer complex: Controllable hierarchical self-assembly of nanoribbons. Langmuir 2010, 26, 14509–14511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, K.; Wang, Z.; Zhang, X. Host-Enhanced π–π Interaction for Water-Soluble Supramolecular Polymerization. Chem. Eur. J. 2011, 17, 9930–9935. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe motif for peptide self-assembly in nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, P.; Li, S.; Wang, C.; Xu, H.; Wang, Z.; Zhang, X. UV-responsive polymeric superamphiphile based on a complex of malachite green derivative and a double hydrophilic block copolymer. Langmuir 2011, 27, 14108–14111. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Williams, R.J.; Mart, R.J.; Ulijn, R.V. Exploiting biocatalysis in peptide self-assembly. Biopolymers 2010, 94, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Gianneschi, N.C. Enzyme-directed assembly and manipulation of organic nanomaterials. Chem. Commun. 2011, 47, 11814–11821. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic hydrogelation of small molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Börner, H.G.; Kühnle, H.; Hentschel, J. Making “smart polymers” smarter: Modern concepts to regulate functions in polymer science. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1–14. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Liu, K.; Wang, Z.; Zhang, X. Enzyme-responsive polymeric supra-amphiphiles formed by the complexation of chitosan and ATP. Langmuir 2012, 28, 14562–14566. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, C.; Han, P.; Wang, Z.; Zhang, X. Acetylcholinesterase responsive polymeric supra-amphiphiles for controlled self-assembly and disassembly. Langmuir 2012, 28, 6032–6036. [Google Scholar] [CrossRef] [PubMed]
- Peltier, R.; Chen, G.; Lei, H.; Zhang, M.; Gao, L.; Lee, S.S.; Wang, Z.; Sun, H. The rational design of a peptide-based hydrogel responsive to H2S. Chem. Commun. 2015, 51, 17273–17276. [Google Scholar] [CrossRef] [PubMed]
- Inouye, H.; Sharma, D.; Goux, W.J.; Kirschner, D.A. Structure of core domain of fibril-forming PHF/Tau fragments. Biophys. J. 2006, 90, 1774–1789. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Makin, O.S.; Serpell, L.C. Structures for amyloid fibrils. FEBS J. 2005, 272, 5950–5961. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. Global analysis of tandem aromatic octapeptide repeats: The significance of the aromatic–glycine motif. Bioinformatics. 2002, 18, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Vaks, L.; Carny, O.; Trudler, D.; Magno, A.; Caflisch, A.; Frenkel, D.; Gazit, E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012, 8, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Shirman, E.; Weissman, H.; Shimoni, E.; Wolf, S.G.; Pinkas, I.; Rybtchinski, B. Supramolecular gel based on a perylene diimide dye: Multiple stimuli responsiveness, robustness, and photofunction. J. Am. Chem. Soc. 2009, 131, 14365–14373. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Roy, B.; Nandi, A.K. A light harvesting Bi-component hydrogel with a riboflavin acceptor. Chem. Commun. 2012, 48, 10850–10852. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kang, Y.; Liu, K.; Li, Z.; Wang, Z.; Zhang, X. pH and enzymatic double-stimuli responsive multi-compartment micelles from supra-amphiphilic polymers. Polym. Chem. 2012, 3, 3056–3059. [Google Scholar] [CrossRef]
- Wurthner, F.; Kaiser, T.E.; Saha-Moller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.E.; Carmieli, R.; Mickley, S.M.; Vura-Weis, J.; Wasielewski, M.R. Photoinitiated charge transport through π-stacked electron conduits in supramolecular ordered assemblies of donor-acceptor triads. J. Am. Chem. Soc. 2009, 131, 11919–11929. [Google Scholar] [CrossRef] [PubMed]
- Malinovskii, L.V.; Wenger, D.; Haener, R. Nucleic acid-guided assembly of aromatic chromophores. Chem. Soc. Rev. 2010, 39, 410–422. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Peng, Y.; Liu, W.; Fan, Y.; Wu, Y.; Li, X.; Fan, X. Hierarchical Self-Assembly of Amino Acid Derivatives into Enzyme-Responsive Luminescent Gel. Chemosensors 2017, 5, 6. https://doi.org/10.3390/chemosensors5010006
Li Y, Peng Y, Liu W, Fan Y, Wu Y, Li X, Fan X. Hierarchical Self-Assembly of Amino Acid Derivatives into Enzyme-Responsive Luminescent Gel. Chemosensors. 2017; 5(1):6. https://doi.org/10.3390/chemosensors5010006
Chicago/Turabian StyleLi, Yibao, Yu Peng, Wei Liu, Yulan Fan, Yongquan Wu, Xun Li, and Xiaolin Fan. 2017. "Hierarchical Self-Assembly of Amino Acid Derivatives into Enzyme-Responsive Luminescent Gel" Chemosensors 5, no. 1: 6. https://doi.org/10.3390/chemosensors5010006




