Electrochemical Study of Trametes Versicolor Laccase Compatibility to Different Polyphenolic Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Preparation of Carbon Paste Electrode Modified by Laccase
2.4. Electrochemical Experiments
3. Results
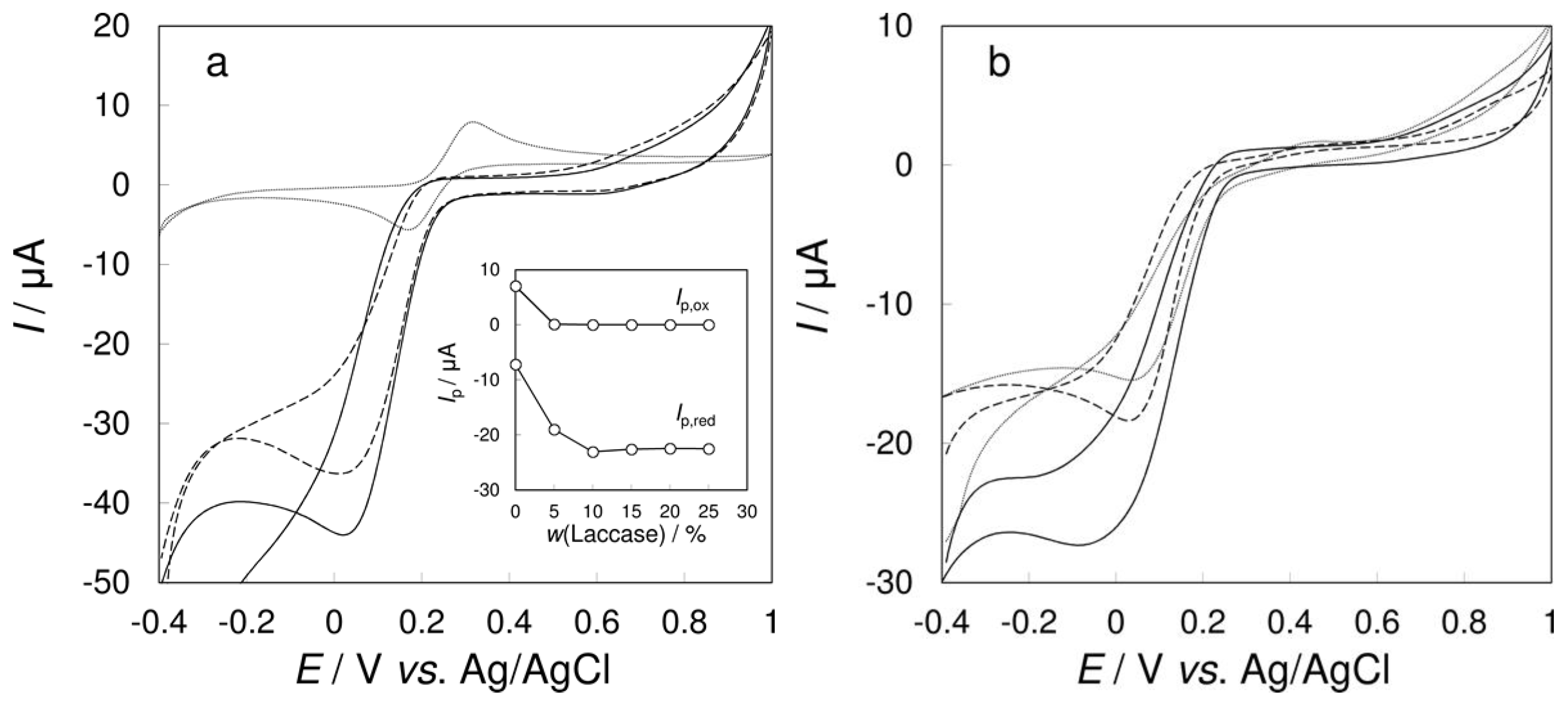
3.1. Effect of the Amount of Laccase in Carbon Paste on Sensitivity
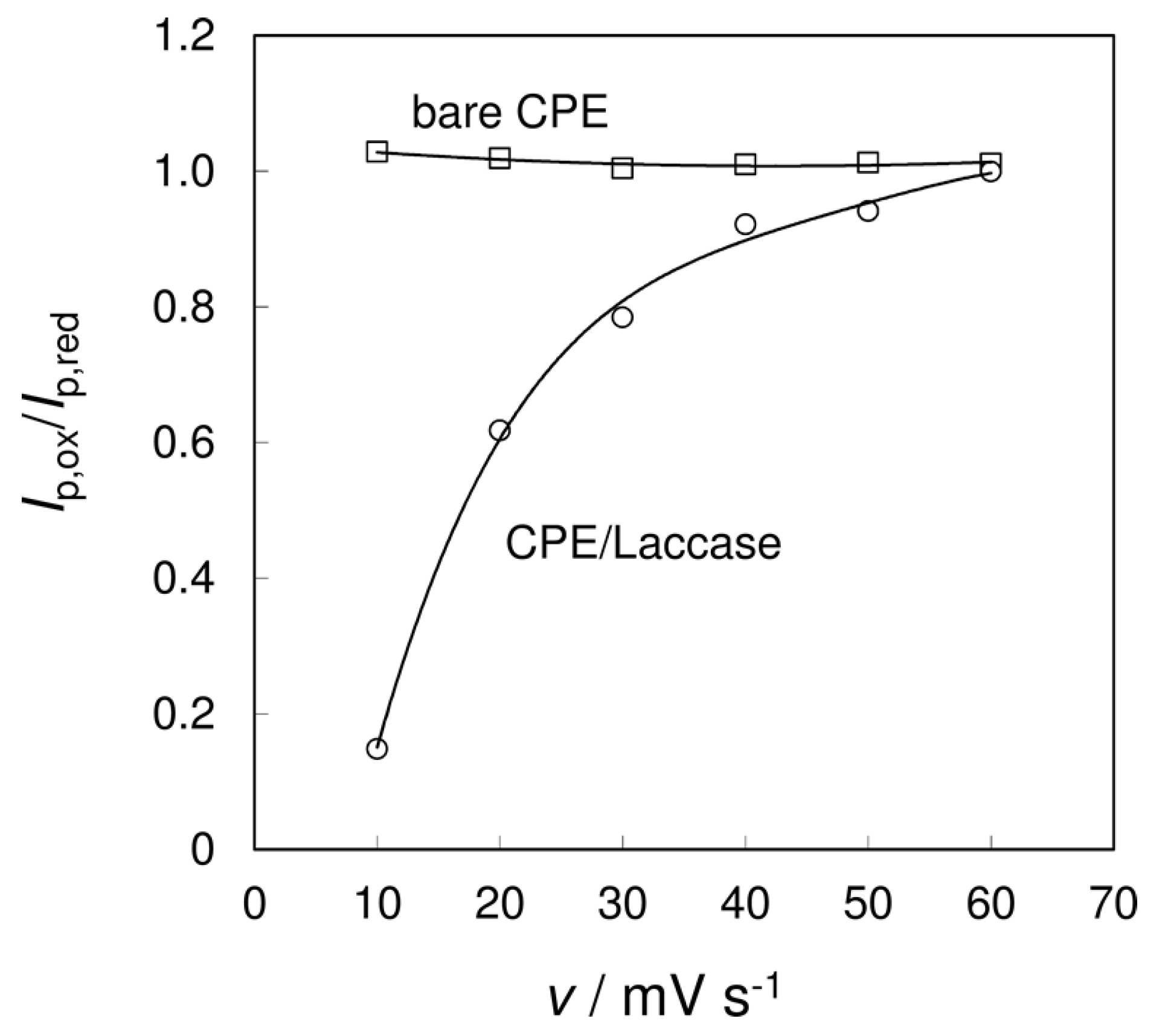
3.2. Effect of Scan Rate on the Electrochemical Detection of Biocatalysis
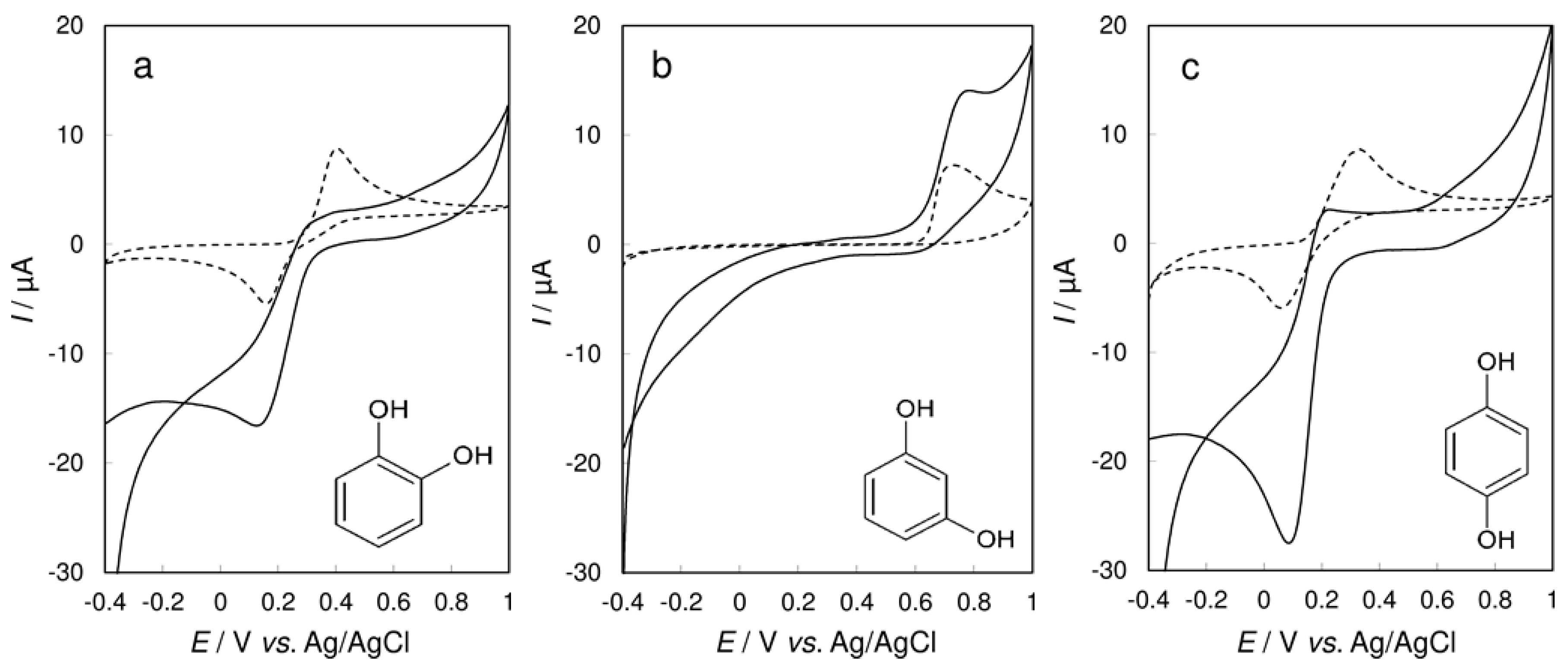
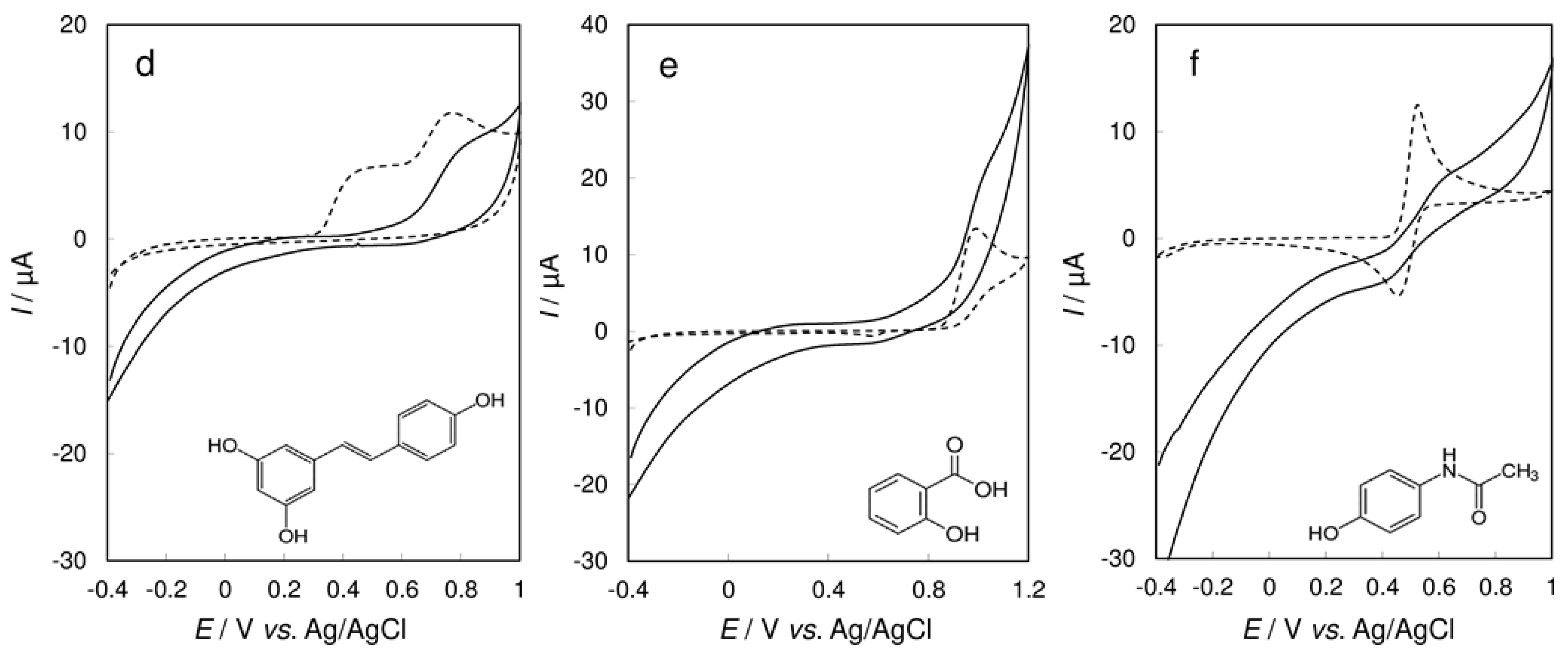
3.3. Cyclic Voltammetry of Phenol at CPE/Laccase vs. Bare CPE
3.4. Cyclic Voltammetry of Cresols at CPE/Laccase vs. Bare CPE
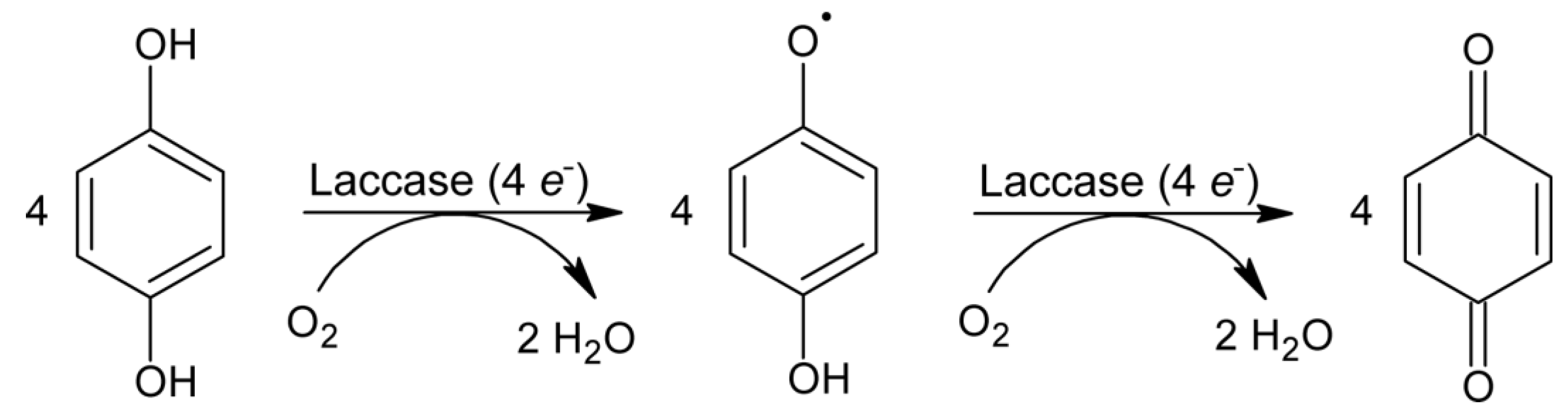
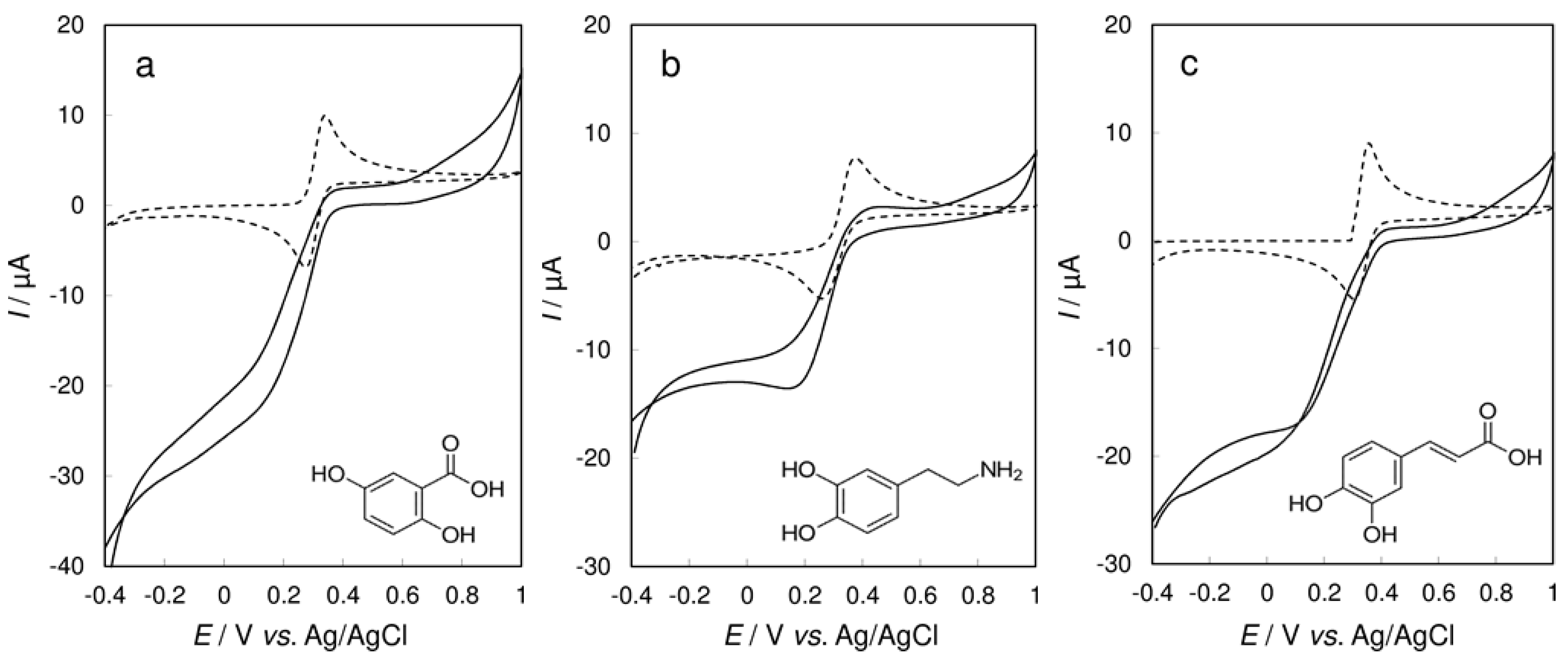
3.5. Cyclic Voltammetry of Benzenediols at CPE/Laccase vs. Bare CPE
3.6. Final Discussion and Confirmation of Obtained Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPE | Carbon paste electrode |
| CPE/Laccase | Carbon paste electrode modified with enzyme laccase |
References
- Kalcher, K. Chemically modified carbon paste electrodes in voltammetric analysis. Electroanalysis 1990, 2, 419–433. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A. Electroanalysis with Carbon Paste Electrodes; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Pohanka, M.; Skladal, P. Electrochemical biosensors—Principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar]
- Gorton, L. Carbon-paste electrodes modified with enzymes, tissues, and cells. Electroanalysis 1995, 7, 23–45. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Cairney, J.W.G. Laccases and other polyphenol oxidases in ecto- and ericoid mycorrhizal fungi. Mycorrhiza 2002, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef] [PubMed]
- Witayakran, S.; Ragauskas, A.J. Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Thurston, C.F. The Structure and Function of Fungal Laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Haghighi, B.; Rahmati-Panah, A.; Shleev, S.; Gorton, L. Carbon ceramic electrodes modified with laccase from Trametes hirsuta: Fabrication, characterization and their use for phenolic compounds detection. Electroanalysis 2007, 19, 907–917. [Google Scholar] [CrossRef]
- Jarosz-Wilkolazka, A.; Ruzgas, T.; Gorton, L. Amperometric detection of mono- and diphenols at Cerrena unicolor laccase-modified graphite electrode: Correlation between sensitivity and substrate structure. Talanta 2005, 66, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Gorton, L.; Ruzgas, T.; Jonsson, L.J. Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta 2003, 487, 3–14. [Google Scholar] [CrossRef]
- Haghighi, B.; Jarosz-Wilkolazka, A.; Ruzgas, T.; Gorton, L.; Leonowicz, A. Characterization of graphite electrodes modified with laccases from Trametes hirsuta and Cerrena unicolor and their use for flow injection amperometric determination of some phenolic compounds. Int. J. Environ. Anal. Chem. 2005, 85, 753–770. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Lele, S.S. Laccase: Properties and Applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Lante, A.; Crapisi, A.; Krastanov, A.; Spettoli, P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem. 2000, 36, 51–58. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, Q.; Yang, M.; Jiao, K. Electrochemical behaviors of hydroquinone on a carbon paste electrode with ionic liquid as binder. Bull. Korean Chem. Soc. 2008, 29, 915–920. [Google Scholar]
- Janeiro, P.; Brett, A.M.O. Catechin electrochemical oxidation mechanisms. Anal. Chim. Acta 2004, 518, 109–115. [Google Scholar] [CrossRef]
- Bark, K.M.; Yeom, J.E.; Yang, J.I.; Yang, I.J.; Park, C.H.; Park, H.R. Spectroscopic Studies on the Oxidation of Catechin in Aqueous Solution. Bull. Korean Chem. Soc. 2011, 32, 3443–3447. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E. Enzyme Initiated Radical Polymerizations. Polymers 2012, 4, 759–793. [Google Scholar] [CrossRef]
- Rolff, M.; Schottenheim, J.; Decker, H.; Tuczek, F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011, 40, 4077–4098. [Google Scholar] [CrossRef] [PubMed]
- Eicken, C.; Krebs, B.; Sacchettini, J.C. Catechol oxidase—Structure and activity. Curr. Opin. Struct. Biol. 1999, 9, 677–683. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Stratford, M.R.L.; Ramsden, C.A.; Riley, P.A. Mechanistic studies of the inactivation of tyrosinase by resorcinol. Bioorg. Med. Chem. 2013, 21, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Yaropolov, A.I.; Shleev, S.V.; Morozova, O.V.; Zaitseva, E.A.; Marko-Varga, G.; Emneus, J.; Gorton, L. An amperometric biosensor based on laccase immobilized in polymer matrices for determining phenolic compounds. J. Anal. Chem. 2005, 60, 553–557. [Google Scholar] [CrossRef]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sýs, M.; Metelka, R.; Frangu, A.; Vytřas, K.; Arbneshi, T. Electrochemical Study of Trametes Versicolor Laccase Compatibility to Different Polyphenolic Substrates. Chemosensors 2017, 5, 9. https://doi.org/10.3390/chemosensors5010009
Sýs M, Metelka R, Frangu A, Vytřas K, Arbneshi T. Electrochemical Study of Trametes Versicolor Laccase Compatibility to Different Polyphenolic Substrates. Chemosensors. 2017; 5(1):9. https://doi.org/10.3390/chemosensors5010009
Chicago/Turabian StyleSýs, Milan, Radovan Metelka, Arbër Frangu, Karel Vytřas, and Tahir Arbneshi. 2017. "Electrochemical Study of Trametes Versicolor Laccase Compatibility to Different Polyphenolic Substrates" Chemosensors 5, no. 1: 9. https://doi.org/10.3390/chemosensors5010009





